SUMMARY
Increasing ambient temperature reorganizes the Drosophila sleep pattern in a way similar to the human response to heat, increasing daytime sleep while decreasing nighttime sleep. Mutation of core circadian genes blocks the immediate increase in daytime sleep, but not the heat-stimulated decrease in nighttime sleep, when animals are in a light:dark cycle. The ability of per01 flies to increase daytime sleep in light:dark can be rescued by expression of PER in either LNv or DN1p clock cells and does not require rescue of locomotor rhythms. Prolonged heat exposure engages the homeostat to maintain daytime sleep in the face of nighttime sleep loss. In constant darkness, all genotypes show an immediate decrease in sleep in response to temperature shift during the subjective day, implying that the absence of light input uncovers a clock-independent proarousal effect of increased temperature. Interestingly, the effects of temperature on nighttime sleep are blunted in constant darkness and in cryOUT mutants in light:dark, suggesting that they are dependent on the presence of light the previous day. In contrast, flies of all genotypes kept in constant light sleep more at all times of day in response to high temperature, indicating that the presence of light can invert the normal nighttime response to increased temperature. The effect of temperature on sleep thus reflects coordinated regulation by light, the homeostat, and components of the clock, allowing animals to reorganize sleep patterns in response to high temperature with rough preservation of the total amount of sleep.
INTRODUCTION
Both internal states and environmental factors can affect sleep. Disruptions of sleep architecture in individuals suffering from circadian disorders and in blind individuals have been well documented [1, 2]. In general, circadian rhythms schedule wakefulness and activity at specific times of day depending on whether the animal is diurnal or nocturnal. Light has a dual role in the regulation of sleep-wake cycles. First, it is the major Zeitgeber (time giver) that entrains and synchronizes the circadian system. Second, light regulates locomotor activity. The latter effects of light are independent of the circadian system and are observed even in animals without a functional circadian clock [3]. Similar to light, temperature also acts as a Zeitgeber to the circadian system and has circadian-independent effects on sleep-wake cycles [4]. In contrast, homeostatic sleep drive promotes sleep as a function of accumulated wakefulness and, as such, dissipates during sleep [5]. The regulation of sleep-wake cycles by the balance between homeostatic, environmental, and circadian forces has important implications for human health.
The fruit fly, Drosophila melanogaster, is an ideal model for dissection of the relationships between genes, environment, and behaviors [6]. As in mammals, sleep in Drosophila is associated with reduced sensory responsiveness, is under circadian and homeostatic regulation [7, 8], and is associated with altered oscillatory brain activity [9–11]. Researchers have identified a number of genes [12], circuits [13], and biological processes [14, 15] that regulate fly sleep. However, the mechanisms underlying the regulation of Drosophila sleep by temperature have not been extensively investigated.
Temperature has been shown to have a plethora of potentially sleep-related effects in both flies and humans. In flies it has been shown to effect locomotor activity at mid-day, during siesta [16, 17] and sleep itself [18]. The level of splicing of the circadian clock gene period is associated with this modulation [19, 20]. In humans, increased ambient temperatures correlate with decreases in REM [21], and optimal temperatures have been suggested to improve nighttime sleep [22]. Interestingly, thermoregulation has also been shown to influence human sleep patterns [23, 24]; people suffering from insomnia have aberrant core body temperature at bedtime compared to normal sleepers [25]. Here, we show that in flies too sleep patterns are sensitive to temperature and investigate the processes that mediate this effect. We find that both the daytime and nighttime effects of an acute increase in temperature in normal light:dark (LD) conditions are gated by daytime light exposure, but only the effects of temperature on daytime sleep are regulated by circadian clock genes. Prolonged exposure to high temperature engages the homeostat to further shift sleep into the daytime. In constant light (LL) or constant darkness (DD), temperature has acute and chronic effects on sleep that are independent of the circadian clock.
RESULTS
Acute Increases in Temperature Alter Sleep Architecture and Distribution
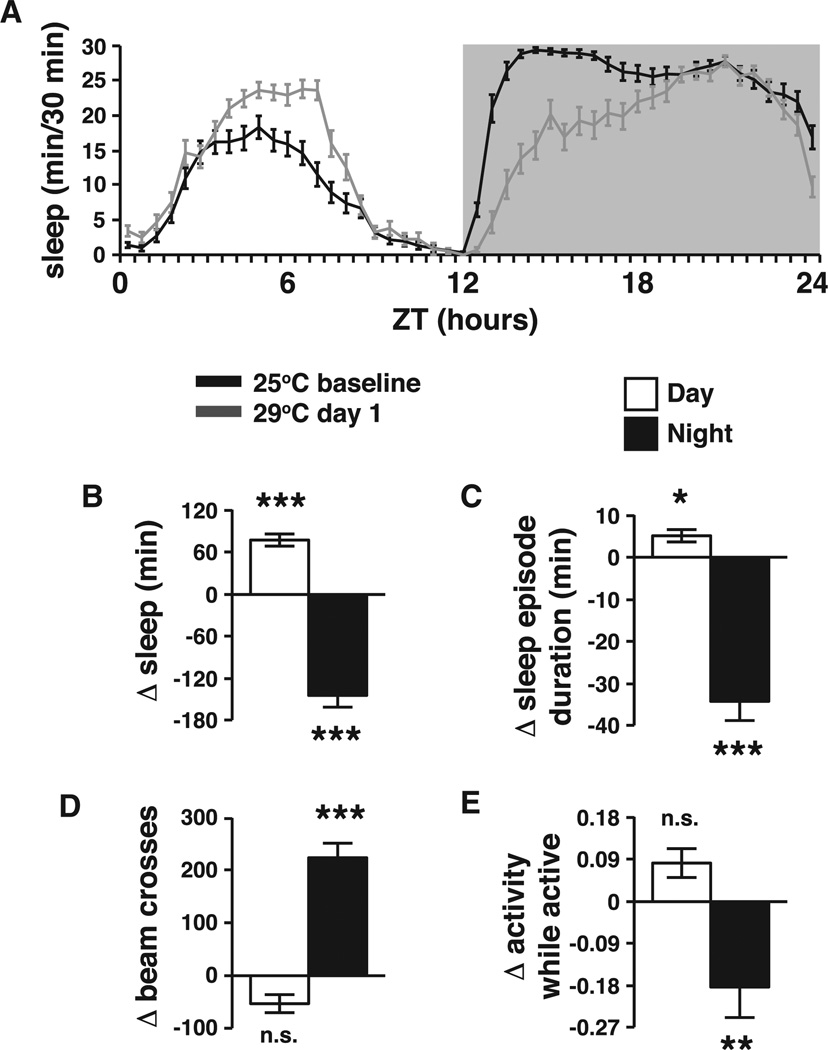
Previous studies have shown that increasing ambient temperature from 25°C to 29°C affects circadian regulation of locomotor activity by decreasing daytime locomotion and increasing nighttime locomotion [16, 26]. This change was interpreted as arising solely from changes in clock regulation of motor function, and it was unknown how much of this activity change is due to changes in sleep. To evaluate the effect of temperature on sleep, we assayed the sleep pattern of wild-type Canton S female flies in a (12 hr:12 hr) light:dark cycle for 3 days at 25°C using beam break data (DAM system, Trikinetics), defining a sleep bout as a period of >5 min of inactivity [27]. On day 4, animals were shifted to 29°C at the start of the light period (ZT0). Figure 1A shows a comparison of sleep at 25°C to sleep on the first day after the shift to 29°C in wild-type flies. Daytime sleep increased on average by more than 1 hr in wild-type flies at 29°C, compared to baseline, while nighttime sleep is decreased by over 2 hr during the temperature elevation (Figure 1B), resulting in a rough preservation of total sleep after the temperature shift. Similar results were seen with males, though the magnitude of daytime changes was limited by the high baseline level of male daytime sleep (Figure S1).
