Abstract
Corneal scarring is the result of a disease, infection or injury. The resulting scars cause significant loss of vision or even blindness. To-date, the most successful treatment is corneal transplantation, but it does not come without side effects. One of the corneal dystrophies that are correlated with corneal scarring is keratoconus (KC). The onset of the disease is still unknown; however, altered cellular metabolism has been linked to promoting the fibrotic phenotype and therefore scarring. We have previously shown that human keratoconus cells (HKCs) have altered metabolic activity when compared to normal human corneal fibroblasts (HCFs). In our current study, we present evidence that quercetin, a natural flavonoid, is a strong candidate for regulating metabolic activity of both HCFs and HKCs in vitro and therefore a potential therapeutic to target the altered cellular metabolism characteristic of HKCs. Targeted mass spectrometry-based metabolomics was performed on HCFs and HKCs with and without quercetin treatment in order to identify variations in metabolite flux. Overall, our study reveals a novel therapeutic target OF Quercetin on corneal stromal cell metabolism in both healthy and diseased states. Clearly, further studies are necessary in order to dissect the mechanism of action of quercetin.
Keywords: metabolomics, cornea, fibrosis, cell metabolism, urea cycle, quercetin, keratoconus
INTRODUCTION
Cellular metabolism involves a series of biochemical reactions that occur within the cells of living organisms and enable energy production, assembly and breakdown of key metabolites and biomolecules.1 Metabolism allows organisms to grow, reproduce and respond to external stimuli.2,3 Because the environment of most tissues is incredibly dynamic, cellular metabolism must be accurately regulated to ensure certain optimal conditions within cells, thereby maintaining a condition known as homeostasis.
The study of the collective metabolome, termed metabolomics, is a useful tool to measure changes in bioenergetics within different cell types and with changes in local microenvironments. Changes in cellular metabolism within a single cell can promote numerous processes including cellular proliferation, differentiation, quiescence, and apoptosis.3–6 A number of previous studies have utilized metabolomics to identify variations in concentration of metabolites as they relate to human disease.7–9 Moreover, metabolomics has also been used to characterize a variety of ocular diseases, including KC,10,11 retinitis pigmentosa,12 uveitis13 and a parasite-derived disease termed onchocerciasis.14 Understanding the role of cellular metabolism in promoting disease is crucial to developing novel therapeutics to target a central regulatory system that affects multiple signalling pathways and ultimately determines cellular fate. Moreover, changes in metabolism also affect the extracellular environment, including extracellular matrix (ECM) composition and assembly, thereby regulating tissue structure and endocrine signalling pathways.
The human cornea is a highly organized and transparent tissue that is directly affected by changes in ECM structure.15,16 Resident cells, termed keratocytes, are normally quiescent until there is an invasion such as disease, infection and/or injury. In response to these conditions, keratocytes become ‘activated’ and differentiate to what are known as myofibroblasts.17,18 These cells are responsible for corneal wound healing and the reestablishment of the damaged corneal ECM. Without external intervention, myofibroblasts will secrete and assemble a dense, disorganized ECM leading to scar formation and vision impairment. Corneal dystrophies, such as KC, also exhibit characteristics of scar formation.19,20 While the aetiology and onset of this disease is unknown, it is clear that cellular metabolism is abnormal in KC, as previously reported.11,21
Given the importance of cellular bioenergetics within the cornea, we report here the effect of a novel metabolic modulator known as quercetin. Quercetin is a flavonoid widely distributed in nature. In terms of its biosynthesis, quercetin is born from the phenylpropanoid pathway in a series of highly regulated reactions.22,23 Studies have shown that flavonoids, including quercetin, play a protective role against UV-light damage in plants, whereby their biosynthesis is altered dependent on cellular stress by exposure to UV-light.24 We have recently reported quercetin as a key regulator of corneal fibrosis and ECM assembly in KC.25 As a natural progression to our recent findings, this study establishes the metabolic activity of quercetin in both HCFs and HKCs. To the authors’ knowledge, this is the first report of metabolic alterations in corneal cells following quercetin treatment.
Overall, our results show that quercetin significantly affects concentrations of critical metabolites in both HCFs and HKCs, suggesting that quercetin treatment may modulate energy production in corneal fibroblasts thereby modifying cell function and likely differentiation. Further investigation of the potential therapeutic role of quercetin may have important clinical implications to reduce scarring following corneal injury or KC dystrophy. Furthermore, our study suggests that targeting specific enzymes involved in glucose metabolism may be an approach to treat corneal dystrophies, such as KC.
MATERIALS AND METHODS
Primary cultures of human corneal fibroblasts (HCF) and keratoconus (HKC)
The research adhered to the tenets of the Declaration of Helsinki. HCFs and HKCs were cultured as previously described.11,26,27 Briefly, HCFs were collected from normal patients from the National Disease Research Interchange (NDRI; Philadelphia, PA), and HKCs were obtained following isolation from KC corneas obtained from Aarhus University Hospital (Aarhus, Denmark). Epithelium and endothelium were removed, and the stromal tissue was sliced into small pieces and incubated in Eagle’s Minimum Essential Medium (EMEM) with 10% foetal bovine serum (Biologicals: Lawrenceville). After 2 weeks of incubation, cells were passaged into a 100 mm cell culture plate and grown to confluency prior to seeding into six-transwell plates.
3D constructs
3D constructs were developed as described previously.11,26 Briefly, HCFs were seeded at a density of 106 cell/well into six-well transwell plates containing a 0.4-μm pore polycarbonate membrane in each well (Corning Costar; Charlotte, NC). Constructs were grown in 10% FBS EMEM media and stimulated with a stable Vitamin C derivative (0.5 mM 2-O-α-D-glucopyranosyl-L-ascorbic acid, American Custom Chemicals Corporation) for 4 weeks with media changes 3× per week. 3D constructs containing HCFs and HKCs were treated with vehicle (dimethylsulfoxide, DMSO) or 10-μM quercetin (Sigma-Aldrich, St. Louis, MO) for the entire 4 week period. At the end of the 4 week time point, constructs (cells + self-assembled ECM) were isolated and submitted for metabolite extraction.
