Abstract
Background
Maternal prenatal stress during pregnancy is associated with fetal growth restriction and adverse neurodevelopmental outcomes, which may be mediated by impaired placental function. Imprinted genes control fetal growth, placental development, adult behaviour (including maternal behaviour) and placental lactogen production. This study examined whether maternal prenatal depression was associated with aberrant placental expression of the imprinted genes paternally expressed gene 3 (PEG3), paternally expressed gene 10 (PEG10), pleckstrin homology-like domain family a member 2 (PHLDA2) and cyclin-dependent kinase inhibitor 1C (CDKN1C), and resulting impaired placental human placental lactogen (hPL) expression.
Method
A diagnosis of depression during pregnancy was recorded from Manchester cohort participants’ medical notes (n = 75). Queen Charlotte's (n = 40) and My Baby and Me study (MBAM) (n = 81) cohort participants completed the Edinburgh Postnatal Depression Scale self-rating psychometric questionnaire. Villous trophoblast tissue samples were analysed for gene expression.
Results
In a pilot study, diagnosed depression during pregnancy was associated with a significant reduction in placental PEG3 expression (41%, p = 0.02). In two further independent cohorts, the Queen Charlotte's and MBAM cohorts, placental PEG3 expression was also inversely associated with maternal depression scores, an association that was significant in male but not female placentas. Finally, hPL expression was significantly decreased in women with clinically diagnosed depression (44%, p < 0.05) and in those with high depression scores (31% and 21%, respectively).
Conclusions
This study provides the first evidence that maternal prenatal depression is associated with changes in the placental expression of PEG3, co-incident with decreased expression of hPL. This aberrant placental gene expression could provide a possible mechanistic explanation for the co-occurrence of maternal depression, fetal growth restriction, impaired maternal behaviour and poorer offspring outcomes.
Key words: Human placental lactogen, PEG3, prenatal depression
Introduction
Maternal psychological stress (or prenatal stress) during pregnancy, such as symptoms of anxiety and/or depression, has been associated with fetal programming of adverse long-term consequences for the child including an increased risk of fetal growth restriction (for example, Steer et al. 1992; Rondo et al. 2003; Khashan et al. 2008; Liu et al. 2012), emotional and behavioural problems, learning difficulties, cognitive impairment and psychopathology (for reviews, see Van den Bergh et al. 2005; Talge et al. 2007). In addition, there is evidence that prenatal depression can impair the normal development of postnatal maternal behaviour and mother–infant interactions (Pearson et al. 2012).
Located at the boundary between the maternal and fetal environments, the placenta supports fetal growth and development through numerous functions including transport of nutrients and oxygen and the production and metabolism of hormones (Gude et al. 2004). The placenta has been proposed as a potential mechanism mediating the association between maternal prenatal stress and adverse infant outcomes (O'Donnell et al. 2009; Janssen et al. 2016). Consistent with this hypothesis, prenatal stress has previously been associated with altered placental function in both animal models (Mairesse et al. 2007; Jensen Pena et al. 2012) and in humans (O'Donnell et al. 2012; Blakeley et al. 2013; Reynolds et al. 2015).
Imprinted genes, which are monoallelically expressed with expression depending on the parent of origin (Surani, 1998), are regulated by epigenetic marks that may respond to in utero environmental stimuli. These genes have been suggested as potential mediators of adverse infant outcomes because of their well-established roles in controlling fetal growth, placental development, adult behaviour and metabolism (Lefebvre et al. 1998; Li et al. 1999; Curley et al. 2005; Smith et al. 2006; Tunster et al. 2013; Jensen et al. 2014; McNamara & Isles, 2014). Aberrant expression of imprinted genes in the placenta has also been demonstrated to be associated with impaired infant neurobehavioural development in humans (Green et al. 2015). Recent studies demonstrate that a subset of imprinted genes converge on the endocrine lineages of the mouse placenta to regulate placental hormone production (John, 2013). This may be of particular relevance to maternal prenatal stress as the placenta is a significant source of hormones, such as placental lactogens (Glynn & Sandman, 2011). These lactogenic hormones act on the maternal brain, priming the mother for pregnancy and postnatal care (Bridges et al. 1985, 1990, 1997; Bridges & Freemark, 1995; Glynn & Sandman, 2011). In addition, impaired placental lactogen production has been associated with adverse infant outcomes such as fetal growth restriction (Roh et al. 2005; Dutton et al. 2012). Thus, it is possible that aberrant placental imprinted gene expression and resulting impaired placental lactogen production mediate the association between maternal prenatal stress and adverse infant outcomes. In support of such a hypothesis, epigenetic changes in cord blood DNA at imprinted loci have been associated with both depressed maternal mood (Liu et al. 2012) and maternal stress (Vidal et al. 2014). The expression of imprinted genes in the placenta has not previously been examined in relation to maternal prenatal stress.
In this study we examined the expression levels of the paternally expressed gene 3 (PEG3), paternally expressed gene 10 (PEG10), pleckstrin homology-like domain family a member 2 (PHLDA2) and cyclin-dependent kinase inhibitor 1C (CDKN1C). These four imprinted genes were chosen based on the conserved imprinting status between mouse and human and their predicted role in regulating the production of placental hormones, including the placental lactogens, known to induce physiological changes in pregnant women (John, 2013). Expression of these genes was first analysed in a pilot cohort of women (the Manchester cohort) with clinically diagnosed depression during pregnancy, with results suggesting a significant alteration in placental PEG3. Based on these results, we further analysed placental PEG3 expression in two additional independent cohorts of mothers reporting prenatal symptoms of depression. Finally, given the proposed link between PEG3 expression and placental hormones, we quantified expression of human placental lactogen (hPL, also known as chorionic somatomammotropins; CSH) in all three cohorts.
Method
Manchester cohort
Placental gene expression was first analysed in a pilot cohort. Women (n = 75) presenting with maternal perception of reduced fetal movements (RFM) at St Mary's Hospital (Manchester, UK) were approached to participate in the study as previously described (Dutton et al. 2012; Warrander et al. 2012). Written informed consent was obtained and the study approved by Oldham and Greater Manchester North Research Ethics Committees (REC no. 08/1011/83 and 11/NW/0664). A diagnosis of depression during pregnancy, including any treatment prescribed, was recorded from the participant's medical notes.
Queen Charlotte's and My Baby and Me study (MBAM) cohorts
For both cohorts, women awaiting an elective Caesarean section (with no known complications of pregnancy) were recruited from Queen Charlotte's maternity hospital, London. Participants in the Queen Charlotte's cohort (n = 40) were recruited between 2010 and 2011, while an independent cohort of women participating in the MBAM (n = 81) were recruited in 2014. Written informed consent was obtained. The Queen Charlotte's study was approved by the Ethics Committee of Hammersmith and Queen Charlotte's Hospital, London (REC no. 08/H0708/126) and MBAM was approved by the Research Ethics Committee of London (Chelsea) (REC no. 13/LO/1436).