Figure 1. Temperature Shift Induces Sleep Restructuring in Wild-Type Flies.
(A) Baseline sleep per 30 min comparing 25°C (black line) to 29°C (gray line) after a temperature shift at ZT0 in wild-type Canton S flies (n = 35).
(B–E) Changes from baseline in light and dark periods are shown for the following sleep parameters: (B) total sleep, (C) mean sleep episode duration, (D) total number of beam breaks, and (E) activity level while awake (beam breaks/min active). Data in (B)–(E) are presented as means ± SEM and 25°C compared to 29°C using type 2, two-tail Student’s t test. *p < 0.05, **p < 0.005, ***p < 0.0005; n.s., not significant.
See also Figures S1 and S2A–S2D.
Changes in the amount of sleep were accompanied by changes in its structure. Daytime sleep became more consolidated, with sleep episode duration increasing by 7 min (Figure 1C). Conversely, nighttime sleep became more fragmented with a significant decrease (over 30 min shorter) in mean episode duration (Figure 1C). These data suggest temperature is altering sleep structure as well as its day/night distribution. This supports the idea that temperature has a primary effect on sleep and that these effects may be upstream of temperature-dependent changes in locomotion. The chronic effects of this temperature shift (assessed on day 3 after the shift) were similar (Figures S2A–S2C).
Consistent with previous reports [16, 26], we observed a decrease in the number of daytime beam crosses at 29°C, and an increase in the number of beam crosses at night both immediately after the shift and after prolonged exposure to higher temperature (Figures 1D and S2D). To determine whether the observed changes in locomotor activity were due to hyperlocomotion or to differences in arousal, we measured the average level of activity exclusively during wake periods. We found that the intensity of locomotor activity was not significantly altered during daytime waking, but that it was significantly decreased during nighttime wake periods (Figures 1E and S2E). This is the opposite of what would be expected if increased temperature was simply causing hyperlocomotion, indicating that the observed changes in activity at night are due to arousal changes affecting sleep and not hyperlocomotion.
Acute Upregulation of Daytime Sleep, but Not Nighttime Sleep, by Heat Requires Core Clock Proteins
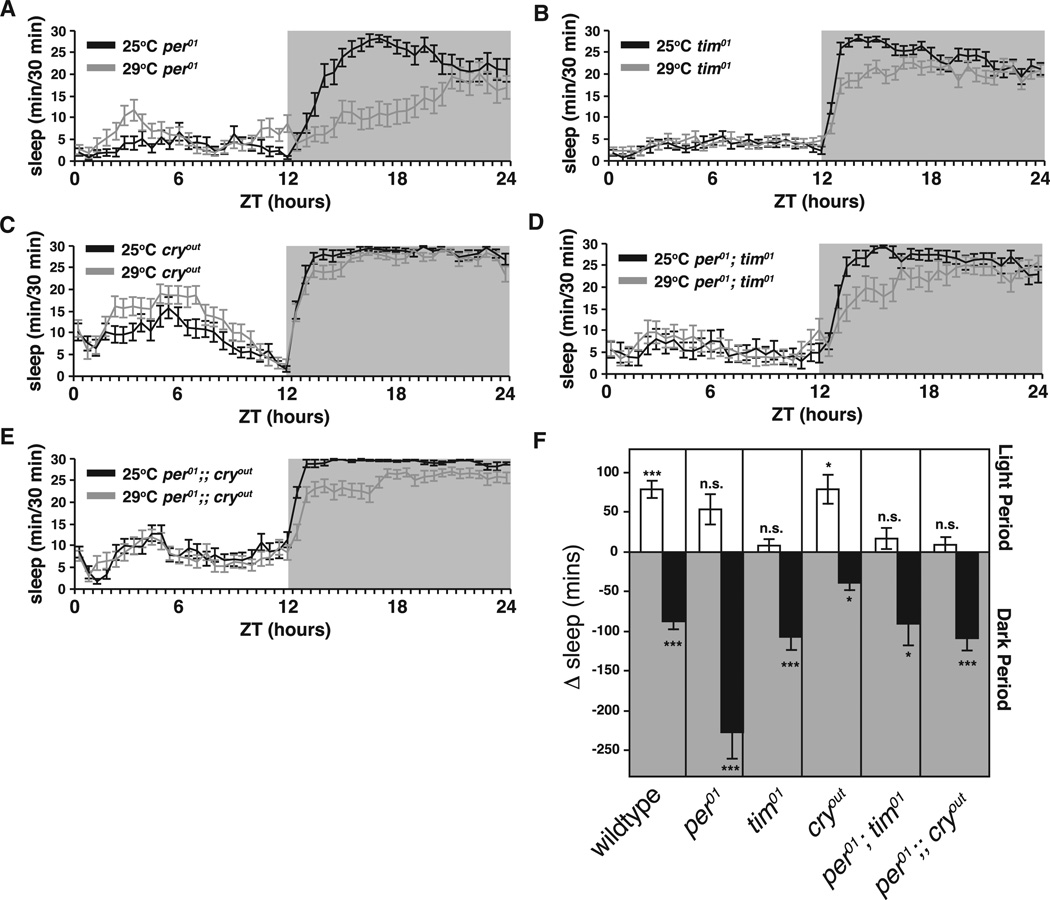
The apportioning of sleep between day and night is in part a function of the circadian clock. To determine whether temperature-dependent redistribution of sleep required a fully functional clock, we evaluated the effects of temperature shifts in circadian mutants by testing flies carrying loss of function alleles of the core clock genes period (per01) and timeless (tim01) and flies mutant for cryptochrome (cryOUT), whose gene product conveys light information to the clock [28]. Baseline sleep was measured at 25°C in LD, and then animals were shifted to 29°C at the start of the light period (ZT0). In per01, tim01, and the per01;tim01 double mutants, elevated temperature failed to elicit a significant increase in daytime sleep on the day after temperature shift (Figures 2A, 2B, 2D, and 2F), indicating that the molecular clock is required. In cryOUT mutants, however, elevated temperature elicited a normal increase in daytime sleep on the day of the shift (Figures 2C and 2F) suggesting that CRY is not necessary for the daytime heat response. These results imply that temperature-dependent increases in daytime sleep require the molecular clock.
Figure 2. Temperature Shift at ZT0 Fails to Increase Siesta in Circadian Mutants.
(A–E) Sleep plots for each genotype comparing 25°C baseline sleep (black line) to first day after a ZT0 29°C temperature shift (gray line).
(F) Changes in total daytime (white bars) and nighttime (black bars) sleep, 25°C versus 29°C, for wild-type and circadian mutants are shown. Canton S wild-type, n = 37; per01, n = 20; tim01, n = 33; per0;tim01, n = 17; cryOUT, n = 26; per0;cryOUT, n = 28. Data are presented as means ± SEM and are compared within genotype (25°C versus 29°C) using Student’s t test. *p < 0.05, **p < 0.005, ***p < 0.0005; n.s., not significant.
See also Figure S2F.
The effects of temperature on nighttime sleep were not reduced by mutations in per or tim, indicating that the ability of temperature to modulate nighttime sleep does not require a functional clock. Notably, however, mutations in cry, almost completely eliminated the nighttime decrease in sleep. This raises the possibility that light during the previous day may gate the ability of high temperature to inhibit nighttime sleep.
