Abstract
BACKGROUND
The development of intrinsic and acquired resistance to antineoplastic agents is a major obstacle to successful chemotherapy in ovarian cancers. Identification and characterization of chemoresponse-associated biomarkers is of paramount importance for novel therapeutic development.
METHODS
Global RNA expression profiles were obtained by high-throughput microarray analysis. Cell cycle, proliferation rate, and paclitaxel sensitivity of ovarian cancer cells harboring cyclin A1 inducible expression construct was compared with and without tetracyclin induction, as well as when the cyclin A1 expression was suppressed by short inhibiting RNA (siRNA). Cellular senescence was evaluated by β-galactosidase activity staining.
RESULTS
Global RNA expression profiling and subsequent correlation studies of gene expression level and drug response has identified that elevated expression of cyclin A1 (CCNA1) was significantly associated with cellular resistance to paclitaxel, doxorubicin and 5-fluorouracil. The role of cyclin A1 in paclitaxel resistance was confirmed in ovarian cancer cells that harbor an inducible cyclin A1 expression construct, which showed reduced paclitaxel-mediated growth inhibition and apoptosis when cyclin A1 expression was induced, whereas down-regulation of cyclin A1 expression in the same cell lines using cyclin A1-specific siRNAs sensitized the cells to paclitaxel toxicity. However, ovarian cancer cells with ectopic expression of cyclin A1 demonstrated slow down of proliferation and senescence-associated β-galactosidase activity.
CONCLUSIONS
Our profiling and correlation studies have identified cyclin A1 as one chemoresistance-associated biomarker in ovarian cancer. The results of the characterization studies suggest that cyclin A1 functions as an oncogene that controls proliferative and survival activities in tumorigenesis and chemo-resistance of ovarian cancer.
Keywords: ovarian cancer, tumorigenesis, chemoresistance, paclitaxel, microarray
INTRODUCTION
Ovarian cancer is the fifth leading cause of cancer death in females in the United States and accounts for more than half of the deaths due to gynecological malignancy (1). Currently, clinical management of epithelial ovarian cancers is surgical cytoreduction followed by consolidative chemotherapy. First line chemotherapy in ovarian cancer consists of a platinum analogue in combination with Taxol® (paclitaxel) (2). The clinical response rates of ovarian cancer patients to chemotherapy can be as high as 80%. However, most of the tumors will either recur after definitive treatment or be chemo-resistant from the outset, with a dismal 5-year survival rate of 30% (1).
Several patient and disease characteristics including disease stage, volume of residual tumor following cytoreductive surgery, performance status, sites of metastases, presence of ascites, serum cancer antigen 125 (CA125) half-life during early chemotherapy, and tumor histology (3, 4) have been proposed as prognostic indicators for ovarian cancer. Among all of these clinical prognostic indicators, disease-free interval after the first-line treatment has emerged as an important prognostic factor for clinical outcome and predictor of response to second-line chemotherapy in a number of clinical studies conducted over the past two decades (5). Patients who responded to first-line therapy and demonstrated a significant treatment-free interval had a favorable clinical outcome and high probability of responding to second-line treatment. According to the guidelines of Gynecologic Oncology Group (6), patients who remain relapse-free for more than six months after completing prior therapy are considered as having a responsive disease and may achieve a long-term remission. Patients who relapse within six months after prior therapy are considered as having a resistant disease and the expected response rate to retreatment is less than 20%. Finally, refractory disease can be defined as disease that progressed or was stable during chemotherapy.
To understand the mechanisms of drug resistance and identify novel molecular targets, we have performed a high-throughput microarray expression profiling study to compare gene expression between primary and recurrent ovarian cancers, as well as between chemo-responsive and chemo-resistant ovarian cancer cell lines and tumor tissues. Validation of target gene expression and functional studies have demonstrated that the expression of cyclin A1 (CCNA1) was significantly correlated with resistance to Taxol-mediated apoptosis in ovarian cancer cell lines and negatively correlated with relapse time of ovarian cancer patients.
MATERIALS AND METHODS
Clinical specimens and ovarian cell lines
Ovarian carcinomas were obtained from patients with an IRB approved protocol at Brigham and Women’s Hospital in Boston. All the surgical specimens were collected with patient consents. Twenty-four ovarian cancer cell lines were employed in this study. ALST, DOV13, CAOV3, OVCA3, OVCA420, OVCA429, OVCA432, OVCA433, OVCA633, OVCA680, OVCA702, OVCA712, OVCA810 and OVCA882 were established in our laboratory from ovarian carcinomas obtained from different patients. SKOV3, ES-2, TOV-112D, and TOV21G were purchased from American Tissue Culture Collection (ATCC, Manassas, VA, USA). RMG-1, MCAS, RMUG-S and RMUG-L were purchased from Japanese Collection of Research Bioresources (JCRB Cell Bank, Osaka, Japan). OV2008 and PEOH were obtained from National Cancer Institute (Frederick, MD, USA). All the culture media were supplemented with 10% fetal calf serum (FCS) and were purchased from Thermo Fisher Scientific (Waltham, MA, USA) unless stated otherwise. ES-2 was maintained in McCoy’s SA medium, while RMG-1 and RMUG-L were cultured in DMEM-F12 HAM medium. OV2008 and PEOH were cultured in RPMI media. Other ovarian cancer cell lines were grown in medium 199 and MCDB 105 (Sigma-Aldrich, Natick, MA, USA) (1:1) supplemented with 10% FCS.
cDNA microarray analysis
Total RNA was extracted from the primary and recurrent tumor cells by Trizol reagent (Thermo Fisher Scientific, MA, USA). Poly (A) RNA was purified from total RNA by Oligotex mRNA kit (Qiagen Inc., Valencia, CA, USA). cDNA synthesis and microarray hybridization was performed through a subcontract to the Center for Genomics Research (CGR) of Harvard University. The first microarray experiment with primary and recurrent tumors was performed on a UniGEM V2.0 microarray and the results have been described previously (7). The microarray data of the subsequent two microarray experiments between early and late relapse tumors, as well as drug-sensitive and drug-resistant cancer cell lines have been deposited in NCBI GEO repository with the series record number GSE2058.