At the time of recruitment, maternal prenatal depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (EPDS). This questionnaire has been validated for use during pregnancy (Cox et al. 1996), with total EPDS scores ranging from 0 (low depression) to 30 (high depression). An EPDS score ⩾13 is used to identify women at risk of a depressive disorder (Cox et al. 1987).
Placental dissection
All placentas were collected immediately after delivery and sampled within 1 h (O'Donnell et al. 2012; Warrander et al. 2012). Villous trophoblast tissue samples were taken from the maternal surface of the placenta, midway between the cord and distal edge. Sampling methods were comparable across all three independent cohorts. Tissue samples were washed in phosphate-buffered saline to remove maternal blood and stored in RNAlater (Sigma-Aldrich, UK) prior to storage at −80°C.
Gene expression analysis
Total RNA was extracted from the placental tissue samples from the Manchester and MBAM cohorts using the GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, UK). RNA quantity and quality (based on the 260:280 absorbance ratio) were assessed using a NanoDrop ND-1000 spectrophotometer. From the Queen Charlotte's cohort, RNA was extracted from placental tissue samples using RNeasy Mini Kits (Qiagen, UK). In addition, for this cohort RNA integrity was examined using a Bioanalyzer 2100 (Agilent, UK), according to the protocol for a RNA 6000 Nano Assay; samples with a RNA integrity number (RIN) value of ⩾5 were considered of sufficient quality for reverse transcription and quantitative polymerase chain reaction (qPCR).
For the Manchester cohort, 5 µg of RNA were reverse transcribed using M-MuLV reverse transcriptase (Promega, UK) with random hexamers, according to the manufacturer's instructions. For both the Queen Charlotte's and MBAM cohorts, 2 µg of RNA were reverse transcribed using the Superscript II first strand complementary DNA (cDNA) synthesis system (Invitrogen, UK), according to the manufacturer's instructions.
Quantitative RT-PCR was performed using a Chromo 4 Four Colour Real Time Detector (MJ Research) in a 20 µl reaction containing 5 µl of cDNA (diluted 1 in 50), 1X Buffer 2 mm MgCl2, 2 mm deoxynucleotides (dNTPs), 0.65 units Taq [Fermentas (Thermo), UK], 1 µm of each primer (Sigma-Aldrich, UK) and 0.12X Sybr Green (Invitrogen, UK). All samples were run in triplicate and duplicate plates were performed for MBAM. Conditions for amplification were: (1) 15 min at 94°C; (2) 30 s at 94°C; (3) 30 s at 60°C; (4) 30 s at 72°C; and (5) 30 s 75°C, with steps 2–5 repeated for a total of 40 cycles. Melt Curve was performed from 70 to 94°C, reading every 0.5°C and holding for 2 s.
Primer sequences were as follows: YWHAZ forward: TTCTTGATCCCCAATGCTTC and reverse: AGTTAAGGGCCAGACCCAGT; PEG3 forward: CTCACAACACAATCCAGGAC and reverse: TAGACCTCGACTGGTGCTTG (Feng et al. 2008); PEG10 forward: AAATTGCCTGACATGAAGAGGAGTCTA and reverse:AAGCCTAGTCACCACTTCAAAACACACTAAA (Diplas et al. 2009); PHLDA2 forward: GAGCGCACGGGCAAGTA and reverse: CAGCGGAAGTCGATCTCCTT (Apostolidou et al. 2007); CDKN1C forward: CCCATCTAGCTTGCAGTCTCTT and reverse: CAGACGGCTCAGGAACCATT (Diplas et al. 2009); hPL forward: CATGACTCCCAGACCTCCTTC and reverse: TGCGGAGCAGCTCTAGATTG (Dutton et al. 2012). hPL primers were designed to analyse the expression of CSH1/hPL-A and CSH2/hPL-B, from which the majority of circulating placental lactogens is derived (Newbern & Freemark, 2011). Primer specificity was assessed based on gel electrophoresis product band size and qPCR melt curves.
Gene expression data are presented as the ∆CT (target gene expression relative to the housekeeping gene YWHAZ) and as the fold change in expression, calculated using the 2−∆∆CT (Livak & Schmittgen, 2001) where the ∆∆CT is the target gene expression relative to expression in the control group. The housekeeping gene YWHAZ has previously been demonstrated to be stably expressed in the human placenta (Meller et al. 2005; Murthi et al. 2008; Cleal et al. 2009, 2010). Furthermore, we found no significant difference in YWHAZ expression between control and depressed participants in the Manchester cohort (p = 0.76) in an initial pilot study.
Data analysis
Placental gene expression and maternal EPDS data were normally distributed and parametric statistical tests were used to analyse the data. Placental gene expression data were not significantly associated with any maternal demographics listed in Table 1. The effect of potential confounders (infant birth weight, sex and gestational age) was examined using multiple linear regression analysis. To ease interpretation, ∆CT values representing gene expression values have been inverted [x(−1)] such that lower values represent decreasing gene expression. Mediation analysis was carried out in IBM SPSS Statistics Version 20 and PROCESS for SPSS version 2.15 (Hayes, 2013). Bootstrap confidence intervals (CIs) were used to assess statistical significance of the indirect effect (Hayes, 2013).
Table 1.