Interestingly, the inability of circadian clock mutants to mount a daytime increase in sleep is gone after longer exposure to high temperatures. Figure S2F shows data from the third day after ZT0 shift (daytime sleep is elevated on the second day as well; data not shown). This suggests that some other process is being rapidly engaged to compensate for these mutants’ inability to mount an increase in daytime sleep. One possible scenario was that sleep loss during the night was stimulating a homeostatic increase in the following day’s sleep. This would result in homeostatically generated rebound sleep masking the circadian mutant phenotype after the first day of exposure.
Heat-Induced Suppression of Nighttime Sleep Can Induce Homeostatic Rebound Sleep the following Day
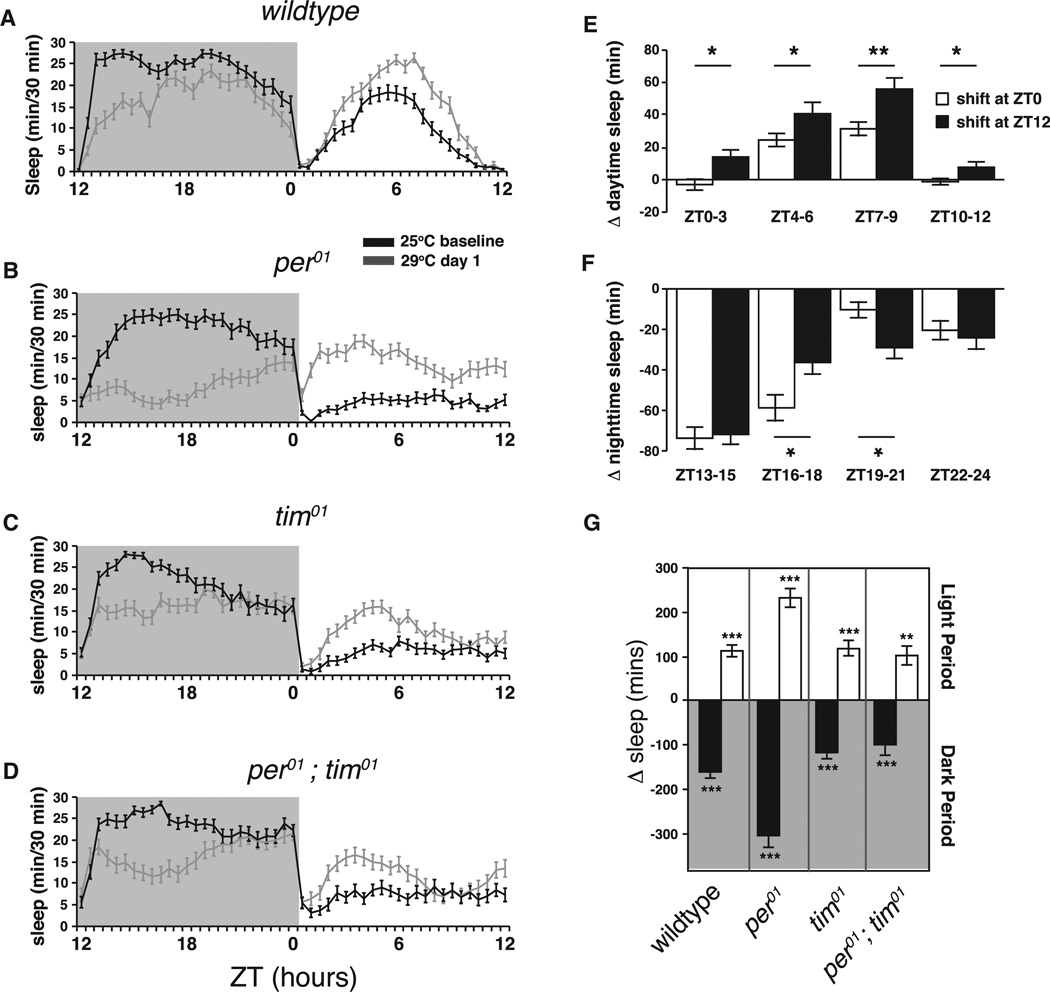
To test the idea that homeostatic processes compensate for loss of clock-dependent daytime sleep, we needed to first determine whether heat-induced nighttime sleep loss could drive daytime rebound sleep. We shifted wild-type flies to 29°C at ZT12 at the beginning of the dark period to assess both the acute effect of high temperature on nighttime sleep and the effect on the subsequent day’s sleep. Wild-type flies shifted to 29°C at the beginning of the dark period displayed significantly reduced nighttime sleep followed by an increase in sleep the following day that was independent of ambient temperature during the rebound period (Figures 3A and S3). The decrease in nighttime sleep after a ZT12 shift implies that the effect of temperature on nighttime sleep is direct and does not require increased temperature during the previous light period. As shown for the ZT0 shift in Figure 1, changes in the distribution of sleep were accompanied by changes in sleep structure; mean episode duration increased during the day and decreased at night (Figure S4A). Locomotor activity during daytime waking periods was unchanged and actually decreased at night, suggesting that, as with ZT0 shift, the overall changes in activity were a result of changes in arousal and not changes in locomotion (Figure S4B).
Figure 3. Homeostatic Rebound from Temperature-Induced Sleep Loss is Intact in Circadian Mutants.
(A–D) Sleep plots comparing sleep at baseline (25°C black line) to sleep after a shift to 29°C at ZT12 (gray line) for wild-type and mutant animals. Shaded background area indicates dark period, ZT12-ZT24.
(E) Comparison of sleep changes in 3-hr bins during the day for wild-type animals experiencing a ZT0 shift (white bars, data from Figure 1) or a ZT12 shift (black bars). Data are presented as means ± SEM and ZT0 compared to ZT12 for each bin using Student’s t test.
(F) Comparison of sleep changes in 3-hr bins during the night as in (E).
(G) Changes in total daytime (white bars) and nighttime (black bars) sleep after a 25°C to 29°C temperature shift at ZT12 for wild-type and circadian mutants are shown.
Data are presented as means ± SEM and are compared within genotype (25°C versus 29°C) using Student’s t test. Canton S wild-type, n = 35; per01, n = 40; tim01, n = 44; per0;tim01, n = 37. *p < 0.05, **p < 0.005, ***p < 0.0005; n.s., not significant.
See also Figures S3 and S4.
Consistent with homeostasis having a large role in the generation of excess daytime sleep when temperature elevation is prolonged, the change in daytime sleep during the first 24 hr after a shift was larger when the 29°C regimen was started at ZT12 (the beginning of the dark period) than if it was started at ZT0 (53% increase for the ZT12 shift compared to 37% for the ZT0 shift p = 0.02 Student’s t test). Visual comparison of Figures 1A and 3A suggests that, in addition to the difference in the amount of extra sleep, the distribution across the day induced by the temperature shift is different depending on when the shift occurred. To examine this quantitatively, we calculated the change in sleep (baseline 25°C versus 29°C) for 3-hr windows across the day (Figures 3E and 3F). Indeed, shifting flies at the start of the light period (ZT0) does not produce an increase in sleep in the ZT0–ZT3 window, whereas shifting flies the previous night (at ZT12) induces an immediate increase in total sleep in the ZT0–ZT3 period the following day. Importantly, rebound sleep has been shown to occur most profoundly in the beginning of the day [7]. This implies that the overall higher levels of daytime sleep observed in wild-type animals at 29°C in the first day after a ZT12 shift are due to a combination of sleep rebound caused by the negative effect of high temperature on the previous nighttime sleep plus a direct effect of temperature on late daytime sleep. A further implication is that in wild-type flies, when temperature is chronically elevated, the increase seen in daytime sleep will come from two separate processes: rebound sleep from nighttime sleep loss and a direct, clock-dependent, effect of temperature on daytime sleep. In contrast, the magnitude and temporal distribution of changes in nighttime sleep are almost identical after ZT0 and ZT12 shifts during both ZT13–ZT15 and ZT22–ZT24 windows (Figure 3F), suggesting little homeostatic component to nighttime sleep changes.