Metabolite extraction
Metabolites were isolated from cells as previously described.11 Briefly, cells were washed with 1×PBS and scraped into clean centrifuge tubes containing ice-cold 80% methanol. Samples were incubated on dry ice for 15 min and centrifuged overnight at 4 °C (14 000 g). Metabolites were isolated and stored at −80 °C until further use.
Targeted mass spectrometry
Dried metabolites were dissolved in 20-μl HPLC-graded water and analysed by targeted microcapillary liquid chromatography-tandem mass spectrometry (LC-MS/MS) using a hybrid 5500 QTRAP triple quadrupole mass spectrometer (AB/SCIEX) coupled to a Prominence UFLC system (Shimadzu) and analysed with selected reaction monitoring (SRM) with positive/negative polarity switching. Label-free quantification was used to quantify and determine the differential expression levels of metabolites between samples.7,11 Metabolites with significant up- or downregulation from MultiQuant 2.1 software (AB/SCIEX) were uploaded to MetaboAnalyst (http://www.metaboanalyst.ca/MetaboAnalyst) for statistical and pathway analysis according to previously published data.11,28
Statistical analysis
All data was analysed using an unpaired Student T-test. P-values of less than 0.05 were considered statistically significant.
RESULTS
Metabolic predicted pathways
We have recently identified quercetin as a potential inhibitor of corneal fibrotic markers, including α-smooth muscle actin (α-SMA) and Collagen III.25 In our current study, we explored the effects of quercetin on modulating cellular metabolism in HCFs and HKCs in vitro. Using metabolomics, we tested each sample for 295 metabolites, of which 85 were identified in all samples. These metabolites are listed in Table 1. The differences in the metabolite levels in HCFs and HKCs following quercetin treatment were assessed and quantified. Only the metabolites that were up/downregulated by more than a 2:1 ratio were included in our analysis. Table 2 shows the number of metabolites up or downregulated in both HCFs and HKCs following quercetin treatment. We found 16/85 metabolites upregulated in HCFs where 44/85 in HKCs upon quercetin treatment. On the other hand, 23/85 metabolites were downregulated in HCFs and 3/85 in HKCs.
Table 1.
Comprehensive list of detected metabolites present in all samples measured by targeted LC-MS/MS
| Identified metabolites | ||
|---|---|---|
| Glycolate | Uric acid | Creatinine |
| 2-Oxobutanoate | Dihydroxy-acetone-phosphate | Proline |
| Acetoacetate | D-Glyceraldehdye-3-phosphate | Betaine |
| Glycerate | sn-Glycerol-3-phosphate | Threonine |
| Fumarate | Aconitate | Creatine |
| Maleic acid | Ascorbic acid | Nicotinamide |
| 2-Keto-isovalerate | 2-Isopropylmalic acid | DL-Pipecolic acid |
| Guanidoacetic acid | Pyrophosphate | Leucine-isoleucine |
| Succinate | 4-Pyridoxic acid | Glutamine |
| Methylmalonic acid | 3-Phosphoglycerate | Glutamate |
| Nicotinate | Indoleacrylic acid | Methionine |
| Taurine | Isocitrate | Histidine |
| Citraconic acid | Citrate | Carnitine |
| 2-Ketohaxanoic acid | D-Erythrose-4-phosphate | Arginine |
| N-Acetyl-L-alanine | Xanthurenic acid | Phosphorylcholine |
| Oxaloacetate | Deoxyribose-phosphate | Acetylcarnitine DL |
| Hydroxyisocaproic acid | Pantothenate | Tryptophan |
| Malate | Ribose-phosphate | Cystine |
| Hypoxanthine | Hexose-phosphate | Biotin |
| Anthranilate | Glucose-1-phosphate | Glycerophosphocholine |
| p-Aminobenzoate | Glucose-6-phosphate | Glutathione |
| Carbamoyl phosphate | Inosine | Orotate |
| Phenylpropiolic acid | D-Sedoheptulose-1-7-phosphate | Dihydroorotate |
| 2-Oxo-4-methylthiobutanoate | Glutathione-nega | Allantoin |
| 2-Hydroxy-2-Methylbutanedioic acid | Trehalose-sucrose | Aminoadipic acid |
| Orotidine-5-phosphate | Phenyllactic acid | |
| Xanthine | ADP-nega | Quinolinate |
| 2,3-Dihydroxybenzoic acid | 2-Hydroxygluterate | Serine |
| Choline | Alanine | |
Table 2.
Total number of upregulated and downregulated metabolites identified in normal HCFs and diseased HKCs following quercetin treatment
| Cell type + treatment | Total # of upregulated metabolites | Total # of downregulated metabolites |
|---|---|---|
| HCF + quercetin | 16 | 23 |
| HKC + quercetin | 44 | 3 |
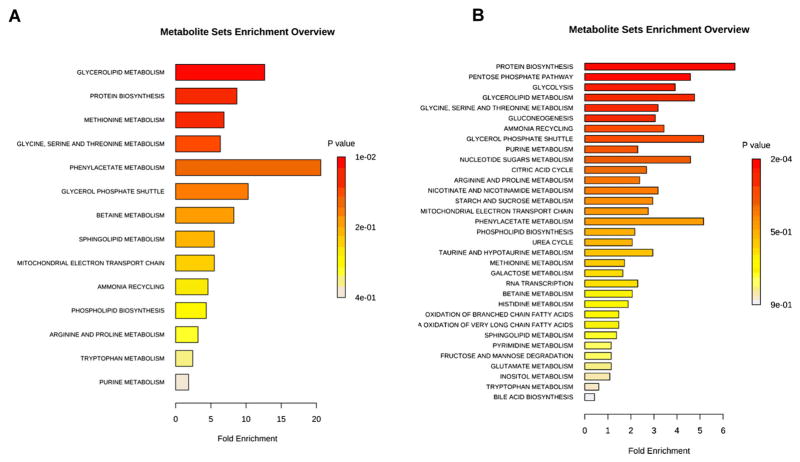
In order to determine the major biochemical pathways targeted by quercetin, we utilized MetaboAnalyst28 to identify pathway enrichment promoted by quercetin treatment. Interestingly, our results show a differential response to quercetin by HCFs and HKCs that may provide clues into defects in HKCs that can be targeted. We saw an increase in pathways associated with glycerolipid metabolism (8-fold), protein biosynthesis (6-fold), methionine metabolism (5-fold) and phenyllacetate metabolism (12-fold) in quercetin-treated HCFs (Figure 1A, p <0.02). Quercetin treatment increased sphingolipid metabolism and phospholipid biosynthesis in both HCFs (fourfold, threefold, respectively, p <0.05, Figure 1A) and HKCs (1.5-fold, 2.5-fold, respectively, p <0.05, Figure 1B) suggesting that quercetin affects lipid metabolism in both normal and diseased cells in a similar mechanism. We measured a significant increase in pathways associated with glycolysis (4-fold), pentose phosphate pathway (5-fold) and the citric acid cycle (i.e. tricarboxylic acid cycle (TCA)) (3.8-fold) in quercetin-treated HKCs (Figure 1B, p <0.05). These results show that quercetin directly modulates cellular metabolism by affecting glucose metabolism.
