Abstract
Background
Mechanical ventilation (MV) during a cardio-thoracic surgery contributes to diaphragm muscle dysfunction that impairs weaning and can lead to the ventilator- induced diaphragm dysfunction. Especially, it is critical in older adults who have lower muscle reparative capacity following MV. Reports have shown that the intraoperative intermittent hemidiaphragm electrical stimulation can maintain and/or improve post-surgery diaphragm function. In particular, from a molecular point of view, intermittent ES may reduce oxidative stress and increase regulatory autophagy levels, and therefore improve diaphragm function in animal studies. We have recently shown in humans that intraoperative ES attenuates mitochondrial dysfunction and force decline in single diaphragm muscle fibers. The aim of this study was to investigate an effect of ES on oxidative stress, antioxidant status and autophagy biomarker levels in the human diaphragm during surgery.
Methods
One phrenic nerve was simulated with an external cardiac pacer in operated older subjects (62.4 ± 12.9 years) (n = 8) during the surgery. The patients received 30 pulses per min every 30 min. The muscle biopsy was collected from both hemidiaphragms and frozen for further analyses. 4-hydroxynonenal (4-HNE), an oxidative stress marker, and autophagy marker levels (Beclin-1 and the ratio of microtubule-associated protein light chain 3, I and II-LC3 II/I) protein concentrations were detected by the western blot technique. Antioxidant enzymatic activity copper-zinc (CuZnSOD) and manganese (MnSOD) superoxide dismutase were analyzed.
Results
Levels of lipid peroxidation (4-HNE) were significantly lower in the stimulated side (p < 0.05). The antioxidant enzyme activities (CuZnSOD and MnSOD) in the stimulated side of the diaphragm were not different than in the unstimulated side (p > 0.05). Additionally, the protein concentrations of Beclin-1 and the LC3 II/I ratio were higher in the stimulated side (p < 0.05).
Conclusion
These results suggest that the intraoperative electrical stimulation decreases oxidative stress levels and upregulates autophagy levels in the stimulated hemidiaphragm. These results may contribute future studies and clinical applications on reducing post-operative diaphragm dysfunction.
Background
Mechanical ventilation (MV) is a life-saving component of modern intensive care and surgery. However, diaphragmatic unloading induced by MV may result in deleterious changes on the cellular level even within the first few hours from the intubation [1]. Diaphragm unloading may contribute to diaphragm muscle dysfunction that impairs weaning and can lead to the ventilator- induced diaphragm dysfunction (VIDD) [2]. Studies have shown that the intermittent intraoperative hemidiaphragm electrical stimulation may maintain or improve post-surgery diaphragm function [3–6]. For example, recently, we were the first to report that intraoperative hemidiaphragm electrical stimulation improved state III (25 %) and state IV (42 %) mitochondrial respiration in comparison with the unstimulated hemidiaphragm in older adults who underwent cardio-thoracic surgeries [6]. Moreover, single fiber force was improved by 30 % in the stimulated side of the diaphragm muscle in the same group of patients [5]. These results warranted further biochemical analyses investigating molecular pathways of these beneficial effects of electrical stimulation on diaphragm function.
Animal studies showed that diaphragmatic inactivity promotes reactive oxygen species (ROS) formation and mitochondrial dysfunction [7, 8] and may contribute to VIDD [9]. Potentially, excessive production of reactive oxygen species may be caused by mechanical inactivity of the respiratory muscles during MV—a state of metabolic oversupply [9, 10]. On the other hand, MV induces oxidative stress that may also reduce mitochondrial oxygen phosphorylation and may lead to reduced overall energy supply to muscle and to cell apoptosis (showed in cultured human diaphragm muscle cells) [11]. However, these molecular pathways have not been well studied in humans undergoing MV.
It has also been shown that autophagy is an important process in maintaining cell homeostasis by disposing cytotoxic elements of malfunctioned cell components e.g. mitochondrial turnover—mitophagy and thereby reducing pathophysiological ROS formation [12]. Importantly, levels of autophagy diminish with age, which may be an additive factor to diaphragm mitochondrial dysfunction in post-operative weaning from MV in older adults [13].
We hypothesized that intermittent electrical stimulation may reduce deleterious oxidative stress levels and increase basal regulatory autophagy levels, which maybe one of the key factors to improve diaphragm function during cardio-thoracic surgeries in mechanically ventilated patients [5, 6].