Participant characteristics of the Manchester, Queen Charlotte's and MBAM cohorts
| Manchester cohort | Queen Charlotte's cohort | MBAM cohort | |
|---|---|---|---|
| Birth outcomes | |||
| Fetal sex, n % | |||
| Male | 37 (49) | 22 (55) | 44 (54) |
| Female | 38 (51) | 18 (45) | 37 (46) |
| Mean gestational age, weeks (s.d.)/range | 40 (1.18)/36–42 | 39 (0.55)/38–41 | 39 (0.78)/37–41 |
| Mean birth weight, g (s.d.)/range | 3382 (461)/2350–4680 | 3451 (421)/2640–4700 | 3384 (415)/2212–4392 |
| Mean placental weight, g (s.d.)/range | 604 (131)/354–854 | 619 (134)/387–932 | 564 (111)/299–873 |
| Mean Apgar score: 1 min (s.d.)/range | 9 (0.97)/6–10 | 9 (0.48)/8–10 | 9 (0.68)/5–10 |
| Mean Apgar score: 5 min (s.d.)/range | 10 (0.12)/9–10 | 10 (0.34)/9–10 | 10 (0.34)/9–10 |
| Mode of delivery, n (%) | |||
| Elective Caesarean section | 6 (8) | 40 (100) | 81 (100) |
| Emergency Caesarean section | 9 (12) | 0 (0) | 0 (0) |
| Vaginal | 39 (52) | 0 (0) | 0 (0) |
| Instrumental | 21 (28) | 0 (0) | 0 (0) |
| Maternal characteristics | |||
| Ethnicity, n (%) | |||
| Caucasian | 50 (67) | 29 (72.5) | 47 (58) |
| African/Afro-Caribbean | 7 (9) | 2 (5) | 6 (7) |
| Indian/Pakistani/Bangladeshi | 13 (17) | 1 (2.5) | 8 (10) |
| Middle Eastern | 2 (3) | 1 (2.5) | 4 (5) |
| Far Eastern | 0 (0) | 2 (2.5) | 3 (4) |
| Other | 3 (4) | 2 (5) | 10 (12) |
| Do not wish to say | 0 (0) | 3 (7.5) | 3 (4) |
| Maternal education, n (%) | |||
| Left before GCSE/O level | Not recorded | 1 (2.5) | 0 (0) |
| GCSE/O level | Not recorded | 3 (7.5) | 6 (7) |
| A levels | Not recorded | 6 (15) | 6 (7) |
| Vocational training | Not recorded | 5 (12.5) | 4 (5) |
| University degree | Not recorded | 16 (40) | 42 (52) |
| Higher degree | Not recorded | 9 (22.5) | 19 (24) |
| Do not wish to say | Not recorded | 0 (0) | 4 (5) |
| Household income, n (%) | |||
| <£18 000 per year | Not recorded | 3 (7.5) | 9 (11) |
| £18–25 000 per year | Not recorded | 3 (7.5) | 3 (4) |
| £25–43 000 per year | Not recorded | 8 (20) | 8 (10) |
| >£43 000 per year | Not recorded | 18 (45) | 37 (46) |
| Do not wish to say | Not recorded | 8 (20) | 24 (29) |
| Mean age, years (s.d.)/range | 29 (5.50)/17–42 | 35 (4.48)/26–42 | 34 (3.86)/26–41 |
| Mean maternal BMI, kg/m2 (s.d.)/range | 25 (4.16)/19–38 | 24.26 (4.08)/19–38 | Not recorded |
| Mean parity (s.d.)/range | 1 (1.13)/0–5 | 1 (1.45)/0–9 | 2 (1.80)/0–10 |
| Smoking during pregnancy, n (%) | |||
| Yes | 11 (15) | 3 (7.5) | 2 (2) |
| No | 64 (85) | 37 (92.5) | 79 (98) |
| Alcohol consumption, n (%) | |||
| None | 74 (99) | 32 (80) | 76 (94) |
| 1–5 units/week | 1 (1) | 8 (20) | 5 (6) |
| Mean maternal EPDS score, total (s.d.)/range | Not assessed | 8.9 (5.46)/0–25 | 7.3 (4.66)/0–18 |
| Clinical depression during pregnancy, n (%)a | |||
| No | 68 (91) | 40 (100) | Not recorded |
| Yes | 7 (9) | 0 (0) | Not recorded |
MBAM, My Baby and Me study; s.d., standard deviation; GCSE, General Certificate of Secondary Education; O level, Ordinary level; A level, Advanced level; BMI, body mass index; EPDS, Edinburgh Postnatal Depression Scale.
A diagnosis of clinical depression was recorded from participants’ medical notes.
Results
Manchester cohort
In an initial pilot study, expression of the imprinted genes PEG3, PEG10, PHLDA2 and CDKN1C was analysed in a cohort of placentas (n = 75), which included a subset of women (n = 7) who had diagnosed depression during pregnancy. Characteristics of the study participants in the Manchester cohort are shown in Table 1. Participants with diagnosed depression did not differ significantly in terms of maternal demographics from control participants (results not shown).
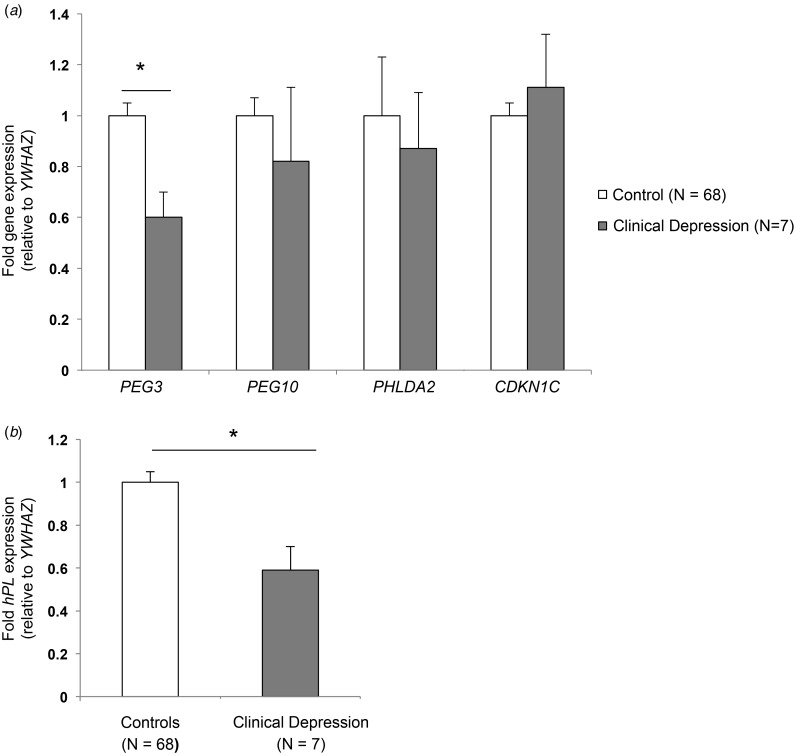
There was no significant alteration in the expression of PEG10, PHLDA2 or CDKN1C in association with maternal depression (Fig. 1a). However, placental PEG3 expression was significantly decreased (by 41%) in placentas from depressed participants compared with controls (Fig. 1a). The association between placental PEG3 expression and maternal diagnosed depression remained significant after controlling for infant birth weight, offspring sex and gestational age (F4,73 = 3.43, p = 0.01, R2 = 0.16), with only maternal depression diagnosis significantly predicting placental PEG3 expression (p = 0.003). Three participants reported prescribed anti-depressant use during pregnancy; the observed decrease in placental PEG3 expression remained significant with the exclusion of these participants (p = 0.02, n = 75).
Fig. 1.
Manchester cohort – maternal diagnosed depression and placental gene expression. (a) There was a significant decrease in paternally expressed gene 3 (PEG3) expression in women with diagnosed depression during pregnancy. Placental paternally expressed gene 10 (PEG10), pleckstrin homology-like domain family a member 2 (PHLDA2) and cyclin-dependent kinase inhibitor 1C (CDKN1C) expression was not significantly altered. (b) Human placental lactogen (hPL) expression was also significantly reduced in depressed participants. Values are means of fold gene expression, with standard errors represented by vertical bars. * p < 0.05.
Given the proposed regulation of placental lactogens by PEG3, expression of hPL was also analysed in this cohort. Placental PEG3 and hPL expression were significantly positively correlated (r = 0.26, p = 0.03, n = 75). There was a significant 44% decrease in placental hPL expression in depressed participants compared with controls (Fig. 1b).
Queen Charlotte's cohort
Results from the pilot study indicated a significant decrease in placental PEG3 and hPL expression in a small number of women with clinically diagnosed depression during pregnancy. To determine whether more general symptoms of prenatal depression were similarly associated with aberrant placental gene expression of these two genes, we made use of a second independent cohort (the Queen Charlotte's cohort; n = 40) with information on maternal prenatal depression symptoms. The characteristics of the study participants in the Queen Charlotte's cohort are shown in Table 1. Indication for elective Caesarean section (ELCS) included previous section (n = 26, 65%), breech presentation (n = 7, 18%), maternal request (n = 2, 5%) and other (e.g. previous tear, low-lying placenta, n = 5, 12%). No participant reported prescribed anti-depressant use during pregnancy.