Quantitative real time PCR analysis (qRT-PCR)
SYBR Green-based qRT-PCR was performed as described before (7). Cyclophilin A was used as the internal control. The sequences of the primers used were: 5′-AATTGTGCCTTGCCTGAGTGA-3′ and 5′-AAGAACTGCAGGTGGCTC CAT-3′ for cyclin A1, 5′-CTTAATAGCCCTCCCCCTCAA-3′ and 5′-CCACAACAGT TTCCCATGGTG-3′ for TGM2,. 5′-CAAAGACATTATCCGGCAGCC-3′ and 5′-TGGTCTGATGAAGGTCCAACC-3′ for PEA-15, 5′-AGACTGAGTGGTTGGATGGCA-3′ and 5′-TGTCCACAGTCAGCAATGGTG-3′ for cyclophilin A. All primers were designed using PrimerExpress™ 2.0 software (Thermo Fisher Scientific, MA, USA). All the forward and reverse primers were designed on different exons separated by long introns in order to prevent noise signals generated from contaminated genomic DNA during the PCR reactions.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on a panel of 21 archived formalin-fixed, paraffin-embedded high-grade malignant tissue samples with known survival data, including 12 high-grade serous, 5 endometrioid, 1 clear cell and 3 mucinous ovarian tumors. Standard xylene deparaffinization, rehydration with a descending series of ethanol solutions, antigen retrieval (Vector Laboratories, Burlingame, CA), and blocking of endogenous peroxidases in 0.3% H2O2 were performed. We used both a mouse monoclonal Cyclin A1 antibody B88-2 from BD Biosciences (San Diego, CA, USA) and a rabbit antibody ab118897 from Abcam Inc. (Cambridge, MA). 3, 3–diaminobenzidine (DAB) horseradish peroxidase substrate kit was used for color development (Vector Laboratories, Burlingame, CA). Staining was graded semiquantitatively by multiplying the proportion of the stained epithelial area (from 0 for absence to 3 for more than 95% of the total epithelial area) with the intensity of the stain (from 0 for negative staining to 3 for strongly positive staining).
Establishment of cyclin A1-inducible cell lines, TOV112D-CCNA1
Full-length cDNA encoding cyclin A1 was produced by reverse transcription polymerase chain reaction using a primer pair (Forward primer: 5′-GCTCGTCACTTGGGATGGAGACCGG-3′, reverse primer: 5′-CCATTCAGAAACTGATTGTAGAAGAAGAAC-3′), with the stop codon mutated to a serine (the changed nucleotide in the reverse primer sequence is in bold) in order to add a MYC epitope tag to the carboxyl terminus of the cyclin A1 recombinant protein. The PCR product was first cloned into the TOPO TA cloning vector, pCRII-TOPO (Thermo Fisher Scientific, MA, USA). The cDNA was then restricted from the vector by EcoRI digestion and cloned into T-REx mammalian inducible vector pcDNA4/TO/mycHisC (Thermo Fisher Scientific, MA, USA) at the EcoRI site. Clones with proper orientation of cyclin A1 cDNA relative to the CMV promoter were selected by restriction mapping and sequencing. The resulting construct was transfected into the TOV112D cell line that also expresses regulator protein TetR from an integrated plasmid, pcDNA6/TR (Thermo Fisher Scientific, MA, USA). Positive sublines were selected using antibiotics blasticidin and zeocin as described in the manufacturer’s protocol. The vector control clone (TOV112D-pcDNA4) was established similarly, except that the pcDNA4/TO/mycHisC parental vector was transfected into the TOV112D cells.
siRNA preparation and transfection
Cyclin A1 and luciferase control siRNAs were produced by in vitro transcription using Silencer siRNA construction kit (Thermo Fisher Scientific, MA, USA). The siRNA sequences were designed using the computational algorithm available at the vendor’s website. The cyclin A1-specific siRNA sequences are 5′-AACCAGAATAACACCTGATTCCCTGTCTC-3′ and 5′-AAGAATCAGGTGTTATTCTGGCCTGTCTC-3′. The luciferase-control siRNA sequences are 5′-AATCGAAGTATTCCGCGTACGCCTGTCTC-3′ and 5′-AACGTACGCGGAATACTTCGACCTGTCTC-3′. siRNAs were synthesized and purified according to the manufacturer’s manual. For cyclin A1 knockdown experiment, cells were seeded in six-well plates (2 × 105 cells/well) and were transfected on the following day with 12 nM siRNA using Oligofectamine reagent (Qiagen Inc, CA, USA). The concentration of siRNA used was based on the recommendation from the manual of Silencer siRNA construction kit. Cyclin A1 expression was induced by tetracycline (1μg/ml) addition, whereas equal volume of the solvent ethanol was added to the control. The siRNA containing media were replaced with normal growth media 24 hr after transfection and the cells were harvested 24 hrs after medium replacement.
Western blot analysis
Standard sodium dodecyl sulphate-polyacrylamide gel electrophoresis and Western blot analysis was performed. Primary antibodies used were anti-cyclin A1 (BD Biosciences, San Diego, CA, USA), anti-cyclin A2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-c-MYC (Roche Diagnostics, Indianapolis, IN, USA) and anti-beta-actin (Sigma-Aldrich, Natick, MA, USA). Peroxidase-based pico-chemiluminescence kit (Thermo Fisher Scientific, MA, USA) was used to reveal antibody signal.
5-bromo-2′-deoxyuridine (BrdU) Incorporation assay
BrdU incorporation assay was performed using the chemiluminescence Cell Proliferation ELISA kit from Roche Diagnostics (Indianapolis, IN, USA). Ten thousand cells in the presence or absence of tetracycline were seeded in the wells of a black 96-well multititer plate. After 24 hours of growth, 10 μM of BrdU was added for 2 hours. The cells were then fixed and the DNA was denatured with FixDenat buffer according to the manufacturer’s instruction. The incorporated BrdU was bound by peroxidase-conjugated anti-BrdU monoclonal antibody and the immune complexes were quantified by measuring the luminescence generated by the peroxidase activity on the substrate luminol, using a Reporter microplate luminometer (Turner Designs Inc, Sunnyvale, CA, USA).
Flow cytometry
Cells treated with tetracycline or ethanol were harvested after 24 hr, washed once with PBS, and were fixed by pipeting with vortex into equal volume of ice-cold 80% ethanol. After 30 min fixation, cells were washed with PBS and resuspended with PBS containing 20 μg/ml of propidium iodide and 40 μg/ml of RNase A and stained at 37 °C for 1 hr. After the staining, cells were analyzed by a Becton Dickinson FACScans at the Flow Cytometry Core facility at Dana-Farber Harvard Cancer Center.
Cytoxicity assays
Paclitaxel, Doxorubicin, 5-Fluorouracil, Carboplatin Cisplatin, Etoposide and Camptothecin were purchased from Sigma-Aldrich (MA, USA). Topotecan (Hycamtin) was purchased from the pharmacy of Brigham and Women’s Hospital, Boston, MA. For the assay, 5000 cells were seeded in 100 μl of medium per well in a 96-well microtiter plate. On the following day, 100 μl of medium containing serial dilutions of drugs were added and cell viability was determined after 48 hr of incubation, using a MTT cell proliferation assay kit (Roche Diagnostics, IN, USA). The IC50 value is the concentration of drug resulting in a 50% reduction in absorbance at 550 nm to that of untreated cells. Every assay was performed in triplicates and the drug IC50 value for each cell line was the average of six independent experiments. For the experiments with siRNA transfection, 20,000 cells/well were seeded and drug treatment was performed 24 hr after siRNA transfection.