Figure 1.
Metabolite enrichment analysis showing pathways upregulated in (A) HCFs and (B) HKCs following 10-μM quercetin treatment. Data was analysed using MetaboAnalyst using only metabolites upregulated >twofold. Data is representative of three independent experiments, n = 3
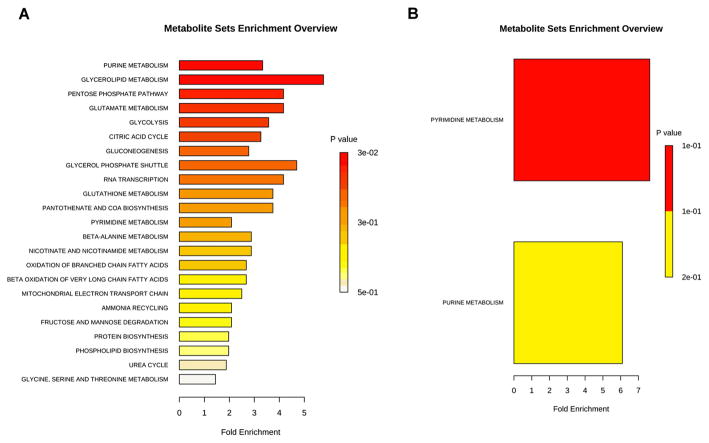
After mapping the metabolites into general biochemical pathways according to Metaboanalyst, it was apparent that the common pathway downregulated in both HCFs and HKCs following quercetin treatment was pyrimidine metabolism (2.5-fold, 7-fold, respectively, p <0.03, Figure 2A–B). Quercetin-treated HCFs showed a significant reduction in biochemical pathways associated with purine metabolism (3.5-fold, p <0.01), glycerolipid metabolism (6-fold, p <0.01), pentose phosphate pathway (4.5-fold, p <0.01), glycolysis (3.8-fold, p <0.01) and TCA cycle (3.5-fold, p <0.01, Figure 2A). We saw a reduction in purine metabolism (6-fold, p <0.02) in HKCs, but not HCFs, following quercetin treatment (Figure 2B). These results show that quercetin has an opposing effect on the major metabolic pathways involved in glucose metabolism (glycolysis, pentose phosphate pathway and TCA) in normal HCFs compared to HKCs. In order to explore the biochemical effect of quercetin on each pathway that may promote its antifibrotic properties,25 we examined variations in metabolic flux of key reactions in each metabolic pathway to explore the molecular mechanism of quercetin.
Figure 2.
Metabolite enrichment analysis showing pathways downregulated in (A) HCFs and (B) HKCs following 10-μM quercetin treatment. Data was analysed using MetaboAnalyst using only metabolites downregulated >twofold. Data is representative of three independent experiments, n = 3
Glycolysis
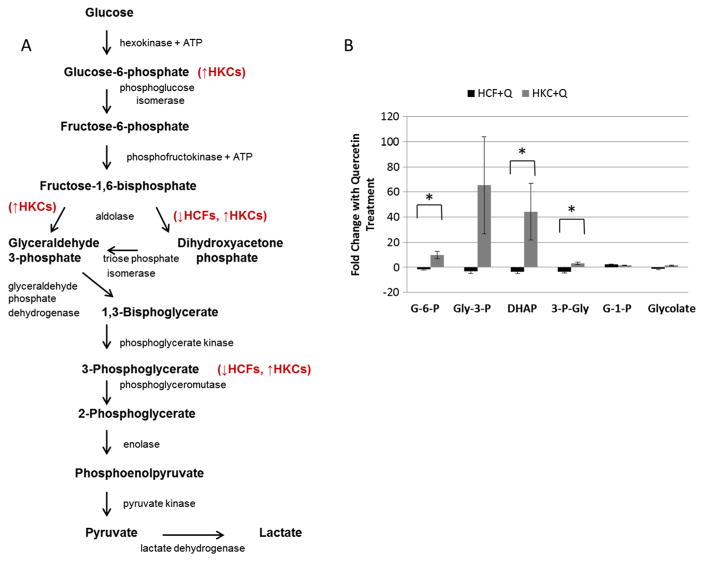
A key metabolic pathway for energy production and glucose metabolism in cells is glycolysis. During this process, glucose is converted to pyruvate, and the free energy released is used to generate a net gain of 2ATPs and 2NADH.29 Figure 3A shows a schematic representation of this pathway.
Figure 3.