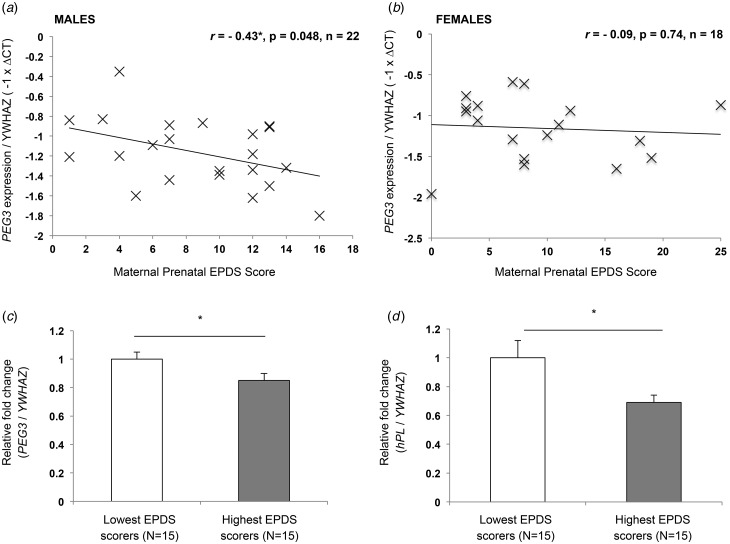
Despite promising pilot data, the modest correlation between placental PEG3 expression and maternal prenatal EPDS scores (r = −0.23, p = 0.16, n = 40) failed to reach statistical significance. Placental PEG3 expression does not differ between male and female placentas from normal, uncomplicated pregnancies (Janssen et al. 2015) but male and female human fetuses are known to respond differently to an adverse intra-uterine environment (Clifton, 2010). Therefore, the association between placental PEG3 expression and maternal depression was also analysed independently in male and female pregnancies. When the PEG3 expression data were analysed according to fetal sex, this revealed a significant negative association between placental PEG3 expression and maternal EPDS scores in males (p = 0.048) but not females (p = 0.74) (Fig. 2a and b). A 13% decrease in PEG3 expression was observed in placentas from participants with an EPDS score ⩾13, highlighting them as at risk of a depressive disorder, although this difference did not reach statistical significance (p = 0.16) possibly due to the relatively small number of women scoring above this cut-off (n = 9). However, when participants in the current study were grouped into lowest (mean EPDS 2.7, n = 15) and highest (mean EPDS 14.5, n = 15) EPDS scorers there was a significant 15% decrease (p = 0.03) in PEG3 expression in placentas from the highest EPDS scorers compared with the lowest EPDS scorers (Fig. 2c).
Fig. 2.
Queen Charlotte's cohort – maternal depression symptoms and placental gene expression. The association between maternal depression and placental paternally expressed gene 3 (PEG3) expression appears to be sex specific with a significant inverse association between Edinburgh Postnatal Depression Scale (EPDS) scores and PEG3 expression in male (a) but not female (b) placentas. There was a significant decrease in PEG3 (c) and human placental lactogen (hPL) (d) expression between 15 participants with the lowest and highest EPDS scores. Values are means of fold gene expression, with standard errors represented by vertical bars. * p < 0.05.
Placental hPL expression was also negatively correlated with maternal EPDS scores (r = −0.27, p = 0.13, n = 40) but without statistical significance in the full cohort or when analysed independently in male (r = −0.25, p = 0.25, n = 22) and female (r = −0.29, p = 0.26, n = 17) placentas. However, as with PEG3, a significant 31% decrease (p = 0.03) in placental hPL expression was observed in the highest EPDS scorers compared with the lowest EPDS scorers (Fig. 2d). There was no significant correlation between placental PEG3 and hPL expression (r = −0.13, p = 0.43, n = 40).
MBAM cohort
We next sought to replicate these findings in the MBAM cohort (n = 81), a larger independent cohort of samples with similar information on maternal prenatal depression symptoms. Characteristics of the study participants in the MBAM cohort are shown in Table 1. Indication for ELCS included previous Caesarean section (n = 43, 53%), breech presentation (n = 15, 19%), maternal request (n = 11, 14%), placenta previa (n = 6, 7%) and obstetric history (n = 6, 7%).
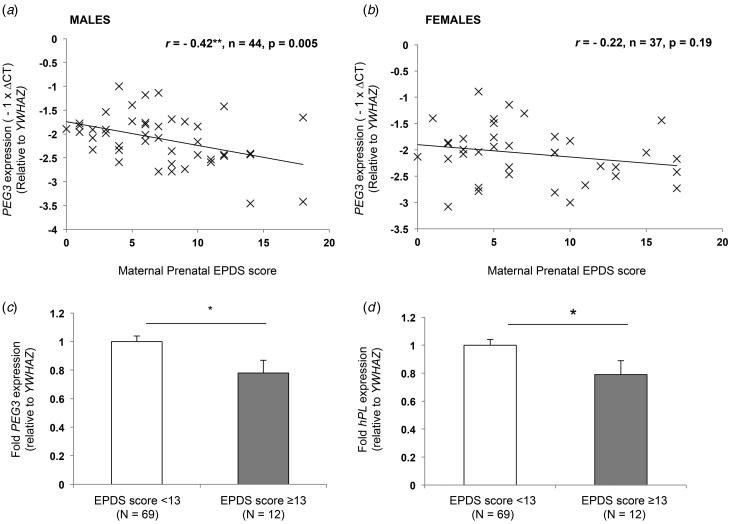
Placental PEG3 expression was significantly inversely associated with maternal prenatal EPDS scores (r = −0.32, p = 0.003, n = 81). As with the Queen Charlotte's cohort, when gene expression was analysed according to fetal sex, there was a significant inverse association between PEG3 expression and maternal EPDS scores in male (r = −0.42, p = 0.005, n = 44) but not female placentas (r = −0.22, p = 0.19, n = 37) (Fig. 3a and b). Multiple linear regression analysis showed that the association between placental PEG3 expression and maternal depression symptoms remained significant after controlling for infant birth weight, offspring sex and gestational age (F4,76 = 2.45, p = 0.05, R2 = 0.11), with only maternal prenatal EPDS scores significantly predicting placental PEG3 expression (p = 0.005). Only two participants reported prescribed anti-depressant use during pregnancy; the association between placental PEG3 expression and maternal EPDS scores remained significant with the exclusion of these participants (r = −0.30, p = 0.006, n = 79). Finally, a significant 22% decrease in PEG3 expression was observed in placentas from participants with EPDS scores ⩾13, highlighting them as at risk of a depressive disorder (Fig. 3c).
Fig. 3.