Apoptosis assay
Homogeneous Caspases Assay (Roche Diagnostics, IN, USA) was used to determine the activity levels of caspases 2, 3, 6, 7, 8, 9, 10 in the apoptotic cells. For the assay, cells were treated for 48 hr with or without tetracycline and different concentrations of paclitaxel or DMSO, respectively. 2X104 of cells were then mixed with equal volume of lysis buffer/substrate in a microtiter plate for 24 hr. Caspase activities in the cell lysates cleaved a substrate containing the DEVD tetrapeptide sequence (Asp-Glu-Val-Asp-Rhodamine 110). The fluorescence intensity (Ex 499 nm, Em 521 nm) of the cleaved products was measured with a SpectraMax Gemini fluorescence plate reader (Molecular Devices Corp, Sunnyvale, CA, USA).
Senescence-associated β-galactosidase assay
β-Galactosidase staining was performed essentially as described by van der Loo et al. (8). Briefly, cell cultures were first fixed with 4% paraformaldehyde in PBS and then incubated for 24 h at 37 °C in freshly prepared SA-β-gal staining solution containing 1 mg/ml 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-gal) (Sigma-Aldrich, MA, USA), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, 2 mM MgCl2 and 40 mM citric acid, titrated with sodium phosphate to pH 6.0. Lysosomal β-galactosidase was detected using the same solution, but at pH 4.0. At the end of incubation, cultures were washed with water and examined for the blue β–Galactosidase precipitate under an Olympus CK40 microscope (Olympus Corp, PA, USA).
Statistical analysis
Correlation analysis of the relationship between cyclin A1 RNA level and the drug IC50 (or relapse time) was carried out using the MINITAB statistical package (Minitab Inc., PA, USA). The Pearson correlation coefficient calculated between any two variables provides a measure of the strength of their linear relationship. Its significance is tested using a two-tailed t-test. P = 0.05 is accepted as statistically significant. For the survival analysis, overall survival was defined as the time from the date of operation to the date of the patient’s death (uncensored) or the date of last visit (censored). We defined the low and high Cyclin A1 expression groups using the cutoff score given by the median score of all samples plus one standard deviation (Score 3.7). The choice of cutoff score was based on the ability to group cases with significant differences in overall survival. Overall survivals of patients with high and low Cyclin A1 expression were estimated using the Kaplan-Meier method, and compared with a log rank test. For the apoptosis assay, one-way ANOVA was applied to determine if there was significant difference between cells under induced and non-induced conditions.
RESULTS
cDNA microarray analysis
To understand the mechanisms of drug resistance and identify novel molecular targets, we have employed cDNA microarrays to compare global gene expression profiles. The first one has been described previously, which was to compare the expression profiles between a paired primary ovarian tumor and recurrent tumor from the same patient (7). The second microarray experiment was performed to analyze the expression profiles between pooled RNAs prepared from two groups of serous primary ovarian tumors defined by response to the Taxol®-Carboplatin treatment (Table 1). The resistant group was composed of 3 primary tumors with early relapses (within 6 months after chemotherapy), whereas the responsive group was composed of 3 primary tumors with long disease-free periods (longer than 12 months after chemotherapy). The third microarray experiment was performed for a pair of cell lines derived from tumors with clear cell subtype. Although the two cell lines have the same morphology and growth characteristics, they are distinguished by completely different drug responses (Table 1).
Table 1.
Genes that demonstrate higher levels of expression in both recurrent and chemo-resistant ovarian tumors and cancer cell lines.
| Gene Name | Accession Number | Array 1§ | Array 2¶ | Array 3£ |
|---|---|---|---|---|
| CCNA1 | U97680 | 1.5* | 4.8* | 3.9* |
| TGM2 | AL031651 | 2.0 | 2.7 | 3.3 |
| PEA15 | Y13736 | 2.1 | 2.3 | 1.1 |
| APPBP1 | NM_003905 | 1.5 | 1.5 | 1.4 |
| EIF3S3 | NM_003756 | 1.9 | 2.0 | 1.6 |
| EIF3S5 | NM_003754 | 1.7 | 1.6 | 1.4 |
| EIF4A2 | NM_001967 | 1.5 | 1.6 | 1.1 |
| GTF3A | NM_002097 | 2.2 | 2.1 | 1.8 |
| TARS | M63180 | 2.8 | 1.6 | 1.1 |
| DHCR24 | NM_014762 | 1.5 | 1.6 | 1.1 |
Primary and recurrent ovarian cancer array (7).
Chemo-sensitive and chemo-resistant ovarian cancer array (GSM36719).
Drug-sensitive and drug-resistant ovarian cancer cell line array (GSM93732).
Folds of increase of expression in either receurrent (array 1), chemo-resistant cancer (array 2), or drug-resistant ovarian cancer cell line (array 3).
Notably, there are a group of genes that demonstrated consistent and high levels of differential expression in the three array analyses. The genes that showed significantly elevated expression in resistant/recurrent samples of all three microarray experiments are listed in Table 1. There are three genes (CCNA1, TGM2, and PEA15) that showed the highest levels of overexpression and have been described for being related to tumorigenesis and drug resistance. Transgenic mice overexpressing cyclin A1 (CCNA1) had been reported to have a higher frequency of developing acute myeloid leukemia (9); the transamidase activity of transglutaminase II (TGM2) has been reported for the protection of cancer cells against doxorubicin- and cisplatin-induced apoptosis (10); over-expression of PEA-15 was reported in human malignant glioma cells to suppress TNF-induced apoptosis (11). Hence, these three genes were chosen for further validation.