(A) Schematic depicting the role of quercetin in modulating metabolic flux in glycolysis in HCFs and HKCs. (B) Quantification of fold changes in glucose-6-phosphate (G-6-P), glyceraldehyde-3-phosphate (Glyc-3-P), dihydroxyacetone phosphate (DHAP), 3-phosphoglycerate (3-P-Gly), glucose-1-phosphate (G-1-P) and glycolate in HCFs and HKCs following quercetin treatment. Statistical significance denoted by * = p <0.05 determined by an unpaired Student T-test. Q = quercetin. Data is representative of three independent experiments, n = 3
We identified several metabolites that were differentially regulated by quercetin in both HCFs and HKCs (Figure 3B). Glucose-6-phosphate was significantly upregulated (9-fold, p <0.016) in HKCs following quercetin treatment compared to HCFs which showed a slight reduction (1.7-fold). Glyceraldehyde-3-phosphate was also upregulated in HKCs (65-fold) following quercetin treatment compared with a slight reduction by 3-fold in HCFs. Dihydroxyacetone phosphate was downregulated in HCFs upon quercetin treatment (3.2-fold), where it was upregulated, though not significantly, in HKCs (44-fold, p <0.15). Similar regulation of the 3-phosphoglycerate metabolite was seen with downregulation by 3.4-fold in HCFs and upregulation by 3-fold in HKCs (p <0.001). Both glucose-1-phosphate and glycolate had similar metabolic flux following quercetin treatment in both cell types suggesting that quercetin is selectively targeting specific enzymes involved in glycolysis, including hexokinase, aldolase and phosphoglycerate kinase.
Pentose phosphate pathway
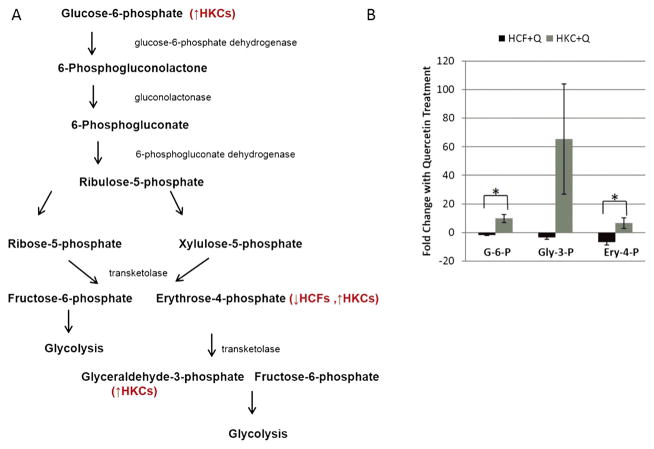
In biochemistry, the pentose phosphate pathway is a metabolic pathway parallel to glycolysis that results in generation of NADPH and 5-carbon pentose groups. The pentose phosphate pathway is known to play an important role in corneal development.30,31 In order to determine the effect of quercetin in modulating energy production, we measured changes in metabolite flux within the pentose phosphate pathway, which is a secondary consumer of glucose.
Figure 4A shows a schematic of the pathway, where Figure 4B shows quantification of the metabolites that were significantly regulated upon quercetin treatment in both HCFs and HKCs. Glucose-6-phosphate, a metabolite common to both glycolysis and the pentose phosphate pathway, was found to be upregulated ninefold (p<0.016) in HKCs following quercetin stimulation compared to a downregulation of 1.7-fold reduction by HCFs. This increase in glucose-6-phosphate in HKCs results in an increase in glycolytic metabolites, as well as metabolite flux in the pentose phosphate pathway, including an upregulation of glyceraldehyde-3-phosphate (65-fold) and erythrose-4-phosphate (6.5-fold, p <0.03). The increase in erythrose-4-phosphate observed in HKCs contributes to the increase in glyceraldehyde-3-phosphate, an intermediate that is shuttled into glycolysis for conversion into 1,3-bisphosphate (Figure 3A).
Figure 4.
(A) Schematic depicting the role of quercetin in modulating metabolic flux in the pentose phosphate pathway in HCFs and HKCs. (B) Quantification of fold changes in glucose-6-phosphate (G-6-P), glyceraldehyde-3-phosphate, erythrose-4-phosphate (Ery-4-P) and glyceraldehyde-3-phosphate (Gly-3-P) following quercetin treatment. Statistical significance denoted by * = p <0.05 determined by an unpaired Student T-test. Q = quercetin. Data is representative of three independent experiments, n = 3
TCA cycle
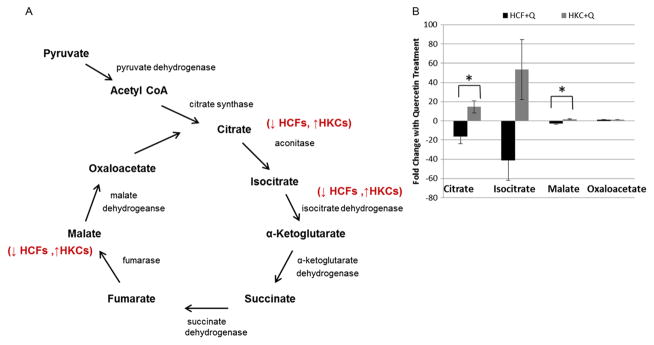
The TCA cycle is a key metabolic pathway for generating NADH and FADH2, which is then converted to ATP during oxidative phosphorylation (1). The end product of glycolysis, pyruvate, is converted to acetyl CoA, which serves as the initial substrate for the TCA cycle. In order to determine if the upregulation in glycolytic intermediates in HKCs induced by quercetin is shuttled into the TCA cycle, we measured metabolite flux of key TCA intermediates. As shown in the simplified scheme in Figure 5A, the TCA cycle has nine vital elements: citrate, cis-aconitate, isocitrate, α-ketoglutarate, succinyl CoA, succinate, fumarate, malate and oxaloacetate. Several of those metabolites were significantly regulated upon quercetin treatment in both HCFs and HKCs. Citrate, isocitrate and malate flux were upregulated (14.6-fold, 53.4-fold and 1.8-fold, respectively) in quercetin-treated HKCs, which was significantly higher (p <0.03) than the downregulated metabolites (16.3-fold, 41.48-fold and 3-fold) measured in quercetin-treated HCFs (Figure 5B). Oxaloactetate, which is generated from malate by malate dehydrogenase, was constant in both HCFs and HKCs with quercetin treatment suggesting that generation of this intermediate may be a limiting step in the TCA cycle. Overall, these results suggest that quercetin not only modulates glucose conversion in glycolysis and the pentose phosphate pathway, but also directly modulates NADH and FADH2 production in the TCA cycle.