Methods
Subjects
Eight patients (at low risk for postoperative complications and weaning difficulties) undergoing cardiothoracic surgery at Shands Hospital at the University of Florida were recruited into the study (Table 1). The phrenic nerve was selected by the surgeons’ convenience and stimulated with an external cardiac pacer (Medtronic 5388) with temporary cardiac pacing wire electrodes. Stimulation was conducted for 1 min (30 pulses per min, 1.5 min duration) as soon as the phrenic nerve and diaphragm were exposed and every 30 min thereafter. Exclusion criteria included: prior surgery to the heart, diaphragm, pleura or phrenic nerves resulting in anatomical changes that would complicate obtaining muscle samples or interfere with phrenic stimulation; neuromuscular or inflammatory muscle diseases; obstructive lung disease (FEV1.0 < 60 % of predicted); other lung disease (bronchiectasis, lung cancer, pulmonary hypertension, tuberculosis or pulmonary fibrosis etc.); NYHA Class IV heart failure; implanted cardiac pacemaker or defibrillators; use of immunosuppressants, corticosteroids or aminoglycoside antibiotics within 60 days of surgery and serum creatinine >1.6 mg/dl. The detailed procedure description has been published elsewhere [6]. Full thickness diaphragm samples (20–50 mg) were obtained 30 min following the last stimulation bout. Muscle biopsies from each hemidiaphragm were frozen immediately and later prepared for protein immunoblotting. The University of Florida IRB approved this study protocol, and all subjects gave their consent for participation.
Table 1.
Patient demographics and surgery description
| Pt # | Age | Sex | Height (cm) | Weight (kg) | MV start to biopsy (min) | Surgical procedure |
|---|---|---|---|---|---|---|
| 1 | 52 | F | 162.6 | 65.5 | 241 | Triple CABG |
| 2 | 47 | M | 175.3 | 84.5 | 209 | Aortic valve replacement |
| 3 | 68 | M | 167 | 90.1 | 331 | Triple CABG |
| 4 | 53 | M | 182.9 | 95.5 | 316 | Ascending aortic aneurysm |
| 5 | 56 | F | 175.1 | 90.0 | 329 | Aortic graft, aortic valve replacement, CABG |
| 6 | 79 | M | 167.6 | 81.8 | 334 | Aortic valve replacement, double CABG |
| 7 | 48 | M | 167.6 | 81.8 | 295 | Triple CABG |
| 8 | 72 | F | 165.1 | 106.8 | 209 | Aortic valve replacement |
| Mean | 62.4 ± 12.9 | 172.6 ± 6.7 | 92.5 ± 10.6 | 284.1 ± 53.8 | ||
Data presented as mean ± SD
CABG coronary bypass graft
Western blot
Equal amounts of protein (50 lg/condition) were resolved by 12 % sodium dodecyl sulfate (SDS)–gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes, according to a partially modified conventional protocol [14]. The immunodetection included the transfer (20 V for 25 min, per each membrane) and blocking of the membrane with western blot (WB) blocking solution (2 h at room temperature). After washing the membranes two times with TBST 1, the blots were incubated with the corresponding primary antibodies (Cell Signaling, USA) against 4-Hydroxynonenal (4-HNE), Peroxysome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), Beclin-1, microtubule-associated protein 1 light chain 3 I and II (LC3) (1:000) and (incubated at 4 °C overnight. The LC3-II/I ratio was calculated based on densitometry analysis of both bands. The ratio is a widely used indicator of autophagy flux [15]. The membranes were washed two times with TBST 1 and subsequently incubated with their respective HRP-conjugated secondary antibodies (1:10,000) (1 h at room temperature). The detection of bound antibodies was visualized by chemiluminescence with enhanced chemiluminescence (ECL) substrate. Finally, a quantification analysis was performed with Image J software (NIH), using Ponceau stain.
Antioxidant enzymatic activity
Superoxide dismutase (SOD) activity was measured as previously described [16].
Data analysis
Two-tailed t-tests for matched pairs were used to compare distributions and statistical significance was set at p < 0.05. Data are shown as mean ± standard deviation.
Results
Stimulation
Stimulation of a hemidiaphragm was well tolerated and the biopsies were obtained without complication. All subjects were extubated between 6.7 and 67.1 h after surgery (27.5 ± 22.8 h). The first stimulation was initialized when the phrenic nerve and diaphragm were visualized by a surgeon (95.9 ± 12 min after intubation). The patients received an average of 6.4 ± 1.8 stimulation bouts with the mean amplitude of 19.6 ± 5.8 mA. Muscle biopsies were harvested 28.3 ± 1.8 min after the last stimulation bout.