Correlation studies of gene expression levels and drug responses
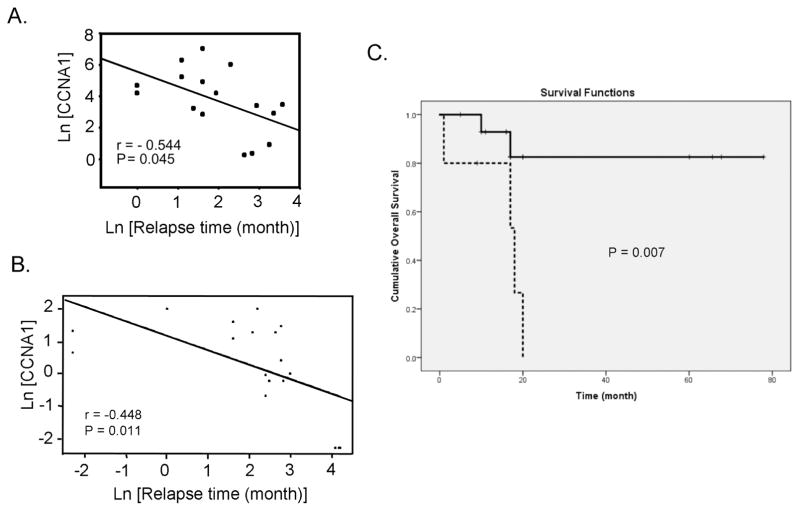
The expression levels of the three selected candidate genes were determined in a panel of 24 ovarian cancer cell lines by quantitative real-time PCR analysis. The histological subtype distribution of the 24 cell lines are listed in Table S1. The correlation statistics between gene expression and sensitivity of the cell lines to eight antioneoplastic agents are shown in Table 2. The cyclin A1 transcript levels were positively correlated with resistance to paclitaxel (P = 0.038), doxorubicin (P = 0.027), and 5-fluorouracil (P = 0.008). In addition, in a study of cyclin A1 expression in a panel of archived tumor samples with known relapse time (7), there was a significant negative correlation between cyclin A1 expression and relapse time of patients (r=−0.544, P = 0.045) (Figure 1A). Immunohistochemical staining with cyclin A1 antibody also showed that the tumors with short relapse time exhibited higher cyclin A1 staining than those with longer relapse time (Figure S1, A and B). To further confirm the correlation, we performed immunohistochemistry on an independent panel of 21 tumor tissues as described in Materials and Methods. In this validation set of samples, there was a significant negative correlation between cyclin A1 protein expression and relapse time of patients (r=−0.448, P = 0.011) (Figure 1B). Kaplan-Meier plot of the overall survival of patients according to cyclin A1 expression showed that the patients with high level of cyclin A1 expression had a significantly worse outcome (P = 0.007) (Figure 1C). Interestingly, the immunohistochemical staining using two different antibodies also showed that cyclin A1 protein was localized in both the nucleus and cytoplasm of the cells (Figure S1). Both qPCR and immunhistochemistry results showed that cyclin A1 appeared to be the most significant gene in our analysis and was chosen for further characterization.
Table 2.
Correlations between the expression levels of 3 candidate genes in 24 ovarian cancer cell lines and the IC50 of 8 anti-cancer agents
| CCNA1 | TGM2 | PEA15 | |
|---|---|---|---|
| Paclitaxel | 0.43 (P = 0.038*) § | 0.33 (P = 0.10) § | 0.19 (P = 0.37) § |
| Doxorubicin | 0.45 (P = 0.027*) | 0.02 (P = 0.93) | 0.05 (P = 0.80) |
| 5-Fluorouracil | 0.53 (P = 0.008*) | 0.21 (P = 0.32) | −0.06 (P = 0.78) |
| Carboplatin | 0.09 (P = 0.69) | 0.20 (P = 0.33) | 0.14 (P = 0.49) |
| Cisplatin | −0.03 (P = 0.88) | 0.09 (P = 0.68) | 0.03 (P = 0.87) |
| Etoposide | 0.29 (P = 0.18) | 0.20 (P = 0.35) | 0.04 (P = 0.85) |
| Topotecan | 0.20 (P = 0.36) | −0.03 (P = 0.88) | −0.06 (P = 0.80) |
| Camptothecin | 0.19 (P = 0.37) | 0.04 (P = 0.84) | 0.006 (P = 0.98) |
Pearson correlation coefficient and P value of two-tailed t-test.
Correlation is significant at P ≤ 0.05.
Figure 1. Association of cyclin A1 expression with early relapse and poor prognosis in ovarian cancer.
(A) Plot of cyclin A1 transcript level versus relapse time of ovarian cancer patient. (B) Plot of cyclin A1 protein level versus relapse time of ovarian cancer patient. (C) Kaplan-Meier plot of overall survival of patients with high or low level of cyclin A1 protein expression. The criterion to separate the patients into high expression and low expression group is described in Materials and Methods. Solid line represents the patients with low cyclin A1 expression, whereas the dotted line represents the patients with high cyclin A1 expression.
Establishment of inducible cyclin A1-expressing model cell lines
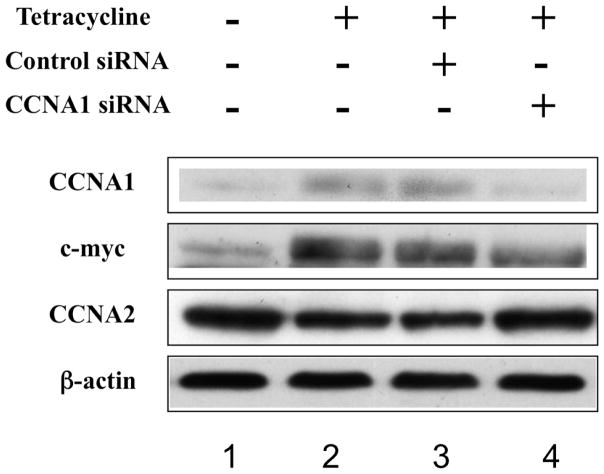
In order to study the possible role of cyclin A1 expression in drug resistance, ovarian cancer cells (TOV112D-CCNA1) that harbor an inducible cyclin A1 expression construct were established as described in the Materials and Methods. The results of two such selected cell lines were very similar and were combined in the presentation. The parental TOV-112D cell line has very little endogenous cyclin A1 expression and is sensitive to Taxol. The integrated cyclin A1 expression construct was designed to express cyclin A1 only when tetracycline was supplemented in the media. A cyclin A1-specific antibody was able to detect the overexpression of cyclin A1 after tetracycline addition (Figure 2, lanes 1 and 2). However, the use of a Myc antibody in the Western blot showed some exogenous Myc epitope-linked cyclin A1 expression in the absence of tetracycline addition, suggesting that some leaky cyclin A1 expression occurs in the absence of tetracycline. In the same assay, a cyclin A1-specific siRNA was used to suppress the expression of cyclin A1 protein. For comparison, a luciferase control siRNA was not able to affect the cyclin A1 expression (Figure 2, lanes 3 and 4). Probing of the same membrane with an antibody specific to cyclin A2 protein did not show the similar changes in the level of the homologous cyclin A2, suggesting that only ectopic cyclin A1 expression was induced, and that the siRNA we employed was solely specific to inhibiting cyclin A1 expression. Interestingly, we found that there was a slight reduction of the cyclin A2 level during cyclin A1 induction (Figure 2, lanes 1 and 2, CCNA2) and this reduction was reversed after the level of cyclin A1 was reduced by cyclin A1-specific siRNA (Figure 2, lanes 3 and 4, CCNA2).
Figure 2. Regulatable expression of cyclin A1 in the TOV112D-CCNA1 cancer cells.
Western blot analysis of total lysates prepared from TOV112D-CCNA1 cells in the presence (lanes 2 through 4) or absence (lane 1) of tetracycline, and after transfection of cyclin A1-specific siRNA (lane 4) or control luciferase-specific siRNA (lane 3). The same membrane was probed sequentially by all of the four antibodies.