Figure 5.
(A) Schematic of the TCA cycle showing the effect of quercetin in modulating concentrations of key metabolites in HCFs and HKCs. (B) Quantification of fold changes in citrate, isocitrate, malate and oxaloacetate in HCFs and HKCs following quercetin treatment. Statistical significance denoted by * = p <0.05 determined by an unpaired Student T-test. Q = quercetin. Data is representative of three independent experiments, n = 3
Urea cycle
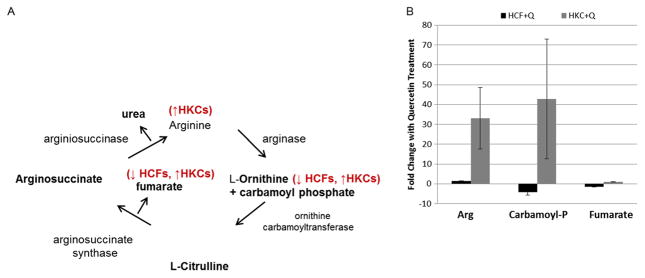
The urea cycle utilizes arginine to generate L-ornithine, which following several reactions is eventually converted to urea. The urea cycle is known to contribute to cataract formation within the lens.32,33 However, its role within the cornea is unknown. We measured variations of key intermediates important in the urea cycle following quercetin treatment. The urea cycle shown in Figure 6A consists of five reactions of which two are mitochondrial and three cytosolic.34 We found altered regulation of arginine and carbamoyl phosphate metabolite upon quercetin treatment, in both HCFs and HKCs. Carbamoyl phosphate reacts with ornithine to give citrulline as shown in Figure 6A. In our system, carbamoyl phosphate was downregulated in HCFs (4.3-fold) and upregulated in HKCs (42.7-fold, Figure 6B). No other metabolite was significantly altered within the urea cycle, except for arginine, a secondary metabolite which is synthesized by citrulline via nitric oxide synthase (NOS). Quercetin treatment on HKCs led to significant upregulation of arginine by 42.7-fold compared to a downregulation by 4.3-fold in HCFs (Figure 6B). Arginine is a known metabolite associated with proline biosynthesis, a key amino acid in collagen structure.35 Increasing intracellular arginine concentration may cause a significant increase in collagen secretion without promoting a myofibroblast phenotype by simply providing a key precursor to collagen assembly.
Figure 6.
(A) Schematic of the urea cycle showing the effect of quercetin in modulating concentrations of key metabolites in HCFs and HKCs. (B) Quantification of fold changes in arginine (Arg), carbamoyl phosphate (Carbamoyl-P) and fumarate. Statistical significance denoted by * = p <0.05 determined by an unpaired Student T-test. Q = quercetin. Data is representative of three independent experiments, n = 3
ATP/ADP/AMP regulation
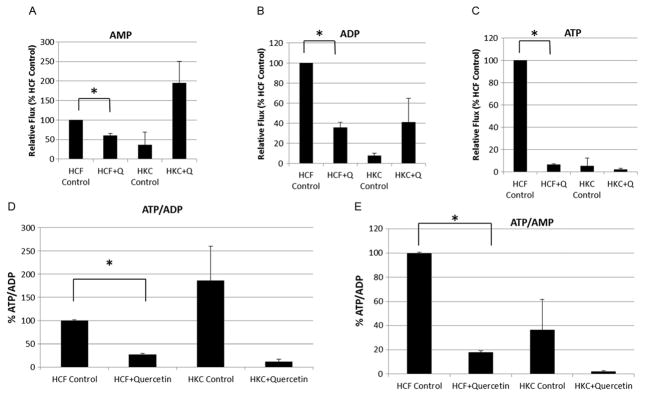
All living cells must continuously maintain a non-equilibrium ratio of ADP to ATP.36 The fact that this ratio in cells usually remains almost constant indicates the importance and efficiency involved in regulating this process. Figure 7 shows the relative flux of AMP, ADP and ATP in both cell types with and without quercetin treatment. AMP levels were reduced by 40% in quercetin-treated HCFs (p<0.002), while quercetin-treated HKCs increased AMP flux by 159% (p = 0.06, Figure 7A). We also measured an increase in ADP flux in quercetin-treated HKCs (32%, Figure 7B). Both ADP and ATP flux were reduced in HCFs with quercetin treatment (65%, 93%, respectively, p <0.0002, Figure 7B–C) supporting the data showing downregulation of glycolysis and TCA intermediates in HCF, which would be expected to directly modulate ATP production in oxidative phosphorylation. Figure 7D–E shows ATP/ADP and ATP/AMP regulation in HCFs and HKCs upon quercetin treatment. Both the ratios of ATP/ADP and ATP/AMP were significantly downregulated in HCFs with quercetin treatment as compared to vehicle-treated controls (74%, p <0.0001, Figure 7D, and 82%, p <0.0001, Figure 6E, respectively). Surprisingly, the two ratios were not significantly altered in HKCs even though there was a trend showing downregulation upon quercetin treatment.
Figure 7.
Relative flux of (A) AMP, (B) ADP and (C) ATP in HCFs and HKCs measured by LC-MS/MS. (D) Ratios of ATP/ADP and (E) ATP/AMP in HCFs and HKCs following quercetin or vehicle (control) treatment, as measured by LC-MS/MS. Values normalized to HCF control. n = 3, error bars represent standard error of the mean. Statistical significance denoted by * = p <0.05 determined by an unpaired Student T-test. Q = quercetin. Data is representative of three independent experiments, n = 3
DISCUSSION
It is well known that the KC dystrophy may lead to corneal fibrosis as well as corneal thinning and bulging.19,20,37 However, the specific mechanism by which defective corneal stroma signalling leads to the disease phenotype remains incompletely understood. While we know more about the corneal fibrosis mechanism because of an injury or trauma, the KC fibrotic phenotype is still under investigation. Rodent models for corneal injury/trauma are plenty and well-studied,38,39 yet we are still to see a rodent model that can recapitulate the human KC dystrophy. Development of such a model will prove very useful in increasing our understanding of the pathogenesis of KC.