My Baby and Me Study (MBAM) cohort – maternal depression symptoms and placental gene expression. The inverse association between paternally expressed gene 3 (PEG3) expression and maternal depression was significant in male (a) but not female (b) placentas. There was a significant decrease in PEG3 (c) and human placental lactogen (hPL) (d) expression in participants with Edinburgh Postnatal Depression Scale (EPDS) scores ⩾13 (cut-off for clinical depression). Values are means of fold gene expression, with standard errors represented by vertical bars. * p < 0.05.
Placental hPL expression was also significantly inversely associated with maternal prenatal EPDS scores (r = −0.36, p = 0.001, n = 81) in the overall cohort. As with PEG3, this association was sex specific, being significant in male (r = −0.51, p < 0.001, n = 44) but not female (r = −0.11, p = 0.53, n = 37) placentas. There was also a significant 21% decrease in hPL expression in placentas from participants with EPDS scores ⩾13 (Fig. 3d). Finally, there was a positive correlation between placental PEG3 and hPL expression although this was not statistically significant in the overall cohort (r = 0.20, p = 0.08, n = 81), being significant in male (r = 0.39, p = 0.01, n = 44) but not female placentas (r = −0.26, p = 0.12, n = 37).
Mediation analysis
Mediation analysis determines the effect of an independent variable on a dependent variable via a third mediating variable and is typically conducted in larger cohort sizes of >100 participants (Fritz & Mackinnon, 2007). In this study mediation analysis was carried out in the two largest cohorts (Manchester and MBAM) with the hypothesis that the association between placental PEG3 and maternal EPDS scores is mediated by placental hPL expression. In the Manchester cohort (n = 75) the indirect effect of placental PEG3 expression on maternal EPDS scores mediated by placental hPL expression was not statistically significant in the overall cohort (B = 0.29, 95% CI −0.08 to 1.13) or when only male placentas were analysed (B = 0.91, 95% CI −0.64 to 5.20). Similarly, in the MBAM cohort (n = 81) the indirect effect of placental PEG3 expression was not statistically significant in the overall cohort (B = −0.53, 95% CI −1.71 to 0.46) or when only male placentas were analysed (B = 1.35, 95% CI −0.26 to 3.64).
Maternal depression and birth weight
Maternal stress is known to be associated with an increased risk of fetal growth restriction (Steer et al. 1992; Rondo et al. 2003; Khashan et al. 2008; Liu et al. 2012) and therefore we analysed birth weight in relation to maternal depression in the three cohorts. Birth weight was not significantly altered in women with diagnosed depression (3.30 kg v. 3.09 kg, p = 0.41, n = 75) in the Manchester cohort. There was also no significant association between maternal prenatal EPDS scores and birth weight in the Queen Charlotte's (r = −0.17, p = 0.28, n = 40) or MBAM (r = 0.12, p = 0.28, n = 81) cohorts. Reduced expression of Peg3 in mice has also been linked to fetal growth restriction (Li et al. 1999). There was no significant association between placental PEG3 expression and birth weight in the Manchester (r = 0.05, p = 0.70, n = 75), Queen Charlotte's (r = 0.09, p = 0.58, n = 40) or MBAM (r = −0.02, p = 0.84, n = 81) cohorts. However, it should be noted that for the Queen Charlotte's and MBAM cohorts women with complications of pregnancy (including fetal growth restriction) were excluded and that previous studies demonstrating an association between maternal prenatal stress and fetal growth restriction examined substantially larger cohorts (Talge et al. 2007).
Discussion
This study has identified a novel association between maternal prenatal depression and decreased placental expression of the imprinted gene PEG3. In our pilot study, placental expression of the imprinted genes PEG3, PEG10, PHLDA2 and CDKN1C was examined in relation to clinically diagnosed depression. Despite the small number of participants, there was a clear significant reduction in placental PEG3 expression but no alteration in expression of the other three imprinted genes examined. Importantly, placental PEG3 expression was also significantly inversely associated with symptoms of maternal prenatal depression in two further independent cohorts, the Queen Charlotte's and MBAM cohorts. This is the first report linking abnormal expression of this imprinted gene in the placenta with maternal depression during pregnancy, which may inform our understanding of the mechanisms underlying the association between prenatal depression and adverse offspring outcomes.
PEG3 exemplifies all the known functions of imprinted genes; it acts to regulate fetal growth, placental development, behaviour and metabolism in mice (Li et al. 1999; Curley et al. 2005; Champagne et al. 2009; Chiavegatto et al. 2012; Kim et al. 2013). We did not observe reduced birth weight in this study, probably as a result of the small study numbers and exclusion of growth-restricted infants. However, it is possible that reduced expression of PEG3 in human pregnancy could contribute to fetal growth restriction, either directly or indirectly via a placental defect, and therefore mediate the previously reported association between maternal prenatal stress and impaired fetal growth.
In this study we also demonstrated a significant inverse association between placental hPL expression and maternal prenatal depression in all three cohorts. hPL is a lactogenic hormone, produced in large quantities by the placenta, and is important for maternal glucose management during pregnancy and fetal growth (Newbern & Freemark, 2011). Maternal serum hPL levels and placental hPL expression have previously been demonstrated to be significantly reduced in pregnancies complicated by fetal growth restriction (Roh et al. 2005; Dutton et al. 2012). Placental lactogens are closely related to pituitary prolactin (Haig, 2008). This hormone family is known to be important for driving the maternal physiological adaptations to pregnancy (John, 2013) but studies in rodents suggest that these hormones may also be important for psychological adaptation to pregnancy. Both prolactin and placental lactogen can induce maternal behaviour in non-pregnant rodents (Bridges et al. 1985, 1990, 1997; Bridges & Freemark, 1995) and prolactin plays an important role in pregnancy-related neurogenesis (Bridges & Grattan, 2003; Shingo et al. 2003; Walker et al. 2012). There has not yet been a comprehensive examination of serum hPL levels in relation to maternal mood in humans. However, decreased maternal serum levels of prolactin have been reported in human mothers with postnatal depression symptoms (Abou-Saleh et al. 1998; Ingram et al. 2003; Groer & Morgan, 2007) and increased levels in mothers with low anxiety scores during pregnancy (Asher et al. 1995). Prolactin contributes to a suppression of anxiety-related behaviours during pregnancy via binding to prolactin receptors, which are also known to bind the placental lactogens (Torner et al. 2001). As with reduced expression of PEG3, reduced placental lactogen production could contribute to a suboptimal pregnancy.
PEG3 has been proposed to regulate the production of placental hormones (John, 2013). We observed a significant correlation between placental PEG3 and hPL expression in the two largest cohorts (Manchester and MBAM). Mediation analysis was carried out in these two cohorts with the hypothesis that the association between placental PEG3 and maternal depression is mediated by placental hPL expression. The indirect effect of placental PEG3 expression on maternal depression mediated by placental hPL expression was not statistically significant in either cohort. However, it should be noted that the sample sizes used in this analysis (Manchester cohort n = 75 and MBAM n = 81) are relatively low given that mediation analysis is typically conducted in studies of >100 participants (Fritz & Mackinnon, 2007). While it is possible that the gene expression changes observed are independently related to maternal prenatal depression, studies in the mouse placenta demonstrate reduced expression of Peg3 drives reduced expression of Prls (mouse placental lactogens) (Broad & Keverne, 2011; Kim et al. 2013). Future studies employing mediation analysis will probably be more informative in larger cohorts and will be crucial in determining the relationship between PEG3 and hPL in the human placenta.