Modulation of cyclin A1 expression in ovarian cancer cells reduced cell proliferation rate and affected cellular response to paclitaxel-mediated growth inhibition
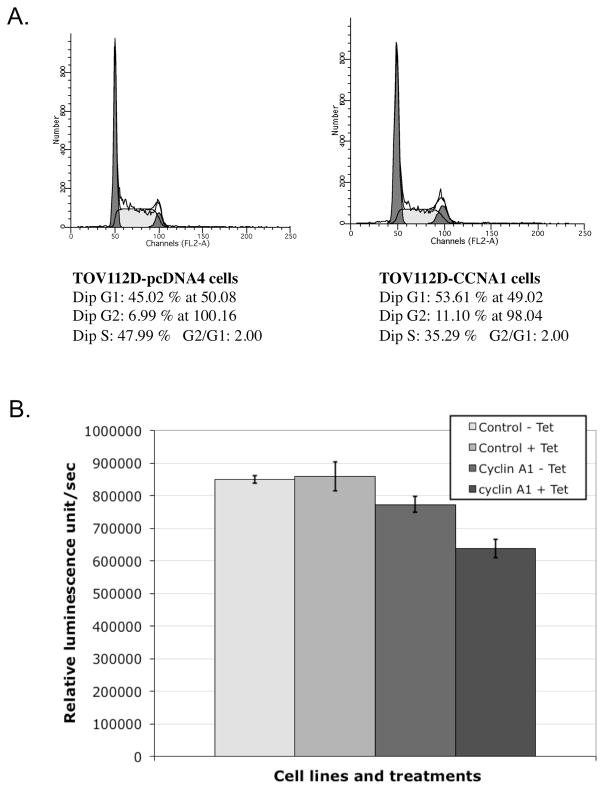
As expression of cyclins is cell cycle-dependent, we tested the effect of cyclin A1 overexpression on cell cycle distribution. As shown in Figure 3A, induction of cyclin A1 expression by tetracycline showed some slight decrease of cells in S phase. We also examined the cell proliferation by BrdU incorporation (Figure 3B). Tetracycline-induced ectopic cyclin A1 expression was associated with 25% reduction of BrdU incorporation.
Figure 3. Cell cycle distribution and proliferation of TOV112D-CCNA1 and TOV112D-pcDNA4 control cells.
(A) Cell cycle distribution of the cancer cells analyzed by propidium iodide staining and flow cytometry. Both cell types were analyzed in the presence of tetracycline induction. The percentages of cells in the various cell cycle phases are presented. (B) Proliferation rates of cells as determined by a BrdU chemiluminescence immunoassay kit.
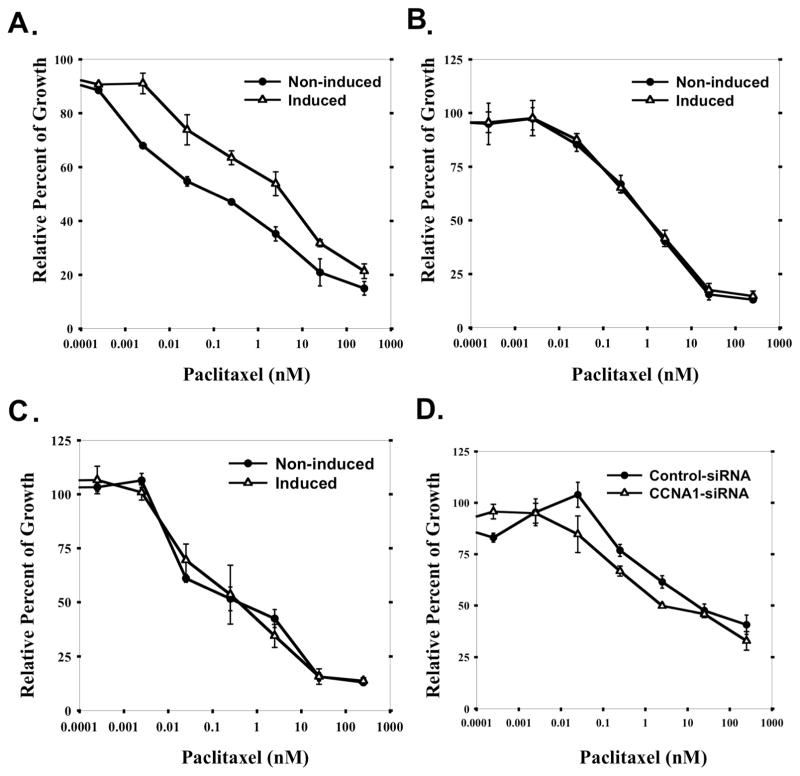
Since Taxol is a first-line therapeutic drug used in treating ovarian cancer, we focused on investigating whether cyclin A1 expression alone is sufficient to influence Taxol response in ovarian cancer cells. Cytotoxicity assay was performed on the TOV112D-CCNA1 cells under induced and non-induced conditions. Paclitaxel IC50 was increased by 40-fold when ectopic cyclin A1 expression was induced by tetracycline (4 nM vs. 0.1 nM) (Figure 4A). The observed change in IC50 was underscored by the leaky expression in the absence of tetracycline. In comparison, tetracycline did not change the paclitaxel IC50 of either the parental cell line (TOV112D) (Figure 4B), or the vector-transfected cell line (TOV112D-pcDNA4) (Figure 4C).
Figure 4. Cellular level of cyclin A1 influences paclitaxel response in ovarian cancer cells.
Growth inhibition of (A) TOV112D-CCNA1 cancer cells, (B) the parental TOV112D cell line, and (C) TOV112D-pCDNA4 cells that harbor mock control vector, pcDNA4/TO/mycHisC, by paclitaxel as measured by MTT assay. Cells were either incubated in medium containing 1μg/ml tetracycline, or without any tetracycline, respectively. In (D), TOV112D-CCNA1 cells were induced by tetracycline incubation, transfected with either cyclin A1-specific siRNA, or control siRNA (luciferase-specific), respectively. After 48 hours of paclitaxel incubation, MTT assays were performed. All the data shown are averages of triplicates of data from three independent experiments.
To further confirm that cyclin A1 is sufficient to change paclitaxel sensitivity, cyclin A1-specific siRNA was used to suppress the level of cyclin A1 expression after tetracycline induction in TOV112D-CCNA1 cells and MTT was performed after 48 hr paclitaxel treatment. Approximately 10-fold reduction of paclitaxel IC50 relative to control siRNA transfection was identified (Figure 4D). These results strongly suggest that the cellular cyclin A1 level has a significant impact on the paclitaxel sensitivity of ovarian cancer cells.