Human corneal stromal cells isolated from KC corneal tissue following corneal transplantation have been widely used over the last decade to model KC in vitro.40,41 It is now widely accepted that cellular metabolism plays a key role in KC disease.10,11,21 Our group established the first 3D culture system in 2012 that mirrors the in vivo phenotype.26 In an attempt to gain insight into the molecular and biochemical mechanisms of the disease process, we have used our 3D model to characterize the global metabolic profiles of human corneal cells from healthy and KC 3D cultures.11 In our current study, we explored the effect of a novel anti-fibrotic compound named quercetin on modulation of cellular metabolism in HKCs, which we hypothesize, drives the disease phenotype of altered ECM assembly giving rise to corneal scarring.
Quercetin is a flavonol and dietary antioxidant found in most fruits and vegetables, such as onions, cranberries and dark grapes.42,43 It has been reported that quercetin has anti-inflammatory,44,45 anti-proliferative46,47 and anti-carcinogenic effects48,49 in a variety of models. In the retina, quercetin was shown to protect retinal pigment epithelium (RPE) from hydrogen peroxide-induced cell death and reverse oxidative damage in the RPE.50,51 In the cornea, we have recently shown that quercetin inhibits the fibrotic phenotype exhibited by HKCs in vitro with a decrease in α-SMA and Collagen III expression.25 Herein, we explored the role of quercetin in modulating cellular metabolism in HKCs as a possible mechanism of action..
Among the most significant metabolic findings were the opposite modulation of both glycolysis and the TCA cycle by quercetin. Glycolysis and the TCA cycle are the dominant mechanisms utilized by cells to produce ATP, NADH and FADH2, which are then used to generate energy to drive biochemical reactions. Our results show that quercetin modulates glycolysis in HKCs and causes a significant increase in glucose-6-phosphate, which is the primary metabolite generated by hexokinase upon glucose uptake.1 Phosphorylation of glucose is fundamental in restricting glucose to the cytosol and enabling metabolism of the sugar to generate ATP.1 Our data suggests that quercetin directly modulates expression of glycolytic enzymes resulting in an increase in glucose-6-phosphate that contributes to driving favourable energy production by HKCs. The increase in glyceraldehyde-3-phosphate and dihydroxyacetone phosphate, both products of aldolase activity, in HKCs following quercetin treatment suggests that quercetin directly modulates glycolytic activity to favour substrate-level phosphorylation to generate ATP production. Moreover, in the normal HCFs we found the opposite effect on production of glycolytic intermediates by quercetin suggesting that inherent gene expression of glycolytic enzymes dominates energy production and response to quercetin.
Two of the most important mechanisms in cellular metabolism were regulated in an opposite manner between HCFs and HKCs with quercetin treatment. First, in HCFs, key glycolytic metabolites, dihydroxyacetone phosphate and 3-phosphoglycerate, were downregulated, where in HKCs the same two metabolites were upregulated. Second, in HCFs, the TCA cycle showed downregulation of citrate while HKCs showed upregulation of this metabolite as well as isocitrate and malate, indicating a faster and rather overloading TCA cycling. These results become even more significant when we consider that quercetin significantly downregulates fibrotic markers in these same cell types and the same 3D culture model, as shown by our group recently.25 This suggests that the metabolic mechanism for corneal fibrosis differs between specific conditions and diseases. In other words, the metabolism in HCFs needs to be modulated in a different manner compared to HKCs in order to promote a non-fibrotic phenotype.
Our findings were further supported by the regulation of the adenine nucleotides, ATP, ADP and AMP. The majority of metabolic pathways are regulated by the relative proportions of ATP, ADP and AMP in the cells. A cell which lacks sufficient ATP and/or accumulates ADP/AMP will almost certainly have altered normal function and cellular behaviour. In this study, we examined healthy HCFs and diseased HKCs isolated from KC patients. It was no surprise to us that there was a massive difference on the ATP availability and the ATP:ADP/ATP:AMP ratios. We have shown previously26,27 that HKCs are terminally differentiated to myofibroblasts, a cell responsible for corneal fibrosis.17,18 HCFs, on the other hand, are from healthy donors, but still not as quiescent as the normal resident corneal cells known as keratocytes.52,53 Despite that, there are extensive differences between HCFs and HKCs, in terms of their role in fibrosis, as reported previously.11,26,27,54 Our data on the adenine nucleotides indicates that ATP levels were higher in HCFs compared to HKCs and massively decreased upon quercetin treatment. HKCs did not show extensive response in ATP, ADP and AMP flux following quercetin treatment. However, the ratios of both ATP/ADP and ATP/AMP flux showed massive upregulation in HKCs when treated with quercetin as compared to all other groups. This indicates accumulation of both ATP and AMP when HKCs are treated with quercetin. Adding these findings to what we know about quercetin and anti-fibrotic effects on HKCs, these results suggest that quercetin helps reestablish the ATP–ADP–AMP balance in these cells and therefore ‘push’ them towards a less fibrotic and potentially healthier state.
Clearly, further studies are required in order to ensure that we can switch HKCs back to a more keratocyte like phenotype, but this is a promising start for this process. Given the unavailability of an animal model for studying KC disease, our 3D model poses huge potential for discovering the key factors involved HKC dysfunction. Our study suggests that targeting cellular metabolism in KC may prove to be a promising approach to treating this corneal disease by inhibiting differentiation to the myofibroblast phenotype. We propose that quercetin may be a useful antioxidant that can potentially be used to inhibit corneal fibrosis by modulating cellular metabolism and inhibiting scar formation.
Acknowledgments
This work was supported by the National Institutes of Health Grants 5R01EY023568 (DK) and 5R01EY020886 (DK) and T32EY023202. We acknowledge the support of the NEI/DMEI Cellular Imaging Core Facility at OUHSC (P30EY021725) and an unrestricted grant (DMEI) from Research to Prevent Blindness (New York, NY USA). We thank Min Yuan for technical help with mass spectrometry experiments and NIH grants 5P01CA120964 (JMA) and NIH DF/HCC Cancer Center Support Grant 5P30CA006516 (JMA).