The association between maternal prenatal depression symptoms and placental PEG3 and hPL expression showed a sex bias for both genes, in both cohorts, with the effect more marked with male fetuses. This result is of interest as previous studies suggest sexual dimorphism in infant outcomes following maternal prenatal stress (for a review, see Glover & Hill, 2012). Boys, but not girls, are at an increased risk of attention-deficit/hyperactivity disorder (Li et al. 2010), schizophrenia (van Os & Selten, 1998) and impaired motor development (Gerardin et al. 2011). There is also some preliminary evidence to suggest that women who give birth to boys are more likely to suffer from postpartum depression than those having girls (de Tychey et al. 2008; Lagerberg & Magnusson, 2012). It will therefore be important to establish whether there is an association between placental PEG3 and hPL expression, fetal sex, infant outcomes and maternal postnatal depression.
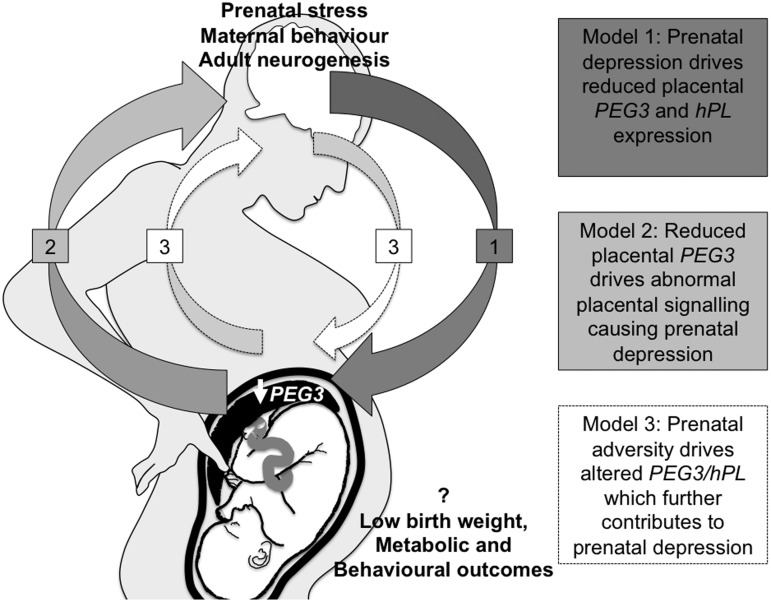
This study was not designed to establish cause-or-effect relationships. Prenatal depression, or other factors associated with prenatal depression, may drive reduced expression of placental PEG3 and hPL in the placenta (Fig. 4: model 1). Peg3 in the rodent placenta is known to be responsive to environmental stimuli, such as maternal diet (Broad & Keverne, 2011; Radford et al. 2012). Alternatively, reduced PEG3 may contribute to or even initiate prenatal depression (Fig. 4: model 2). Loss of function of Peg3 in mice results in abnormal maternal behaviour (Li et al. 1999; Champagne et al. 2009; Chiavegatto et al. 2012). However, in this scenario it is the dams that are mutant for Peg3 and not the placenta. Rather, aberrant placental PEG3 expression may contribute to abnormal maternal mood in human pregnancies, via impaired placental production of hPL (Janssen et al. 2016). It is also possible that both models are correct. A suboptimal maternal environment, which may include maternal prenatal stress, could misprogramme placental expression of PEG3, which in turn may alter placental signalling via hPL, thereby further contributing to the depressed mood (Fig. 4: model 3).
Fig. 4.
Paternally expressed gene 3 (PEG3), human placental lactogen (hPL) signalling and maternal psychological adaptation to pregnancy. Model 1: prenatal depression causes reduced PEG3 and reduced hPL expression. Model 2: reduced PEG3 initiates prenatal depression through regulation of placental lactogen production. Model 3: an adverse intra-uterine environment causes reduced PEG3 expression limiting placental signalling and establishing a cycle of aberrant placental gene expression, aberrant signalling and abnormal maternal mood.
There are limitations to this study, primarily in the number of participants. However, this is countered by the reproducibility of these findings in three independent studies. Also, in the Queen Charlotte's and MBAM cohorts, maternal prenatal depression symptoms were assessed 1 day prior to elective Caesarean section when mothers may be particularly anxious. Ideally, future studies would involve assessment of maternal mood at different time points throughout pregnancy in a larger number of participants to provide a better indication of maternal prenatal stress during pregnancy. Another study limitation is that participants in the Manchester cohort suffered from RFM before delivery and so are not representative of uncomplicated pregnancies. However, both controls and women with depression during pregnancy experienced RFM and therefore this was not causative of the differences in gene expression observed. Finally, as depression diagnosis in the Manchester cohort was based solely on a diagnosis reported within clinical records, we cannot exclude the presence of depression symptoms in the control cases. Future studies with measures of depression for all participants (as with the Queen Charlotte's and MBAM cohorts) as well as more detailed information on the nature of depression and medication during pregnancy will be important in confirming the association between aberrant placental gene expression and diagnosed depression.
Conclusions
In summary, this study is the first to report reduced expression of both PEG3 and hPL in the human placenta in relation to adverse maternal mood. Substantial indirect data from rodent models suggest that this relationship is pathologically relevant and may indeed be causal. Animal studies will be important in further establishing cause-and-effect relationships. Meanwhile, it will be important to validate our findings in a larger study cohort and, importantly, to examine the outcomes of pregnancies with reduced PEG3 expression, both for the child and for the mother. Measuring maternal serum levels of hPL will be instrumental in determining whether reduced placental hPL expression in the term placenta reflects reduced hormone serum level during pregnancy. This is of clinical relevance since it may be possible to use maternal serum hPL levels as a biomarker in combination with self-report questionnaires to identify mothers at high risk of maternal depression. Finally, our current findings are of broader interest as reduced expression of PEG3 could provide a mechanistic explanation for the co-occurrence of maternal depression, low birth weight and poorer outcomes for the offspring, a finding that will lead to a greater understanding of both the causes and consequences of prenatal depression.
Acknowledgements
The authors would like to acknowledge all the women who participated in this study and the midwives at St Mary's Hospital and Queen Charlotte's Hospital for their help with sample collection. The Manchester cohort was supported by Manchester National Institute for Health Research (NIHR) Biomedical Research. The Queen Charlotte's cohort was supported by the Medical Research Council (MRC) (Eurostress), National Institutes of Health (R01MH073842) and the Genesis Research Trust. The MBAM cohort was supported by the Genesis Research Trust. A.B.J. was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) Doctoral Training Grants (DTG) studentship and subsequently MRC project grant MR/M013960/1. S.J.T. was supported by BBSRC project grant BB/J015156/1. L.E.C. was supported by an Imperial College London Ph.D. studentship and both L.E.C. and P.G.R were supported by the NIHR Imperial Biomedical Research Centre.