Ectopic cyclin A1 expression reduced paclitaxel-mediated apoptosis but caused senescence-associated β-galactosidase activity
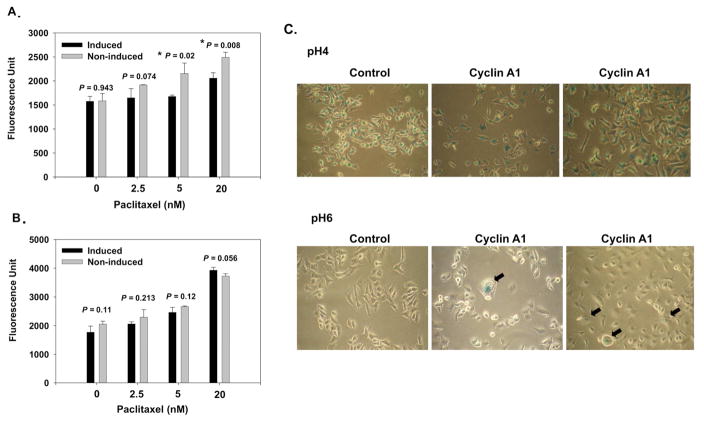
Since apoptosis is a major cell death mechanism induced by chemotherapeutic agents, the extents of apoptosis induction by paclitaxel on the TOV112D-CCNA1 cells were compared under induced and non-induced conditions. At the concentrations of 5 nM, and 20 nM of paclitaxel, significant reduction of caspase activities was found under the condition of cyclin A1 induction (5 nM, P = 0.02; 20 nM, P = 0.008) (Figure 5A). No significant differences in the caspase activities were found in the same analysis with the vector control cell line (Figure 5B).
Figure 5. Apoptosis assay and senescence-associated beta-galactosidase assay.
TOV112D-CCNA1 (A) and TOV112D-pCDNA4 (B) were treated with various concentrations of paclitaxel for 48 hours either in the induced (1μg/ml tetracycline), or non-induced (without tetracycline) conditions, respectively, and then subject to apoptosis assay as described in the Materials and Methods. (C) Cytochemical detection of lysosomal beta-galactosidase activity at pH 4.0 (upper panel) and senescence-associated beta-galactosidase activity at pH 6.0 (bottom panel) of control (TOV112D-pcDNA4) cells, and cylin A1-induced (TOV112D-CCNA1) cells. The senescence-associated beta-galactosidase stained TOV112D-CCNA1 cells are marked by arrow blocks.
As our study showed that ectopic expression of cyclin A1 slowed down cell proliferation in ovarian cancer cells and other studies have shown that overexpressed oncogenes in normal and cancer cells can indcue premature senescence, which may protect cells from apoptosis and is involved in cellular response to chemotherapy and radiation (12), we further investigated the cyclin A1-expressing TOV112D cells and vector-transfected control cells by performing senescence-associated β-galactosidase assay in the presence of tetracycline. As shown in Figure 5C, cyclin A1-expressing cells demonstrated senescence-associated β-galactosidase activity at pH 6.0, which was detectable using chromogenic substrate X-gal. This senescence-associated β-galactosidase activity was not observed in the control cells. The presence of senescence-associated β-galactosidase activity in cyclin A1-expressing ovarian cancer cells may be related to the reduction of apoptosis and proliferation rate of these cells.
DISCUSSION
In our global expression profiling analysis using three different classes of cell lines and clinical samples, we have identified that cyclin A1 was consistently overexpressed in recurrent and chemoresistant ovarian tumors and cancer cell lines. Further validation studies with a panel of ovarian cancer cell lines and tumor tissues demonstrated a positive correlation between levels of cyclin A1 and paclitaxel resistance. Involvement of cyclin A1 in human cancers has been implicated in other cancer types (13, 14), and high expression of cyclin A1 is linked to poor patient outcome in acute myeloid leukemia (15) and prostate cancer invasion (16). By using inducible TOV112D-CCNA1 cancer cells, we were able to demonstrate that ectopic expression of cyclin A1 was sufficient to enhance paclitaxel resistance, while depletion of cyclin A1 expression by siRNA could sensitize the cells to paclitaxel cytotoxicity. As there was still some residual cyclin A1 expression in the cells at uninduced state and after cyclin A1-specific siRNA trasnfection (Figure 2, lanes 1 and 4), we reasoned that the modest differences of IC50 values in our experiments were underscored by the residual cyclin A1 levels. Notwithstanding, we further showed that enhanced expression of cyclin A1 significantly attenuated paclitaxel-induced apoptosis. In conjunction with the fact that cyclin A1 overexpression was observed in all three classes of samples we studied, the results of our functional studies using the inducible cells suggest that cyclin A1 might be an important factor in modulating the response of ovarian cancer cells to paclitaxel.
Unlike the ubiquitously expressed cyclin A2, high expression of cyclin A1 is restricted only to the germ cells undergoing meiosis in testis (17). Cyclin A1 knockout male mice were sterile due to the block of spermatogenesis, whereas female mice were normal (18). In contrast, cyclin A2 is essential for DNA replication and cell proliferation in somatic cells and the null genotype of cyclin A2 is lethal for both male and female mice (19). However, the work by Yang et al. has shown that in the mitotic cell cycle, cyclin A1 interacts with cyclin-dependent kinase CDK2, which subsequently phosphorylates important cell cycle regulators such as E2F1, p21, and Rb family of proteins (20). In our TOV112D-CCNA1 epithelial cancer cells, overexpression of cyclin A1 demonstrated a slow down in cell cycle and proliferation rate, which is also consistent with the observations that the level of proliferation-associated cyclin A2 was negatively related to the cellular level of cyclin A1 (Figure 2).
The cytotoxic effects of chemotherapeutic agents depend largely on the activation of apoptosis. Taxol and its derivatives stabilize cytoskeletal microtubules by binding to β-tubulin and preventing depolymerization and consequently blocking the cell cycle in the G2/M phase, causing mitotic arrest at metaphase. Prolonged arrest of cell division eventually activates a checkpoint and induces programmed cell death (21). The mechanisms that are relevant to paclitaxel resistance include overexpression of MDR1 (P-glycoprotein drug efflux pump) (22), mutations of β-tubulin (23), differential expression of β-tubulin isotypes (24), altered cellular localization of mitotic check point factors (25), and modulation of p53 activities (26). Although the exact mechanism underlying cyclin A1-mediated chemoresistance awaits further delineation, regulation of CDK2 activity by cyclin A1 might be the major contributor. Changes of cdc2/CDK2 activity have been reported for the resistance to doxorubicin and Taxol-induced apoptosis (27). In a yeast triple-hybrid screen, G-protein pathway suppressor 2 (GPS2), which can suppress cell growth by inhibiting c-Jun N-terminal Kinase 1 (JNK) (28), was found to be one of the associated partners of cyclin A1-CDK2 complex (29). JNK has been suggested to be responsible for the paclitaxel-mediated hyperphosphorylation of Bcl-2 and inhibition of its anti-apoptotic activity (30). Therefore, cyclin A1 might confer apoptosis-resistance by upregulating a survival pathway via GPS2 activity and prevention of Bcl-2 phosphorylation. Alternatively, cell survival influenced by cellular localization of pro-death transcription factor FOXO1 due to unscheduled CDK2 activity has also been reported (31).