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests. A provisional patent application relating to the use of Quercetin for treating ocular conditions has been filed by the Board of Regents of the University of Oklahoma.
References
- 1.Bea A. Molecular Biology of the Cell. 4. Garland Science; New York: 2002. [Google Scholar]
- 2.Moncada S, Higgs EA, Colombo SL. Fulfilling the metabolic requirements for cell proliferation. Biochem J. 2012;446:1–7. doi: 10.1042/bj20120427. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes AP, Blenis J. A nexus for cellular homeostasis: the interplay between metabolic and signal transduction pathways. Curr Opin Biotechnol. 2015;34c:110–117. doi: 10.1016/j.copbio.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan HX, Xiong Y, Guan KL. Nutrient sensing, metabolism, and cell growth control. Mol Cell. 2013;49:379–387. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locasale JW, Melman T, Song S, Yang X, Swanson KD, Cantley LC, Wong ET, Asara JM. Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol Cell Proteomics. 2012;11:M111.014688. doi: 10.1074/mcp.M111.014688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012;7:872–881. doi: 10.1038/nprot.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janzer A, German NJ, Gonzalez-Herrera KN, Asara JM, Haigis MC, Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:10574–10579. doi: 10.1073/pnas.1409844111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karamichos D, Zieske JD, Sejersen H, Sarker-Nag A, Asara JM, Hjortdal J. Tear metabolite changes in keratoconus. Exp Eye Res. 2015;132c:1–8. doi: 10.1016/j.exer.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karamichos D, Hutcheon AE, Rich CB, Trinkaus-Randall V, Asara JM, Zieske JD. In vitro model suggests oxidative stress involved in keratoconus disease. Sci Rep. 2014;4:4608. doi: 10.1038/srep04608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Fernandez de la Camara C, Salom D, Sequedo MD, Hervas D, Marin-Lambies C, Aller E, Jaijo T, Diaz-Llopis M, Millan JM, Rodrigo R. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS One. 2013;8:e74223. doi: 10.1371/journal.pone.0074223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young SP, Nessim M, Falciani F, Trevino V, Banerjee SP, Scott RA, Murray PI, Wallace GR. Metabolomic analysis of human vitreous humor differentiates ocular inflammatory disease. Mol Vis. 2009;15:1210–1217. [PMC free article] [PubMed] [Google Scholar]
- 14.Globisch D, Moreno AY, Hixon MS, Nunes AA, Denery JR, Specht S, Hoerauf A, Janda KD. Onchocerca volvulus-neurotransmitter tyramine is a biomarker for river blindness. Proc Natl Acad Sci U S A. 2013;110:4218–4223. doi: 10.1073/pnas.1221969110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Linsenmayer TF, Fitch JM, Gordon MK, Cai CX, Igoe F, Marchant JK, Birk DE. Development and roles of collagenous matrices in the embryonic avian cornea. Prog Retin Eye Res. 1998;17:231–265. doi: 10.1016/s1350-9462(97)00010-4. [DOI] [PubMed] [Google Scholar]
- 17.Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torricelli AA, Wilson SE. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp Eye Res. 2014;129:151–160. doi: 10.1016/j.exer.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zadnik K, Barr JT, Edrington TB, Nichols JJ, Wilson BS, Siegmund K, Gordon MO. Corneal scarring and vision in keratoconus: a baseline report from the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2000;19:804–812. doi: 10.1097/00003226-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Barr JT, Wilson BS, Gordon MO, Rah MJ, Riley C, Kollbaum PS, Zadnik K. Estimation of the incidence and factors predictive of corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2006;25:16–25. doi: 10.1097/01.ico.0000164831.87593.08. [DOI] [PubMed] [Google Scholar]
- 21.Kryczka T, Ehlers N, Nielsen K, Wylegala E, Dobrowolski D, Midelfart A. Metabolic profile of keratoconic cornea. Curr Eye Res. 2013;38:305–309. doi: 10.3109/02713683.2012.754904. [DOI] [PubMed] [Google Scholar]
- 22.Brodenfeldt R, Mohr H. Time courses for phytochrome-induced enzyme levels in phenylpropanoid metabolism (phenylalanine ammonia-lyase, naringenin-chalcone synthase) compared with time courses for phytochrome-mediated end-product accumulation (anthocyanin, quercetin) Planta. 1988;176:383–390. doi: 10.1007/bf00395419. [DOI] [PubMed] [Google Scholar]
- 23.Patschke L, Grisebach H. Biosynthesis of flavonoids—XVI: dihydrokaempferol and dihydroquercetin as precursors of kaempferol and quercetin inPisum sativum. Phytochemistry. 1968;7:235–237. doi: 10.1016/S0031-9422(00)86321-9. [DOI] [Google Scholar]
- 24.Möhle B, Heller W, Wellmann E. UV-induced biosynthesis of quercetin 3-O-β-D-glucuronide in dill cell cultures. Phytochemistry. 1985;24:465–467. doi: 10.1016/S0031-9422(00)80748-7. [DOI] [Google Scholar]
- 25.McKay TB, Lyon D, Sarker-Nag A, Priyadarsini S, Asara JM, Karamichos D. Quercetin attenuates lactate production and extracellular matrix secretion in keratoconus. Sci Rep. 2015;5 doi: 10.1038/srep09003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karamichos D, Zareian R, Guo X, Hutcheon AE, Ruberti JW, Zieske JD. Novel model for keratoconus disease. J Funct Biomater. 2012;3:760–775. doi: 10.3390/jfb3040760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karamichos D, Hutcheon AEK, Zieske JD. Transforming growth factor-β3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J Tissue Eng Regen Med. 2011;5:e228–e238. doi: 10.1002/term.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2. 0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012;40:W127–133. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar-Even A, Flamholz A, Noor E, Milo R. Rethinking glycolysis: on the biochemical logic of metabolic pathways. Nat Chem Biol. 2012;8:509–517. doi: 10.1038/nchembio.971. [DOI] [PubMed] [Google Scholar]