Homeostatic Changes in Sleep after Temperature Shift Are Intact in Clock Mutants
Since daytime sleep was relatively unchanged by a ZT0 shift in per01 and tim01 mutants, we were interested in determining whether they could mount a homeostatically driven increase in daytime sleep after a ZT12 shift or whether they were refractory to all sleep-inducing processes during the light period. We found that these circadian mutants were able to increase sleep during the subsequent light period after a ZT12 shift (Figures 3B–3D), consistent with a previous study showing that per01 mutants were able to generate homeostatic sleep after mechanical sleep deprivation [29]. This suggests that while temperature alone cannot induce an increase in daytime sleep in the absence of a PER or TIM in LD, homeostatic drive is still able to increase sleep during the light period. The direct effects of homeostatic rebound and temperature are therefore mechanistically separate, with only the latter requiring these clock proteins. This strongly supports the notion that the recovery of daytime sleep in mutants on the second and third days after a ZT0 shift is due to engagement of the homeostat. Notably, nighttime sleep was decreased in all genotypes regardless of when the shift occurred (Figure 3G).
PER Expression in sLNv or DN1 Cells of the Clock Circuit Is Sufficient to Allow Heat-Induced Increases in Daytime Sleep
To determine the site of action of PER in generating temperature-dependent increases in daytime sleep, we expressed per in specific cell types in per01 flies using the GAL4/UAS system [30]. We assayed sleep in LD before and after a ZT0 25°C to 29°C shift. Both UAS-per and each individual GAL4 on the per01 mutant background were assessed as controls and daytime sleep change in these lines was not significantly different from the per01 mutant alone (Figure S5A). Figure 4 shows that daytime heat response is rescued to a statistically significant level when PER is expressed panneuronally (elav-GAL4) or under control of pdf-GAL4, pdfR-GAL4, or clk4.1-GAL4. All lines, controls and experimental, showed a decrease in nighttime sleep when shifted to 29°C in LD (Figures S5B and S5C).
Figure 4. PER Expression in LNvs or DN1 Circadian Cells Is Sufficient for Recovery of Heat-Induced Siesta.
Change in total daytime sleep after a shift from 25°C to 29°C at ZT0 is shown. GAL4 control lines (all on a per01 background) were compared to experimental rescue lines containing the same GAL4 and UAS-per16 transgenes during the light period. Data are presented as means ± SEM and analyzed using type 2, two-tail, Student’s t test. *p < 0.05, **p < 0.005, ***p < 0.0005; n.s., not significant. Canton S wild-type, n = 24; per01, n = 49; per01;pdfR2-GAL4/+;UAS-per16, n = 26; per01;pdf-GAL4/+; UAS-per16/+, n = 25; per01;elav-GAL4/+; UAS-per16/+, n = 25; per01;c929-GAL4/+; UAS-per16/+, n = 22; per01; Clk4.1GAL4/UAS-per16, n = 17 (male flies were assayed for this genotype).
See also Figure S5.
The GAL4 lines used for rescue and their expression patterns are listed in Table 1. Interestingly, there is no single neuron type that rescues daytime sleep regulation by temperature in LD. The best rescue is with clk4.1-GAL4, which has very limited expression, only labeling a subset of dorsal clock neurons, the DN1ps [38]. DN1ps have previously been implicated in integration of temperature and light signals [39–41] and are also present in elav-GAL4 and pdfR-GAL4. The three lines other than clk4.1-GAL4 that rescue (elav-GAL4, pdf-GAL4, and pdfR-GAL4) have overlapping expression only in the small and large ventrolateral clock neurons (sLNvs and lLNvs). c929-GAL4, which has a statistically insignificant increase in daytime sleep, expresses in lLNvs, but not sLNvs, suggesting that sLNvs are more important for the circuitry that allows heat to increase daytime sleep in LD. These results imply that the presence of a functional clock in either sLNv or DN1 is sufficient to allow temperature to increase daytime sleep.
Table 1.
Expression Patterns and Rhythm Effects of GAL4 Lines Used for UAS-per Rescue of Daytime Sleep
| GAL4 Line | Expression Pattern | Rhythmicity in DD |
|
|---|---|---|---|
| Clock Cell Expression |
Non-clock Expression |
||
| elav-GAL4 | all [31] | panneuronal [31] | yes [32, 33] |
| pdf-GAL4 | all sLNvs and lLNvs [34] |
none [34] | yes [35] |
| pdfR-GAL4 | subsets of all clock groups including sLNvs and DN1ps [36] |
ellipsoid body, optic lobes [36] |
unknown |
| c929-GAL4 | lLNvs [37] | peptidergic neurons [37] |
no [35] |
| Clk4.1-GAL4 | subset of DN1ps [38] |
none [38] | no [39] |
For expression patterns, the reference to the original description of the line is listed. For locomotor rhythmicity in DD, results from papers containing data on rescue of per01 with UAS-per are described along with the reference. There are no published data on rescue of DD rhythms for pdfR-GAL4>UAS-per.
Notably, locomotor rhythms are not rescued in all lines that rescue the ability of heat to increase daytime sleep (Table 1). This suggests that the role of PER in regulation of temperature responses of daytime sleep is not due to its effects on locomotor rhythms, but rather reflects a separate role for the molecular clock, and for specific neurons of the clock circuit, in regulation of sleep by temperature. The finding that both sLNv and DN1p expression can rescue could suggest redundancy, but may reflect reciprocal connectivity between these cell types; cycling in LNvs likely drives cycling in DN1ps and vice versa (see the Discussion).