Aside from the influence on apoptotic pathways, cyclin A1 has also been reported to participate in p53-mediated DNA double-strand break (DSB) repair following radiation damage (32). Ionizing radiation can generate DNA DSB by inducing reactive oxygen species (ROS) in the cells. Topoisomerase II poisons such as etoposide can stabilize topoisomerase-DNA cleavable complex, which if collides with DNA replication complex, can cause DNA DSB. However, in our correlation analysis (Table 2), cyclin A1 expression is not correlated with etoposide response, but rather is correlated with resistance to another weak topoisomerase II poison, doxorubicin. It was postulated that in addition to weak topoisomerase poisoning activity, doxorubicin can also generate ROS and cause significant DNA DSB (33). It is intriguing that paclitaxel treatment will also increase the cellular ROS levels at a clinically relevant concentration (100 nM) (34). However, significant DNA damages caused by paclitaxel was only observed at the concentrations of 10 μM and above (35). Hence, the involvement of cyclin A1 in paclitaxel resistance may be related to ROS, which occurs earlier than the appearance of DNA strand breaks. In conjunction with the survival pathway mentioned above, cyclin A1/CDK2 complex may be crucial to determine the balance between cell death and survival when cells are under genotoxic stress.
Lastly, it is intriguing that cyclin A1-overexpressing ovarian cancer cells demonstrate senescence-associated phenotype. A report by Narita et al. has shown that the high-mobility group A (HMGA) proteins, which can enhance proliferation and promote tumorigenesis, also contribute to proliferative arrest and senescence stabilization (36). It is noted that the anti-proliferative activity of HMGA can be canceled by CDK overexpression. Hence, like HMGA proteins, the properties of cyclin A1 may depend on the presence or absence of some interacting partners. Some recent studies have provided evidence that premature senescence may be a survival strategy that protects cells from undergoing apoptosis after genotoxic stress (37, 38). As mentioned above, one potential outcome of cyclin A1-CDK2 interaction is the blocking of Bcl2 hyperphosphorylation and degradation, and is ROS-related. Bcl2 was found to promote reversible cell cycle arrest and senescence after DNA damage and serum withdrawal (38). Cancer cells with high levels of AKT activation and intracellular ROS undergo senescence instead of apoptosis (37). Furthermore, proinflammatory and mitogenic cytokines secreted by senescent cells were reported to trigger the emergence of chemoresistant and clonogenic cell subpopoulations in mesothelioma cells (39). As a clinically more relevant example between premature senescence and chemoresistance, Sun et al. have found acquired activation of TGF-β signaling and EGFR expression in 6 out of 16 drug resistant BRAF(V700E) mutant melanomas, which led to a slow-growth phenotype and oncogene-induced senescence (40). However, tumors with EGFR expression and TGF-β activation became beneficial for proliferation in the presence of BRAF or MEK inhibitor drugs (40). Hence, cyclin A1 may be one of the many pathways that employ reversible senescence as an adaptive resistance mechanism to chemotherapy. Currently, several inhibitors such as flavopiridol, 17-AGG, and UCN-01 that disrupt the functions of cell cycle regulatory kinases and signal transduction proteins are undergoing phase I and II clinical trials as monotherapy or in combination with traditional chemotherapy agents. It will be of great interest to investigate if these inhibitors can abrogate a critical cyclin A1-mediated survival pathway and rationally enhance the therapeutic efficacy of Taxol in treating ovarian cancer.
Supplementary Material
Elevated cyclin A1 expression is negatively associated with relapse time of ovarian cancer patients and sensitivity of cancer cells to paclitaxel, doxorubicin and 5-fluorouracil.
Ectopic expression of cyclin A1 in ovarian cancer cells contributes to reduced paclitaxel-induced apoptosis and enhanced cell survival, which can be attenuated by suppression of cyclin A1 expression in the same cell line.
Cyclin A1-overexpression, however, slows down cell proliferation and induces premature senescence.
Acknowledgments
This study was supported by an Individual Investigator Award (to SWN) and a grant BWH/BW.03 (to KCH) from Ovarian Cancer Research Fund Inc., and also partly from NCI grants CA94944, CA104743 from National Institute of Health, Department of Health and Human Services. The Laboratory of Gynecologic Oncology at Brigham and Women’s Hospital is also supported by the Robert and Deborah First Fund, the Sperling Family Fund Foundation, Ruth N. White Gynecologic Oncology Research Fund, Women’s Cancer Program and Gillette Center for Women’s Cancer from Dana-Farber Cancer Institute, Adler Foundation, Inc., and Friends of Dana Farber Cancer Institute.
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: 2015. [Google Scholar]
- 2.du Bois A, Neijt JP, Thigpen JT. First line chemotherapy with carboplatin plus paclitaxel in advanced ovarian cancer--a new standard of care? Ann Oncol. 1999;10(Suppl 1):35–41. doi: 10.1023/a:1008355317514. [DOI] [PubMed] [Google Scholar]
- 3.Hoskins P, Tu D, James K, Pater J, Koski B. Factors predictive of survival after first relapse or progression in advanced epithelial ovarian carcinoma: a prediction tree analysis-derived model with test and validation groups. Gynecol Oncol. 1998;70(2):224–30. doi: 10.1006/gyno.1998.5074. [DOI] [PubMed] [Google Scholar]
- 4.Akahira JI, Yoshikawa H, Shimizu Y, Tsunematsu R, Hirakawa T, Kuramoto H, et al. Prognostic factors of stage IV epithelial ovarian cancer: a multicenter retrospective study. Gynecol Oncol. 2001;81(3):398–403. doi: 10.1006/gyno.2001.6172. [DOI] [PubMed] [Google Scholar]
- 5.Chi DS, Sabbatini P. Advanced ovarian cancer. Curr Treat Options Oncol. 2000;1(2):139–46. doi: 10.1007/s11864-000-0058-1. [DOI] [PubMed] [Google Scholar]
- 6.Thigpen JT, Blessing JA, Ball H, Hummel SJ, Barrett RJ. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. J Clin Oncol. 1994;12(9):1748–53. doi: 10.1200/JCO.1994.12.9.1748. [DOI] [PubMed] [Google Scholar]
- 7.Huang KC, Rao PH, Lau CC, Heard E, Ng SK, Brown C, et al. Relationship of XIST expression and responses of ovarian cancer to chemotherapy. Mol Cancer Ther. 2002;1(10):769–76. [PubMed] [Google Scholar]
- 8.van der Loo B, Fenton MJ, Erusalimsky JD. Cytochemical detection of a senescence-associated beta-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res. 1998;241(2):309–15. doi: 10.1006/excr.1998.4035. [DOI] [PubMed] [Google Scholar]