- 30.Masterson E, Edelhauser HF, Chader GJ. The pentose phosphate pathway in developing chick cornea. Biochim Biophys Acta. 1978;542:372–377. doi: 10.1016/0304-4165(78)90368-9. [DOI] [PubMed] [Google Scholar]
- 31.Masterson E, Whikehart DR, Chader GJ. Glucose oxidation in the chick cornea: effect of diamide on the pentose shunt. Invest Ophthalmol Vis Sci. 1978;17:449–454. [PubMed] [Google Scholar]
- 32.Rao GN, Cotlier E. Urea cycle enzymes in retina, ciliary body-iris, lens and senile cataracts. Exp Eye Res. 1984;39:483–495. doi: 10.1016/0014-4835(84)90048-4. [DOI] [PubMed] [Google Scholar]
- 33.Abraham EC, Perry RE, Abraham A, Swamy MS. Proteins of urea-soluble high molecular weight (HMW) aggregates from diabetic cataract: identification of in vivo glycation sites. Exp Eye Res. 1991;52:107–112. doi: 10.1016/0014-4835(91)90135-2. [DOI] [PubMed] [Google Scholar]
- 34.Jackson MJ, Beaudet AL, O’Brien WE. Mammalian urea cycle enzymes. Annu Rev Genet. 1986;20:431–464. doi: 10.1146/annurev.ge.20.120186.002243. [DOI] [PubMed] [Google Scholar]
- 35.Barbul A. Proline precursors to sustain mammalian collagen synthesis. J Nutr. 2008;138:2021s–2024s. doi: 10.1093/jn/138.10.2021S. [DOI] [PubMed] [Google Scholar]
- 36.Brown GC. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J. 1992;284(Pt 1):1–13. doi: 10.1042/bj2840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambekar R, Toussaint KC, Jr, Wagoner JA. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J Mech Behav Biomed Mater. 2011;4:223–236. doi: 10.1016/j.jmbbm.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Stepp MA, Zieske JD, Trinkaus-Randall V, Kyne BM, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A. Wounding the cornea to learn how it heals. Exp Eye Res. 2014;121:178–193. doi: 10.1016/j.exer.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boote C, Du Y, Morgan S, Harris J, Kamma-Lorger CS, Hayes S, Lathrop KL, Roh DS, Burrow MK, Hiller J, Terrill NJ, Funderburgh JL, Meek KM. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Invest Ophthalmol Vis Sci. 2012;53:2786–2795. doi: 10.1167/iovs.11-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson SL, Yang Y, El Haj AJ. Corneal stromal cell plasticity: in vitro regulation of cell phenotype through cell-cell interactions in a three-dimensional model. Tissue Eng Part A. 2014;20:225–238. doi: 10.1089/ten.TEA.2013.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida S, Yokoo S, Yanagi Y, Usui T, Yokota C, Mimura T, Araie M, Yamagami S, Amano S. Sphere formation and expression of neural proteins by human corneal stromal cells in vitro. Invest Ophthalmol Vis Sci. 2005;46:1620–1625. doi: 10.1167/iovs.04-0288. [DOI] [PubMed] [Google Scholar]
- 42.Erlund I, Freese R, Marniemi J, Hakala P, Alfthan G. Bioavailability of quercetin from berries and the diet. Nutr Cancer. 2006;54:13–17. doi: 10.1207/s15327914nc5401_3. [DOI] [PubMed] [Google Scholar]
- 43.Cai X, Fang Z, Dou J, Yu A, Zhai G. Bioavailability of quercetin: problems and promises. Curr Med Chem. 2013;20:2572–2582. doi: 10.2174/09298673113209990120. [DOI] [PubMed] [Google Scholar]
- 44.Guazelli CF, Fattori V, Colombo BB, Georgetti SR, Vicentini FT, Casagrande R, Baracat MM, Verri WA., Jr Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J Nat Prod. 2013;76:200–208. doi: 10.1021/np300670w. [DOI] [PubMed] [Google Scholar]
- 45.Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY, Kooistra T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218:44–52. doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Gupta K, Panda D. Perturbation of microtubule polymerization by quercetin through tubulin binding: a novel mechanism of its antiproliferative activity. Biochemistry. 2002;41:13029–13038. doi: 10.1021/bi025952r. [DOI] [PubMed] [Google Scholar]
- 47.Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M. The antiproliferative effect of quercetin in cancer cells is mediated via inhibition of the PI3K–Akt/PKB pathway. Anticancer Res. 2006;26:1177–1181. [PubMed] [Google Scholar]
- 48.Michaud-Levesque J, Bousquet-Gagnon N, Beliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012;318:925–935. doi: 10.1016/j.yexcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Olson ER, Melton T, Dickinson SE, Dong Z, Alberts DS, Bowden GT. Quercetin potentiates UVB-induced c-Fos expression: implications for its use as a chemopreventive agent. Cancer Prev Res (Phila) 2010;3:876–884. doi: 10.1158/1940-6207.capr-09-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kook D, Wolf AH, Yu AL, Neubauer AS, Priglinger SG, Kampik A, Welge-Lussen UC. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 2008;49:1712–1720. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- 51.Cao X, Liu M, Tuo J, Shen D, Chan CC. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp Eye Res. 2010;91:15–25. doi: 10.1016/j.exer.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jester JV, Budge A, Fisher S, Huang J. Corneal keratocytes: phenotypic and species differences in abundant protein expression and in vitro light-scattering. Invest Ophthalmol Vis Sci. 2005;46:2369–2378. doi: 10.1167/iovs.04-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guerriero E, Chen J, Sado Y, Mohan RR, Wilson SE, Funderburgh JL, Sundarraj N. Loss of alpha3(IV) collagen expression associated with corneal keratocyte activation. Invest Ophthalmol Vis Sci. 2007;48:627–635. doi: 10.1167/iovs.06-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fabre EJ, Bureau J, Pouliquen Y, Lorans G. Binding sites for human interleukin 1 alpha, gamma interferon and tumor necrosis factor on cultured fibroblasts of normal cornea and keratoconus. Curr Eye Res. 1991;10:585–592. doi: 10.3109/02713689109013850. [DOI] [PubMed] [Google Scholar]