Biopsy analysis
Due to the limited sample availability we were only able to measure 4-HNE, Beclin-1, LC3 and PGC-1α protein concentrations and enzyme activity of CuZnSOD and MnSOD. Protein concentration of 4-HNE, a marker of lipid peroxidation levels and oxidative stress was significantly lower (p < 0.05) in the stimulated hemidiaphragm. Antioxidant enzyme activities of CuZnSOD and MnSOD were not different (p > 0.05) between the hemidiaphragms (Fig. 1). Macroautophagy biomarker levels of Beclin-1 and the LC3II/I ratio were significantly higher in the stimulated side (p < 0.05) (Fig. 2). Additionally, there was no difference in protein concentration levels of master regulator of mitochondrial biogenesis—PGC-1α between the hemidiaphragms.
Fig. 1.
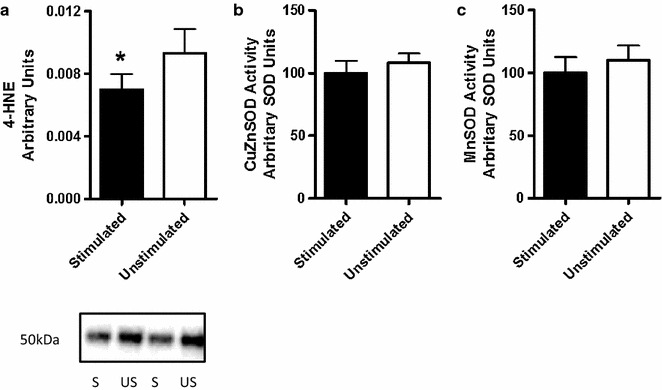
Levels of oxidative stress (a) and enzymatic activity (b, c) in the unstimulated and stimulated hemidiaphragm
Fig. 2.

Protein concentrations of autophagy proteins (a Beclin-1 and b LC3-II/I ratio) in the unstimulated and stimulated diaphragm sides
Discussion
We are the first to report potentially beneficial effects of electrical stimulation on oxidative stress and autophagy levels in human diaphragm in mechanically ventilated older patients during cardio-thoracic surgeries. The main finding of this exploratory study was that levels of oxidative stress were lower in the stimulated hemidiaphragm. Additionally, higher levels of autophagy levels markers i.e. Beclin-1 and the LC3-II/I ratio in the stimulated hemidiaphragm suggest upregulation of macroautophagy levels.
Previous reports suggested that higher levels of oxidative stress may be a key factor of the majority of pathways leading to VIDD in the mechanically ventilated patients, with mitochondrial dysfunction being an essential part [11, 17]. We reported that electrical stimulation improved mitochondrial function (i.e. state III and IV mitochondrial respiration) in comparison with the unstimulated hemidiaphragm [6]. These results suggest improved mitochondrial function in the stimulated hemidiaphragm, which maybe a contributing factor to reducing risk of VIDD in surgery patients. Mitochondrial function can be improved by generating new mitochondrial and/or removing the dysfunctional mitochondria (mitophagy) that are the source of oxidative stress [18]. In the current analysis we showed no difference in the protein concentration of PGC-1α, a biomarker of mitochondrial biogenesis levels. This suggests that autophagy-related mechanisms and reduced oxidative stress levels may have improved mitochondrial function in the stimulated hemidiaphragm [19] and that our observation is not due to an altered level of mitochondrial biogenesis.
The link between increased oxidative stress and MV-related diaphragm dysfunction has also been shown in animal models [17, 20]. Indeed, we found increases in 4-HNE protein concentration, a biomarker of lipid peroxidation, and thus an indication of increased oxidative stress levels in the unstimulated hemidiaphragm. Additionally, the activity levels of endogenous antioxidant defense system (CuZnSOD and MnSOD) were not different between the hemidiaphragms that may be associated with a limited samples size. The future studies may include measurements of other biomarkers of oxidative stress levels e.g. protein carbonylation and 8-isoprostane levels to better interpret the increased 4-HNE levels in the current analysis.