- 9.Liao C, Wang XY, Wei HQ, Li SQ, Merghoub T, Pandolfi PP, et al. Altered myelopoiesis and the development of acute myeloid leukemia in transgenic mice overexpressing cyclin A1. Proc Natl Acad Sci U S A. 2001;98(12):6853–8. doi: 10.1073/pnas.121540098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melino G, Annicchiarico-Petruzzelli M, Piredda L, Candi E, Gentile V, Davies PJ, et al. Tissue transglutaminase and apoptosis: sense and antisense transfection studies with human neuroblastoma cells. Mol Cell Biol. 1994;14(10):6584–96. doi: 10.1128/mcb.14.10.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitsberg D, Formstecher E, Fauquet M, Kubes M, Cordier J, Canton B, et al. Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFalpha-induced apoptosis. J Neurosci. 1999;19(19):8244–51. doi: 10.1523/JNEUROSCI.19-19-08244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76(8):947–57. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Wegiel B, Bjartell A, Ekberg J, Gadaleanu V, Brunhoff C, Persson JL. A role for cyclin A1 in mediating the autocrine expression of vascular endothelial growth factor in prostate cancer. Oncogene. 2005;24(42):6385–93. doi: 10.1038/sj.onc.1208795. [DOI] [PubMed] [Google Scholar]
- 14.Kramer A, Hochhaus A, Saussele S, Reichert A, Willer A, Hehlmann R. Cyclin A1 is predominantly expressed in hematological malignancies with myeloid differentiation. Leukemia. 1998;12(6):893–8. doi: 10.1038/sj.leu.2401051. [DOI] [PubMed] [Google Scholar]
- 15.Ekberg J, Holm C, Jalili S, Richter J, Anagnostaki L, Landberg G, et al. Expression of cyclin A1 and cell cycle proteins in hematopoietic cells and acute myeloid leukemia and links to patient outcome. Eur J Haematol. 2005;75(2):106–15. doi: 10.1111/j.1600-0609.2005.00473.x. [DOI] [PubMed] [Google Scholar]
- 16.Wegiel B, Bjartell A, Tuomela J, Dizeyi N, Tinzl M, Helczynski L, et al. Multiple cellular mechanisms related to cyclin A1 in prostate cancer invasion and metastasis. J Natl Cancer Inst. 2008;100(14):1022–36. doi: 10.1093/jnci/djn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang R, Morosetti R, Koeffler HP. Characterization of a second human cyclin A that is highly expressed in testis and in several leukemic cell lines. Cancer Res. 1997;57(5):913–20. [PubMed] [Google Scholar]
- 18.Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20(4):377–80. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- 19.Murphy M, Stinnakre MG, Senamaud-Beaufort C, Winston NJ, Sweeney C, Kubelka M, et al. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat Genet. 1997;15(1):83–6. doi: 10.1038/ng0197-83. [DOI] [PubMed] [Google Scholar]
- 20.Yang R, Muller C, Huynh V, Fung YK, Yee AS, Koeffler HP. Functions of cyclin A1 in the cell cycle and its interactions with transcription factor E2F-1 and the Rb family of proteins. Mol Cell Biol. 1999;19(3):2400–7. doi: 10.1128/mcb.19.3.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 22.Ling V, Charles F. Kettering Prize. P-glycoprotein and resistance to anticancer drugs. Cancer. 1992;69(10):2603–9. doi: 10.1002/1097-0142(19920515)69:10<2603::aid-cncr2820691034>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272(27):17118–25. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 24.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100(5):1282–93. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasai T, Iwanaga Y, Iha H, Jeang KT. Prevalent loss of mitotic spindle checkpoint in adult T-cell leukemia confers resistance to microtubule inhibitors. J Biol Chem. 2002;277(7):5187–93. doi: 10.1074/jbc.M110295200. [DOI] [PubMed] [Google Scholar]
- 26.Wu GS, El-Diery WS. p53 and chemosensitivity. Nat Med. 1996;2(3):255–6. doi: 10.1038/nm0396-255a. [DOI] [PubMed] [Google Scholar]
- 27.Tan M, Jing T, Lan KH, Neal CL, Li P, Lee S, et al. Phosphorylation on tyrosine-15 of p34(Cdc2) by ErbB2 inhibits p34(Cdc2) activation and is involved in resistance to taxol-induced apoptosis. Mol Cell. 2002;9(5):993–1004. doi: 10.1016/s1097-2765(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 28.Jin DY, Teramoto H, Giam CZ, Chun RF, Gutkind JS, Jeang KT. A human suppressor of c-Jun N-terminal kinase 1 activation by tumor necrosis factor alpha. J Biol Chem. 1997;272(41):25816–23. doi: 10.1074/jbc.272.41.25816. [DOI] [PubMed] [Google Scholar]
- 29.Diederichs S, Baumer N, Ji P, Metzelder SK, Idos GE, Cauvet T, et al. Identification of interaction partners and substrates of the cyclin A1-CDK2 complex. J Biol Chem. 2004;279(32):33727–41. doi: 10.1074/jbc.M401708200. [DOI] [PubMed] [Google Scholar]
- 30.Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci U S A. 1995;92(10):4507–11. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314(5797):294–7. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 32.Muller-Tidow C, Ji P, Diederichs S, Potratz J, Baumer N, Kohler G, et al. The cyclin A1-CDK2 complex regulates DNA double-strand break repair. Mol Cell Biol. 2004;24(20):8917–28. doi: 10.1128/MCB.24.20.8917-8928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurz EU, Douglas P, Lees-Miller SP. Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J Biol Chem. 2004;279(51):53272–81. doi: 10.1074/jbc.M406879200. [DOI] [PubMed] [Google Scholar]
- 34.Park SJ, Wu CH, Gordon JD, Zhong X, Emami A, Safa AR. Taxol induces caspase-10-dependent apoptosis. J Biol Chem. 2004;279(49):51057–67. doi: 10.1074/jbc.M406543200. [DOI] [PubMed] [Google Scholar]
- 35.Branham MT, Nadin SB, Vargas-Roig LM, Ciocca DR. DNA damage induced by paclitaxel and DNA repair capability of peripheral blood lymphocytes as evaluated by the alkaline comet assay. Mutat Res. 2004;560(1):11–7. doi: 10.1016/j.mrgentox.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, Myers MP, et al. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126(3):503–14. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 37.Lee JJ, Kim BC, Park MJ, Lee YS, Kim YN, Lee BL, et al. PTEN status switches cell fate between premature senescence and apoptosis in glioma exposed to ionizing radiation. Cell Death Differ. 2011;18(4):666–77. doi: 10.1038/cdd.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelyudova A, Aksenov N, Pospelov V, Pospelova T. By blocking apoptosis, Bcl-2 in p38-dependent manner promotes cell cycle arrest and accelerated senescence after DNA damage and serum withdrawal. Cell Cycle. 2007;6(17):2171–7. doi: 10.4161/cc.6.17.4610. [DOI] [PubMed] [Google Scholar]
- 39.Canino C, Mori F, Cambria A, Diamantini A, Germoni S, Alessandrini G, et al. SASP mediates chemoresistance and tumor-initiating-activity of mesothelioma cells. Oncogene. 2012;31(26):3148–63. doi: 10.1038/onc.2011.485. [DOI] [PubMed] [Google Scholar]
- 40.Sun C, Wang L, Huang S, Heynen GJ, Prahallad A, Robert C, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508(7494):118–22. doi: 10.1038/nature13121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.