Light Regulates the Ability of Heat to Alter Sleep
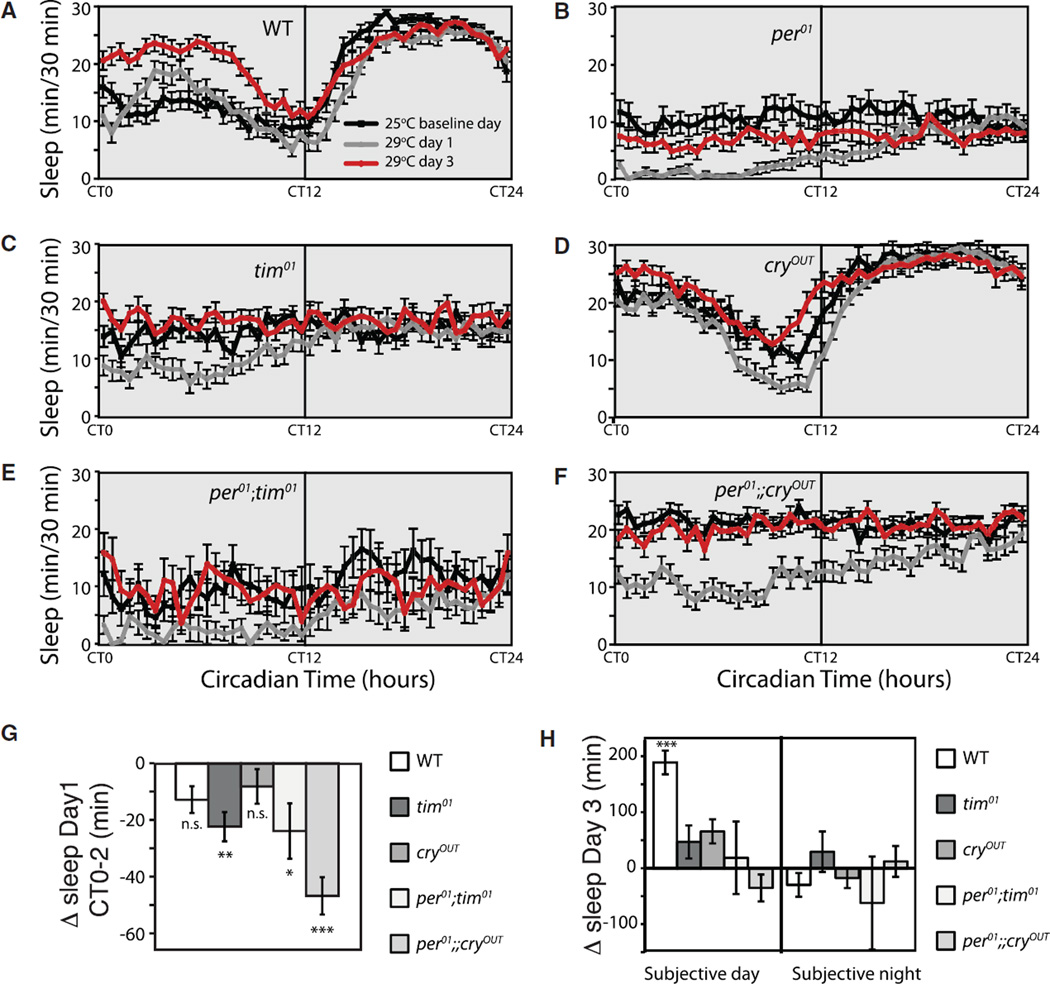
The fact that the major difference between wild-type and clock mutants is their ability to respond to heat during the light period suggested that light itself may have a role in regulating temperature-dependent changes in sleep structure and distribution. To look at the role of light, we tested wild-type and mutant flies in constant darkness (DD) or constant light (LL). Animals were entrained at 25°C in LD for 3 days and then released into constant conditions. On the third day of DD or LL, the temperature was increased to 29°C at CT0, and data were collected for an additional 3 days. Figures 5A–5F (DD) and 6A–6F (LL) show sleep plots demonstrating baseline sleep (25°C, black lines), immediate temperature effects (first day after shift to 29°C, gray lines), and steady-state temperature effects (third day after shift to 29°C, red lines). Because a temperature shift presents a potential entrainment signal, the acute effects of the shift in constant light conditions are more complex than in LD where cycling light is the dominant entrainment signal. Animals that are arrhythmic in DD or LL show a single cycle change in activity but eventually revert to arrhythmicity in the face of constantly elevated temperature. For this reason, we examined both the immediate and chronic effects of temperature for all experiments in which light was held constant. The acute window was very short (CT0–CT2) in order to avoid the locomotor artifacts produced by entrainment processes.
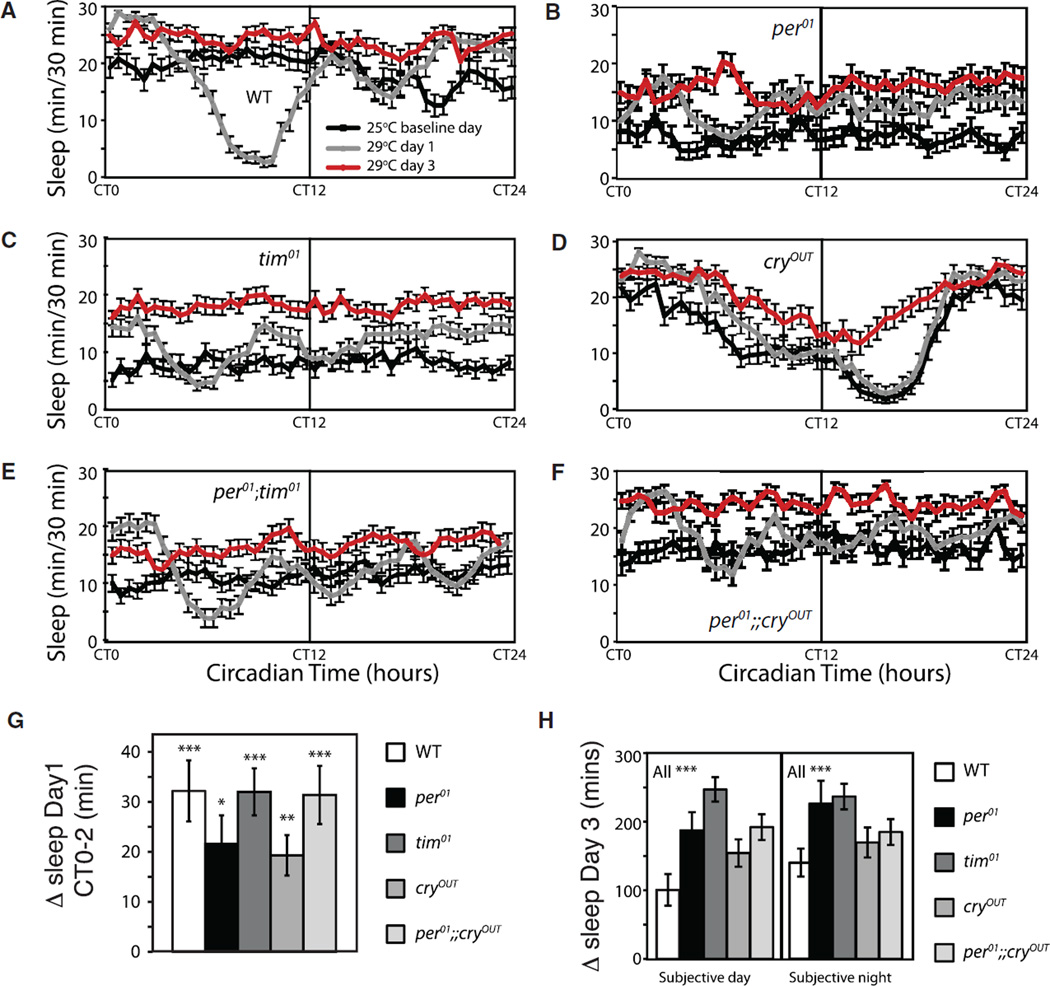
Figure 5. Temperature Shifts in Constant Darkness.
Animals were entrained in LD at 25°C and released into constant darkness (DD) for 3 days before temperature was increased to 29°C at CT0.
(A–F) Sleep plots for the 25°C baseline (black line), day 1 after 29°C shift (gray line) and day 3 after 29°C shift (red line) are shown for the indicated genotypes. Day 1 shows acute effects including entrainment effects, while day 3 shows steady state, after entrainment artifacts have resolved. Canton S wild-type, n = 38; per01, n = 42; tim01, n = 25; per0;tim01, n = 13; cryOUT, n = 28; per0;cryOUT, n = 38. Data for per01 are from an independent experiment run on a different day and included to show qualitative changes are similar.
(G) Acute change in sleep between CT0 and CT2 for indicated genotypes run concurrently. 25°C baseline is compared to day 1 at 29°C.
(H) Chronic change in sleep for indicated genotypes run concurrently. 25°C baseline is compared to day 3 at 29°C.
All comparisons were done using Student’s t test, and data are presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005; n.s., not significant. In (H), only wild-type showed a significant change in sleep; all others were n.s.
Figure 6. Temperature Shifts in Constant Light.
Animals were entrained in LD at 25°C and released into constant light (LL) for 3 days before temperature was increased to 29°C at CT0.
(A–F) Sleep plots for the 25°C baseline (black line), day 1 after 29°C shift (gray line) and day 3 after 29°C shift (red line) are shown for the indicated genotypes. Day 1 shows acute effects including entrainment effects, while day 3 shows steady state, after entrainment artifacts have resolved. Canton S wild-type, n = 39; per01, n = 34; tim01, n = 42; per0;tim01, n = 65; cryOUT, n = 37; per0;cryOUT, n = 37. Data for per0;tim01 are from an independent experiment run on a different day and included to show qualitative changes are similar.