Declaration of Interest
None.
References
- Abou-Saleh MT, Ghubash R, Karim L, Krymski M, Bhai I (1998). Hormonal aspects of postpartum depression. Psychoneuroendocrinology 23, 465–475. [DOI] [PubMed] [Google Scholar]
- Apostolidou S, Abu-Amero S, O'Donoghue K, Frost J, Olafsdottir O, Chavele K, Whittaker J, Loughna P, Stanier P, Moore G (2007). Elevated placental expression of the imprinted PHLDA2 gene is associated with low birth weight. Journal of Molecular Medicine 85, 379–387. [DOI] [PubMed] [Google Scholar]
- Asher I, Kaplan B, Modai I, Neri A, Valevski A, Weizman A (1995). Mood and hormonal changes during late pregnancy and puerperium. Clinical and Experimental Obstetrics and Gynecology 22, 321–325. [PubMed] [Google Scholar]
- Blakeley PM, Capron LE, Jensen AB, O'Donnell KJ, Glover V (2013). Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. Journal of Psychosomatic Research 75, 341–345. [DOI] [PubMed] [Google Scholar]
- Bridges RS, DiBiase R, Loundes DD, Doherty PC (1985). Prolactin stimulation of maternal behavior in female rats. Science 227, 782–784. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Freemark MS (1995). Human placental lactogen infusions into the medial preoptic area stimulate maternal behavior in steroid-primed, nulliparous female rats. Hormones and Behaviour 29, 216–226. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Grattan DR (2003). Prolactin-induced neurogenesis in the maternal brain. Trends in Endocrinology and Metabolism 14, 199–201. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE (1990). Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proceedings of the National Academy of Sciences USA 87, 8003–8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RP, Sturgis JD, Henriquez BM, Mann PE (1997). Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology 138, 756–763. [DOI] [PubMed] [Google Scholar]
- Broad KD, Keverne EB (2011). Placental protection of the fetal brain during short-term food deprivation. Proceedings of the National Academy of Sciences USA 108, 15237–15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley J, Swaney W, Hasen N, Keverne EB (2009). Paternal influence on female behavior: the role of Peg3 in exploration, olfaction, and neuroendocrine regulation of maternal behavior of female mice. Behavioral Neuroscience 123, 469–480. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Sauce B, Ambar G, Cheverud JM, Peripato AC (2012). Hypothalamic expression of Peg3 gene is associated with maternal care differences between SM/J and LG/J mouse strains. Brain and Behavior 2, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal JK, Day P, Hanson MA, Lewis RM (2009). Measurement of housekeeping genes in human placenta. Placenta 30, 1002–1003. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Day PL, Hanson MA, Lewis RM (2010). Sex differences in the mRNA levels of housekeeping genes in human placenta. Placenta 31, 556–557. [DOI] [PubMed] [Google Scholar]
- Clifton VL (2010). Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 31 (Suppl.), S33–S39. [DOI] [PubMed] [Google Scholar]
- Cox JL, Chapman G, Murray D, Jones P (1996). Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. Journal of Affective Disorders 39, 185–189. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Curley JP, Pinnock SB, Dickson SL, Thresher R, Miyoshi N, Surani MA, Keverne EB (2005). Increased body fat in mice with a targeted mutation of the paternally expressed imprinted gene Peg3. FASEB Journal 19, 1302–1304. [DOI] [PubMed] [Google Scholar]
- de Tychey C, Briancon S, Lighezzolo J, Spitz E, Kabuth B, de Luigi V, Messembourg C, Girvan F, Rosati A, Thockler A, Vincent S (2008). Quality of life, postnatal depression and baby gender. Journal of Clinical Nursing 17, 312–322. [DOI] [PubMed] [Google Scholar]
- Diplas AI, Lambertini L, Lee M-J, Sperling R, Lee YL, Wetmur JG, Chen J (2009). Differential expression of imprinted genes in normal and IUGR human placentas. Epigenetics 4, 235–240. [DOI] [PubMed] [Google Scholar]
- Dutton PJ, Warrander LK, Roberts SA, Bernatavicius G, Byrd LM, Gaze D, Kroll J, Jones RL, Sibley CP, Froen JF, Heazell AE (2012). Predictors of poor perinatal outcome following maternal perception of reduced fetal movements – a prospective cohort study. PLOS ONE 7, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Marquez RT, Lu Z, Liu J, Lu KH, Issa JP, Fishman DM, Yu Y, Bast RC Jr. (2008). Imprinted tumor suppressor genes ARHI and PEG3 are the most frequently down-regulated in human ovarian cancers by loss of heterozygosity and promoter methylation. Cancer 112, 1489–1502. [DOI] [PubMed] [Google Scholar]
- Fritz MS, Mackinnon DP (2007). Required sample size to detect the mediated effect. Psychological Science 18, 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardin P, Wendland J, Bodeau N, Galin A, Bialobos S, Tordjman S, Mazet P, Darbois Y, Nizard J, Dommergues M, Cohen D (2011). Depression during pregnancy: is the developmental impact earlier in boys? A prospective case–control study. Journal of Clinical Psychiatry 72, 378–387. [DOI] [PubMed] [Google Scholar]
- Glover V, Hill J (2012). Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: an evolutionary perspective. Physiology and Behavior 106, 736–740. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Sandman CA (2011). Prenatal origins of neurological development: a critical period for fetus and mother. Current Directions in Psychological Science 20, 384–389. [Google Scholar]
- Green BB, Kappil M, Lambertini L, Armstrong DA, Guerin DJ, Sharp AJ, Lester BM, Chen J, Marsit CJ (2015). Expression of imprinted genes in placenta is associated with infant neurobehavioral development. Epigenetics 10, 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer MW, Morgan K (2007). Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology 32, 133–139. [DOI] [PubMed] [Google Scholar]
- Gude NM, Roberts CT, Kalionis B, King RG (2004). Growth and function of the normal human placenta. Thrombosis Research 114, 397–407. [DOI] [PubMed] [Google Scholar]
- Haig D (2008). Placental growth hormone-related proteins and prolactin-related proteins. Placenta 29 (Suppl. A), S36–S41. [DOI] [PubMed] [Google Scholar]
- Hayes A (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. Guilford Press: New York. [Google Scholar]
- Ingram J, Greenwood R, Woolridge M (2003). Hormonal predictors of postnatal depression at 6 months in breastfeeding women. Journal of Reproductive and Infant Psychology 21, 61–68. [Google Scholar]
- Janssen AB, Kertes DA, McNamara GI, Braithwaite EC, Creeth HD, Glover VI, John RM (2016). A role for the placenta in programming maternal mood and childhood behavioural disorders. Journal of Neuroendocrinology. Published online 2 February 2016. doi: 10.1111/jne.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen AB, Tunster SJ, Savory N, Holmes A, Beasley J, Parveen SA, Penketh RJ, John RM (2015). Placental expression of imprinted genes varies with sampling site and mode of delivery. Placenta 36, 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AB, Tunster SJ, John RM (2014). The significance of elevated placental PHLDA2 in human growth restricted pregnancies. Placenta 35, 528–532. [DOI] [PubMed] [Google Scholar]