(G) Acute change in sleep between CT0 and CT2 for indicated genotypes run concurrently. 25°C baseline is compared to day 1 at 29°C.
(H) Chronic change in sleep for indicated genotypes run concurrently. 25°C baseline is compared to day 3 at 29°C.
All comparisons were done using Student’s t test, and data are presented as mean ± SEM *p < 0.05, **p < 0.005, ***p < 0.0005; n.s., not significant. In (H), all genotypes were ***.
In contrast to the increase in daytime sleep seen in LD, wild-type animals in DD show a small immediate decrease in sleep after a CT0 shift. This is quantified in Figure 5G for the first 2 hr following the shift (CT0–CT2). Sleep during subjective night is still decreased, similar to LD. This suggests that, in the absence of light, the default acute response to heat is a decrease in sleep. Does this default pathway require clock proteins? Clock mutants assayed in DD also exhibited an immediate decrease in sleep at the start of a CT0 heat treatment (Figure 5G). Clockless flies therefore also responded to an acute increase in heat during the subjective day with a “night-like” sleep suppression.
We also examined the effects of temperature at steady state by comparing sleep from baseline to the third day after the CT0 shift (Figure 5H). Notably, the effects of temperature are relatively minor in the absence of light. For circadian mutants that are arrhythmic in DD, sleep in both the subjective day and night either did not change or was reduced. Only flies with intact per and tim genes (wild-type and cryOUT) were able to mount increases in sleep in the subjective day in the absence of light. The significant decrease in the ability of heat to cause changes in sleep during the subjective night further supports the idea that, in LD, light during the preceding day enhances the ability of heat to decrease sleep at night.
Heat Increases Sleep in Constant Light
We next wanted to assess the effects of constant light on the ability of temperature to regulate sleep. This manipulation renders wild-type flies arrhythmic since the clock is essentially “frozen” in a ZT12-like state. TIM levels are low and do not cycle [42], while PER in heads continues to cycle for at least 2 days [43]. Flies lacking CRY, however, remain rhythmic in LL [44, 45]. To examine the role of these proteins, we assayed the effects of LL temperature shift in per01, tim01, and cryOUT flies. Flies were shifted to 29°C on the third day of LL. The immediate effect of temperature shift on wild-type and mutant flies was to increase sleep (Figures 6A–6F). Figure 6H quantifies the difference in the first 2 hr after the temperature increases (CT0–CT2). This window was chosen to capture direct effects of temperature before the entraining effects of the shift (which are notable as changes in locomotion on the day of the shift). At steady state on the third day after the CT0 shift, when there is no longer an entrainment artifact, all genotypes show large increases in sleep in both the subjective day and night (Figure 6H). These changes are independent of a functioning clock, since they occur in wild-type flies, and do not require circadian genes.
Temperature Effects on Sleep Architecture Reflect an Integration of Environment and Internal State
These data support the idea that there are three separate processes regulating temperature-dependent changes in sleep and its architecture (Figure S6). The first is a default pathway that acutely decreases sleep in response to increased temperature. This pathway does not require the molecular clock and is used in wild-type flies only in the dark. This process is enhanced at night by light during the preceding day, and this hysteresis serves to link nighttime behavior to conditions present the day before. The second process is light dependent and can suppress the effects of the default pathway and actively promote sleep during the day. This requires per and tim and is mediated by an LNv-DN1p subcircuit of the clock. The third player in the heat response is only important when temperature is chronically elevated. Loss of nighttime sleep activates the homeostat to generate rebound sleep the next day. This serves as a second mechanism for linking changes in daytime and nighttime sleep. The sensors required for heat effects are unknown, but recent studies have suggested that both dTrpA1 and Ir25a are important in communication of temperature information to clock and activity circuits [46, 47].
DISCUSSION
Sleeping at the right time of day is paramount to species survival. At 25°C in LD, wild-type flies display a midday siesta that peaks at ZT6, and they obtain their most consolidated sleep at night. We find that increased temperature reorganizes the distribution of sleep and that the effects of temperature shifts on daytime and nighttime sleep are mechanistically different. At 29°C, flies sleep less at night and more during the daytime. For a poikilothermic organism, environmental temperatures acutely affect physiology; there may be less chance of desiccation if activity is shifted to the nighttime during warm periods. Sleep patterns in homeotherms are also sensitive to environmental conditions; both sleep latency and episode duration are altered by heat [24].
Our results indicate that the main sleep parameter altered by temperature shift is sleep episode duration (Figures 1C, S2C, and S4A). Drawing parallels to human sleep behavior is enticing; we can all relate to the predicament of having difficulties in falling and staying asleep on hot summer nights. It is also common in tropical climates for local populations to partake in midday siesta at the hottest part of the day.
Acute Regulation of Daytime Sleep by Temperature in LD Requires PER in a Subset of Circadian Clock Cells
In LD, animals with a functional clock (wild-type and cryOUT) increase daytime sleep in response to an acute increase in temperature. In contrast, mutants lacking either of the core circadian transcription factor proteins PER or TIM are unable to mount an immediate daytime increase in sleep with temperature. To determine whether the phenotype of these mutants was due to their role in the clock, we rescued per01 by expression of UAS-per in different neuronal populations (Figure 4). We found that immediate heat-induced daytime sleep was restored by expressing PER in either sLNv or DN1p neurons of the clock circuit, indicating that an intact clock in either of these neuron types is sufficient.
The implication of these data is that there is a reciprocally connected sLNv-DN1p subcircuit within the circadian clock circuit controlling the daytime sleep response to temperature. The DN1ps, or the neurons they target, have been shown to be under both photic and thermal regulation-integrating light and temperature inputs to control a variety of behaviors [38, 39, 41, 48]. Significantly, sLNvs have been shown to regulate the activity of DN1ps via release of the neuropeptide PDF [38, 39, 49], and the speeds of the clocks in these two cell types appear to be tethered [32]. The fact that PER expression in either of these cell types is sufficient to rescue daytime sleep effects suggests their functional interaction is a critical feature of sleep regulation by temperature. The ability of this subcircuit to allow temperature regulation of daytime sleep in the absence of locomotor rhythms (Table 1) indicates that sleep is an independent output of the clock rather than a downstream consequence of clock-driven locomotor activity.
Increases in Daytime Sleep after Prolonged Temperature Elevation Are Due to a Combination of Clock-Driven and Homeostatic Processes
While circadian mutants were unable to generate excess daytime sleep immediately after a temperature shift, they were indistinguishable from wild-type by the second day of high temperature exposure. This is due to the ability of these mutants to generate normal rebound sleep after heat-dependent nighttime sleep loss (Figures 3 and S3). This implies that, in wild-type animals, the ability of increased temperature to stimulate daytime sleep relies on two mechanistically separable processes: a clock-gated temperature sensing subcircuit and homeostatic rebound sleep stimulated by heat-dependent nighttime sleep loss (Figure S6).
Regulation of Nighttime Sleep by Temperature in LD Requires Light and CRY
Nighttime sleep is directly decreased by high temperature, and, while this decrease does not require a functioning clock, it is significantly influenced by light during the preceding day. This can be seen by comparison of wild-type nighttime sleep changes in LD and DD conditions (Figures 2F and 5H). This permissive effect of daytime light for suppression of nighttime sleep appears to be partially mediated by CRY, since animals carrying a cry null allele have a blunted heat response during the night in LD: both cryOUT mutants and per01;cryOUT double mutants have a decreased heat-dependent suppression of nighttime sleep compared to wild-type and per01 alone (Figure 2F). This argues that CRY and daytime light are important to setting the amount of sleep the following night or its sensitivity to temperature. The importance of light for sculpting nighttime sleep has been seen previously in the response of animals to activation of dopaminergic pathways [50]. In this study, light was shown to induce expression of an inhibitory dopamine receptor that acted to blunt the wake-promoting effects of dopamine on nighttime sleep. Cycling light conditions can therefore produce a hysteresis that links nighttime sleep to conditions during the preceding day, coordinating the effects of temperature over the course of the 24-hr day (Figure S6). These effects would be predicted to be absent in constant darkness.
Regulation of Sleep by Temperature in Non-cycling Light Conditions Is Clock Independent
Because cycling light clearly had effects that influenced nighttime sleep, it was important to look at constant conditions to identify the underlying processes invoked by light. In the absence of light (DD), an acute increase in temperature immediately decreased sleep regardless of circadian genotype (Figure 5). In wild-type and cryOUT flies, which have an intact clock, this reduction was transient and sleep levels increased within a few hours to a level higher than that seen at 25°C similar to what is seen during the light period in LD. For circadian mutants, the decrease lasted about 12 hr and likely reflects the ability of a temperature shift to produce a single cycle of “entrained” behavior with low subjective daytime sleep and higher subjective nighttime sleep.
Prolonged elevation of temperature in flies with a cycling clock in DD produced effects similar to those seen in LD: slightly higher daytime sleep and slightly lower nighttime sleep with rough preservation of total sleep. Mutants that do not have a clock kept chronically in DD at 29°C had total sleep levels similar to levels at 25°C. This could reflect a homeostatic response to the sleep disrupting effects of heat, or a rundown of the wake-promoting process, perhaps due to the lack of cycling light. The ability of these genotypes to show pronounced decreases in nighttime sleep in LD further supports a role for light during the preceding day in promoting the effects of heat in the dark.
While light at night is an arousal signal for most diurnal animals, maintaining animals in constant light (LL) has a paradoxical sleep-promoting effect for temperature shifts in all genotypes tested. This increase in sleep is seen both acutely, in the first few hours after the temperature increase (Figure 6G), and chronically, several days after the increase in temperature (Figure 6H). The effect of heat in the long term is essentially identical during the subjective day and subjective night in LL. Interestingly, this sleep-promoting effect of light does not require a clock. While similar to what happens during the day in LD, the heat-dependent increase in sleep in LL must be mechanistically distinct. Constant light is known to affect the abundance of a number of clock-related proteins (notably CRY and TIM), and it is possible that differences in the steady-state levels of these proteins account for the differences in clock requirement, though this remains to be tested.
Our data indicate that the mechanisms by which environmental (light and temperature) and internal (circadian clock and homeostatic drive) pressures shape sleep structure are highly coordinated. The fact that temperature exerts effects that are gated by light and modulated by the molecular clock suggests that there is a hierarchical organization to sleep circuits. Using Drosophila, a model in which many of these processes are well defined both genetically and at the circuit level to study such problems will allow us to untangle these complex relationships and achieve a more complete understanding of sleep regulation.
EXPERIMENTAL PROCEDURES
Strains and Fly Rearing
Fly cultures were kept at 25°C in 12-hr:12-hr light/dark conditions (LD) on cornmeal, yeast, sucrose, and agar food. period mutants were obtained from both Toshi Kitamoto’s laboratory (University of Iowa) and Michael Rosbash’s Laboratory (Brandeis University). timeless and cryptochrome mutants were from Jeff Hall’s laboratory (Brandeis University). UAS-per16 was generously provided by Patrick Emery (University of Massachusetts Medical School). GAL4 lines were obtained from Michael Rosbash and then put onto a per01 background.
Behavioral Assays
Flies were collected under CO2 anesthesia 1- to 2-day-old post-eclosion (to allow time for mating of the females), placed in food vials in sets of 30–50 flies each, and placed in behavioral incubator for 2 days of LD. Female flies were used for all experiments except where noted. Flies were loaded into sleep tubes and entrained for 3 days in LD before proceeding into a given experiment (see below). Behavioral assays and analysis were carried out as previously described [36]. For all experiments, flies were placed individually in glass locomotor-monitoring tubes with standard media (5% sucrose, 2% agar). Locomotor activity was monitored in 1-min bins using the Drosophila Activity Monitoring System (TriKinetics). Sleep was defined as 5 consecutive minutes of inactivity. Sleep parameters were calculated and graphed using MATLAB software [27]. In all experiments (except where noted in Figures 5 and 6) in which different genotypes are compared, data are from flies collected and run concurrently. Activity while active (Figures 1E, S2, and S4B) is a measure of the intensity of locomotor activity and is independent of sleep. It is calculated by measuring the number of beam breaks during periods during which the fly is awake. Hyperlocomotor flies would be expected to show an increase in this metric, while hypoactive flies would show a decrease.
Temperature Shifts
Flies was assayed under LD conditions for 3 days at 25°C (to obtain a stable baseline), and then the incubator was set to 29°C for 2–3 days starting at either the beginning of the dark period (ZT12), or at the beginning of the light period (ZT0).
Light Affects
Flies were entrained for 3 days in LD at 25°C and subsequently transferred to constant darkness, remaining at 25°C for 3 days of baseline. After the third full day of DD at CT0, the incubator was shifted to 29°C for 2–3 days. Likewise, for constant light, flies were entrained for 3 days in LD at 25°C and subsequently transferred to constant light, remaining at 25°C for 3 days of baseline. On the third full day of LL at CT0, the incubator was shifted to 29°C for 2–3 days.
Statistical Analysis
Data were analyzed as described in figure legends using JMP software v.5.0.1.2 for the PC and Macintosh (SAS Institute) and using Microsoft Office Excel.
Supplementary Material
Highlights.
Ambient temperature changes the stereotypic sleep profile in Drosophila
Homeostatic rebound sleep is a critical component of the response to heat
Cycling light coordinates the temperature responses of daytime and nighttime sleep
Temperature is integrated with light cycle by a LNv-DN1p clock subcircuit
In Brief.
Why do we sleep more during the day and less at night when it is hot? Parisky et al. show that the reorganization of sleep by increased ambient temperature in Drosophila is controlled by a coordinated interaction of light, the clock, and homeostatic processes. This allows animals to alter the timing of sleep while preserving its approximate amount.
Acknowledgments
This work was supported by NIH R01 MH067284 to L.C.G. K.M.P. and N.C.D. were supported by NIH T32 NS007292 and J.L.A.R. by T32 MH019929.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2016.02.011.
AUTHOR CONTRIBUTIONS
Conceptualization, K.M.P. and J.L.A.R.; Investigation, K.M.P., J.L.A.R., N.C.D., and S.K.; Writing – Original Draft, K.M.P.; Writing – Review & Editing, K.M.P., J.L.A.R., N.C.D., and L.C.G.; Supervision, L.C.G.; Funding Acquisition, L.C.G.