- Jensen Pena C, Monk C, Champagne FA (2012). Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLOS ONE 7, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- John RM (2013). Epigenetic regulation of placental endocrine lineages and complications of pregnancy. Biochemical Society Transactions 41, 701–709. [DOI] [PubMed] [Google Scholar]
- Khashan AS, McNamee R, Abel KM, Pedersen MG, Webb RT, Kenny LC, Mortensen PB, Baker PN (2008). Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosomatic Medicine 70, 688–694. [DOI] [PubMed] [Google Scholar]
- Kim J, Frey WD, He H, Kim H, Ekram MB, Bakshi A, Faisal M, Perera BP, Ye A, Teruyama R (2013). Peg3 mutational effects on reproduction and placenta-specific gene families. PLOS ONE 8, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerberg D, Magnusson M (2012). Infant gender and postpartum sadness in the light of region of birth and some other factors: a contribution to the knowledge of postpartum depression. Archives of Women's Mental Health 15, 121–130. [DOI] [PubMed] [Google Scholar]
- Lefebvre L, Viville S, Barton SC, Ishino F, Keverne EB, Surani MA (1998). Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nature Genetics 20, 163–169. [DOI] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, Obel C (2010). Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. European Child and Adolescent Psychiatry 19, 747–753. [DOI] [PubMed] [Google Scholar]
- Li LL, Keverne EB, Aparicio SA, Ishino F, Barton SC, Surani MA (1999). Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science 284, 330–334. [DOI] [PubMed] [Google Scholar]
- Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, Overcash F, Kurtzberg J, Jirtle R, Iversen ES, Forman MR, Hoyo C (2012). Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics 7, 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O (2007). Maternal stress alters endocrine function of the feto-placental unit in rats. American Journal of Physiology. Endocrinology and Metabolism 292, E1526–E1533. [DOI] [PubMed] [Google Scholar]
- McNamara GI, Isles AR (2014). Influencing the social group: the role of imprinted genes. Advances in Genetics 86, 107–134. [DOI] [PubMed] [Google Scholar]
- Meller M, Vadachkoria S, Luthy DA, Williams MA (2005). Evaluation of housekeeping genes in placental comparative expression studies. Placenta 26, 601–607. [DOI] [PubMed] [Google Scholar]
- Murthi P, Fitzpatrick E, Borg AJ, Donath S, Brennecke SP, Kalionis B (2008). GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction. Placenta 29, 798–801. [DOI] [PubMed] [Google Scholar]
- Newbern D, Freemark M (2011). Placental hormones and the control of maternal metabolism and fetal growth. Current Opinion in Endocrinology, Diabetes and Obesity 18, 409–416. [DOI] [PubMed] [Google Scholar]
- O'Donnell K, O'Connor TG, Glover V (2009). Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Developmental Neuroscience 31, 285–292. [DOI] [PubMed] [Google Scholar]
- O'Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O'Connor TG, Glover V (2012). Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology 37, 818–826. [DOI] [PubMed] [Google Scholar]
- Pearson RM, Melotti R, Heron J, Joinson C, Stein A, Ramchandani PG, Evans J (2012). Disruption to the development of maternal responsiveness? The impact of prenatal depression on mother–infant interactions. Infant Behavior and Development 35, 613–626. [DOI] [PubMed] [Google Scholar]
- Radford EJ, Isganaitis E, Jimenez-Chillaron J, Schroeder J, Molla M, Andrews S, Didier N, Charalambous M, McEwen K, Marazzi G, Sassoon D, Patti ME, Ferguson-Smith AC (2012). An unbiased assessment of the role of imprinted genes in an intergenerational model of developmental programming. PLoS Genetics 8, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RM, Pesonen AK, O'Reilly JR, Tuovinen S, Lahti M, Kajantie E, Villa PM, Laivuori H, Hamalainen E, Seckl JR, Raikkonen K (2015). Maternal depressive symptoms throughout pregnancy are associated with increased placental glucocorticoid sensitivity. Psychological Medicine 45, 2023–2030. [DOI] [PubMed] [Google Scholar]
- Roh CR, Budhraja V, Kim HS, Nelson DM, Sadovsky Y (2005). Microarray-based identification of differentially expressed genes in hypoxic term human trophoblasts and in placental villi of pregnancies with growth restricted fetuses. Placenta 26, 319–328. [DOI] [PubMed] [Google Scholar]
- Rondo PHC, Ferreira RF, Nogueira F, Ribeiro MCN, Lobert H, Artes R (2003). Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. European Journal of Clinical Nutrition 57, 266–272. [DOI] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S (2003). Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299, 117–120. [DOI] [PubMed] [Google Scholar]
- Smith FM, Garfield AS, Ward A (2006). Regulation of growth and metabolism by imprinted genes. Cytogenetic and Genome Research 113, 279–291. [DOI] [PubMed] [Google Scholar]
- Steer RA, Scholl TO, Hediger ML, Fischer RL (1992). Self-reported depression and negative pregnancy outcomes. Journal of Clinical Epidemiology 45, 1093–1099. [DOI] [PubMed] [Google Scholar]
- Surani MA (1998). Imprinting and the initiation of gene silencing in the germ line. Cell 93, 309–312. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V (2007). Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? Journal of Child Psychology and Psychiatry 48, 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID (2001). Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. Journal of Neuroscience 21, 3207–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunster SJ, Jensen AB, John RM (2013). Imprinted genes in mouse placental development and the regulation of fetal energy stores. Reproduction 145, R117–R137. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mulder EJ, Mennes M, Glover V (2005). Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neuroscience and Biobehavioral Reviews 29, 237–258. [DOI] [PubMed] [Google Scholar]
- van Os J, Selten JP (1998). Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. British Journal of Psychiatry 172, 324–326. [DOI] [PubMed] [Google Scholar]
- Vidal AC, Benjamin Neelon SE, Liu Y, Tuli AM, Fuemmeler BF, Hoyo C, Murtha AP, Huang Z, Schildkraut J, Overcash F, Kurtzberg J, Jirtle RL, Iversen ES, Murphy SK (2014). Maternal stress, preterm birth, and DNA methylation at imprint regulatory sequences in humans. Genetics and Epigenetics 6, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TL, Vukovic J, Koudijs MM, Blackmore DG, Mackay EW, Sykes AM, Overall RW, Hamlin AS, Bartlett PF (2012). Prolactin stimulates precursor cells in the adult mouse hippocampus. PLOS ONE 7, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrander LK, Batra G, Bernatavicius G, Greenwood SL, Dutton P, Jones RL, Sibley CP, Heazell AE (2012). Maternal perception of reduced fetal movements is associated with altered placental structure and function. PLOS ONE 7, . [DOI] [PMC free article] [PubMed] [Google Scholar]