REFERENCES
- 1.Skene DJ, Arendt J. Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Med. 2007;8:651–655. doi: 10.1016/j.sleep.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Friedman L, Zeitzer JM, Kushida C, Zhdanova I, Noda A, Lee T, Schneider B, Guilleminault C, Sheikh J, Yesavage JA. Scheduled bright light for treatment of insomnia in older adults. J. Am. Geriatr. Soc. 2009;57:441–452. doi: 10.1111/j.1532-5415.2008.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheeler DA, Hamblen-Coyle MJ, Dushay MS, Hall JC. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J. Biol. Rhythms. 1993;8:67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- 4.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J. Biol. Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 6.Donelson NC, Sanyal S. Use of Drosophila in the investigation of sleep disorders. Exp. Neurol. 2015;274(Pt A):72–79. doi: 10.1016/j.expneurol.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 8.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 9.Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr. Biol. 2002;12:1934–1940. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- 10.van Alphen B, Yap MH, Kirszenblat L, Kottler B, van Swinderen B. A dynamic deep sleep stage in Drosophila. J. Neurosci. 2013;33:6917–6927. doi: 10.1523/JNEUROSCI.0061-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Swinderen B, Nitz DA, Greenspan RJ. Uncoupling of brain activity from movement defines arousal States in Drosophila. Curr. Biol. 2004;14:81–87. [PubMed] [Google Scholar]
- 12.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat. Rev. Neurosci. 2009;10:549–560. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potdar S, Sheeba V. Lessons from sleeping flies: insights from Drosophila melanogaster on the neuronal circuitry and importance of sleep. J. Neurogenet. 2013;27:23–42. doi: 10.3109/01677063.2013.791692. [DOI] [PubMed] [Google Scholar]
- 14.Nall A, Sehgal A. Monoamines and sleep in Drosophila. Behav. Neurosci. 2014;128:264–272. doi: 10.1037/a0036209. [DOI] [PubMed] [Google Scholar]
- 15.Griffith LC. Neuromodulatory control of sleep in Drosophila melanogaster: integration of competing and complementary behaviors. Curr. Opin. Neurobiol. 2013;23:819–823. doi: 10.1016/j.conb.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majercak J, Sidote D, Hardin PE, Edery I. How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron. 1999;24:219–230. doi: 10.1016/s0896-6273(00)80834-x. [DOI] [PubMed] [Google Scholar]
- 17.Blau J, Rothenfluh A. Siesta-time is in the genes. Neuron. 1999;24:4–5. doi: 10.1016/s0896-6273(00)80816-8. [DOI] [PubMed] [Google Scholar]
- 18.Ishimoto H, Lark A, Kitamoto T. Factors that Differentially Affect Daytime and Nighttime Sleep in Drosophila melanogaster. Front. Neurol. 2012;3:24. doi: 10.3389/fneur.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao W, Edery I. A novel pathway for sensory-mediated arousal involves splicing of an intron in the period clock gene. Sleep. 2015;38:41–51. doi: 10.5665/sleep.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Low KH, Lim C, Ko HW, Edery I. Natural variation in the splice site strength of a clock gene and species-specific thermal adaptation. Neuron. 2008;60:1054–1067. doi: 10.1016/j.neuron.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haskell EH, Palca JW, Walker JM, Berger RJ, Heller HC. The effects of high and low ambient temperatures on human sleep stages. Electroencephalogr. Clin. Neurophysiol. 1981;51:494–501. doi: 10.1016/0013-4694(81)90226-1. [DOI] [PubMed] [Google Scholar]
- 22.Togo F, Aizawa S, Arai J, Yoshikawa S, Ishiwata T, Shephard RJ, Aoyagi Y. Influence on human sleep patterns of lowering and delaying the minimum core body temperature by slow changes in the thermal environment. Sleep. 2007;30:797–802. doi: 10.1093/sleep/30.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto-Mizuno K, Mizuno K. Effects of thermal environment on sleep and circadian rhythm. J. Physiol. Anthropol. 2012;31:14. doi: 10.1186/1880-6805-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buguet A. Sleep under extreme environments: effects of heat and cold exposure, altitude, hyperbaric pressure and microgravity in space. J. Neurol. Sci. 2007;262:145–152. doi: 10.1016/j.jns.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 25.Lack LC, Gradisar M, Van Someren EJ, Wright HR, Lushington K. The relationship between insomnia and body temperatures. Sleep Med. Rev. 2008;12:307–317. doi: 10.1016/j.smrv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Raslear TG, Hursh SR, Van Dongen HP. Predicting cognitive impairment and accident risk. Prog. Brain Res. 2011;190:155–167. doi: 10.1016/B978-0-444-53817-8.00010-4. [DOI] [PubMed] [Google Scholar]
- 27.Donelson NC, Kim EZ, Slawson JB, Vecsey CG, Huber R, Griffith LC. High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the “tracker” program. PLoS ONE. 2012;7:e37250. doi: 10.1371/journal.pone.0037250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 29.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 30.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 31.Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 32.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z, Sehgal A. Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron. 2001;29:453–467. doi: 10.1016/s0896-6273(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 34.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 35.Grima B, Chélot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 36.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taghert PH, Hewes RS, Park JH, O’Brien MA, Han M, Peck ME. Multiple amidated neuropeptides are required for normal circadian locomotor rhythms in Drosophila. J. Neurosci. 2001;21:6673–6686. doi: 10.1523/JNEUROSCI.21-17-06673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Chung BY, Lear BC, Kilman VL, Liu Y, Mahesh G, Meissner RA, Hardin PE, Allada R. DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr. Biol. 2010;20:591–599. doi: 10.1016/j.cub.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr. Biol. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gentile C, Sehadova H, Simoni A, Chen C, Stanewsky R. Cryptochrome antagonizes synchronization of Drosophila’s circadian clock to temperature cycles. Curr. Biol. 2013;23:185–195. doi: 10.1016/j.cub.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Head LM, Tang X, Hayley SE, Goda T, Umezaki Y, Chang EC, Leslie JR, Fujiwara M, Garrity PA, Hamada FN. The influence of light on temperature preference in Drosophila. Curr. Biol. 2015;25:1063–1068. doi: 10.1016/j.cub.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busza A, Emery-Le M, Rosbash M, Emery P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science. 2004;304:1503–1506. doi: 10.1126/science.1096973. [DOI] [PubMed] [Google Scholar]
- 43.Marrus SB, DiAntonio A. Preferential localization of glutamate receptors opposite sites of high presynaptic release. Curr. Biol. 2004;14:924–931. doi: 10.1016/j.cub.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 44.Yoshii T, Funada Y, Ibuki-Ishibashi T, Matsumoto A, Tanimura T, Tomioka K. Drosophila cryb mutation reveals two circadian clocks that drive locomotor rhythm and have different responsiveness to light. J. Insect Physiol. 2004;50:479–488. doi: 10.1016/j.jinsphys.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Dolezelova E, Dolezel D, Hall JC. Rhythm defects caused by newly engineered null mutations in Drosophila’s cryptochrome gene. Genetics. 2007;177:329–345. doi: 10.1534/genetics.107.076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C, Buhl E, Xu M, Croset V, Rees JS, Lilley KS, Benton R, Hodge JJ, Stanewsky R. Drosophila Ionotropic Receptor 25a mediates circadian clock resetting by temperature. Nature. 2015;527:516–520. doi: 10.1038/nature16148. [DOI] [PubMed] [Google Scholar]
- 47.Roessingh S, Wolfgang W, Stanewsky R. Loss of Drosophila melanogaster TRPA1 function affects “siesta” behavior but not synchronization to temperature cycles. J. Biol. Rhythms. 2015;30:492–505. doi: 10.1177/0748730415605633. [DOI] [PubMed] [Google Scholar]
- 48.Yoshii T, Hermann C, Helfrich-Förster C. Cryptochrome-positive and -negative clock neurons in Drosophila entrain differentially to light and temperature. J. Biol. Rhythms. 2010;25:387–398. doi: 10.1177/0748730410381962. [DOI] [PubMed] [Google Scholar]
- 49.Choi C, Cao G, Tanenhaus AK, McCarthy EV, Jung M, Schleyer W, Shang Y, Rosbash M, Yin JC, Nitabach MN. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in Drosophila. Cell Rep. 2012;2:332–344. doi: 10.1016/j.celrep.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shang Y, Haynes P, Pírez N, Harrington KI, Guo F, Pollack J, Hong P, Griffith LC, Rosbash M. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat. Neurosci. 2011;14:889–895. doi: 10.1038/nn.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.