Moreover, high levels of oxidative stress and autophagy were linked to apoptotic and proteolytic processes that had consequences in diaphragm dysfunction and developed VIDD in mechanically ventilated patients [2, 21]. However, recent reports suggest that physiological levels of ROS formation and autophagy are essential for cellular signaling and homeostasis [22]. In particular, ROS generated by the mitochondrial respiratory chain at low level stimulate cell signaling, but higher levels of ROS lead to mitochondrial damage that produce more oxidative stress [20]. Therefore, autophagy is suggested to play an important role in down-regulation of oxidative stress [19]. In particular, previous reports have shown that autophagy is essential in maintaining mitochondrial function (mitophagy) by degrading malfunctioning mitochondria that are one of the main sources of deleterious oxidative stress [13, 23]. However, other studies reported a link between higher levels of autophagy and human diaphragm atrophy in mechanically ventilated patients [21]. For examples, Hussain et al. reported higher levels of oxidative stress (4-HNE expression) and upregulated autophagy levels (LC3-II/I ratio) and evidence of autophagosome formation in chronically ventilated patients (average MV time 59 ± 16.5 h) in comparison with a control group (2–4 h of MV) [2, 21]. Also, animal studies have shown that diaphragm function preservation was accompanied by lower levels of oxidative stress and autophagy [20]. However, formation of ROS upregulates autophagy to remove defective proteins and malfunctioning mitochondria that would generate uncontrolled ROS and increase oxidative stress levels [19, 24, 25]. Accordingly, our results demonstrated lower levels of oxidative stress and higher levels of autophagy biomarkers, Beclin-1 and the LC3-II/I ratio, that suggest increased autophagosome formation and expansion levels (Fig. 2). Upregulation of Beclin-1 expression is needed for appropriate autophagy induction phase [22]. Although, the LC3-II/I ratio was higher in the stimulated side, the concentrations of LC3II were lower than LC3I in both hemidiaphragms, which suggests low levels of autophagic flux. For more accurate measurements of autophagy levels in order to answer a question if electrical stimulation promotes an autophagy-induced reduction of oxidative stress in the diaphragm, the future studies will involve electron microscopy documentation of autophagosomes in the surgery patient [26]. Additionally, it has been shown that 4-HNE induced an increase of autophagy levels by accumulation of LC3-II in vascular smooth cells [27, 28]. These results suggest a potential link between ROS-induced autophagy leading to autophagy-induced cellular homeostasis and lower oxidative stress levels as a result of autophagy-related cell survival during oxidative stress in the stimulated hemidiaphragm in surgery patients (Fig. 3).
Fig. 3.
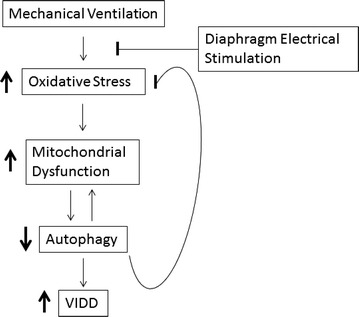
Conceptual figure of a potential mechanism of electrical stimulation and improved diaphragm function
Taken together, we can speculate that decreased oxidative stress levels maybe a product of increased cell-protective autophagy levels (Fig. 3). Although, these results may support our previous report of improved mitochondrial function in the stimulated hemidiaphragms, these exploratory results warrant future studies involving confocal microscopy methods in order to study the autophagy flux and support the clinical value of the diaphragm electrical stimulation.
Study limitations
Although these results suggest ES has a potential protective effect against VIDD, this study has a few limitations. A larger sample size would increase the statistical power and the ability to detect more differences between stimulated and control tissue. Additionally, studying the biological pathways more comprehensively would have been informative, but was limited by small muscle specimens (~20 mg). However, despite the relatively small number of subjects and dependent variables examined, this study presents novel and important findings regarding the effect of electrical stimulation on the human diaphragm during surgery and MV.
Conclusion
Intraoperative hemidiaphragm electrical stimulation may decrease oxidative damage and upregulate autophagy levels. Further studies on molecular bases of the diaphragm electrical stimulation are warranted on a larger population and using confocal technologies. These results may contribute future studies and clinical applications on reducing post-operative diaphragm dysfunction.
Authors’ contributions
RT, CL, and DM participated in the conception, drafting, and revision of the manuscript. TB, PH, BS and TM performed the surgeries, harvested diaphragm muscle biopsies, gave final approval of the version to be published and were involved in revising the manuscripts for important intellectual content. RT, SA, MD, CH and SS were involved in the tissues processing, biochemical analyses, data analysis and revising the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to research nurses for assisting in the recruitment of the subjects. We would also like to thank the subjects for their participation.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets analyzed during the current study could be available from the corresponding author on reasonable request.
Consent to publish
Written informed consent was obtained from the patients for publication of their individual details in this manuscript.
Ethics approval and consent to participate
The University of Florida IRB approved this study protocol, and all subjects gave their consent for participation.
Funding
This study was supported by the University of Florida Clinical Translational Science Institute and Pilot Grant (UL1 RR029890), the Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS): A New Horizon for Surgical Critical Care, P50 Grant (P50GM111152-01), the Claude D. Pepper Older Americans Independence Center (OAIC) Metabolism and Translational Science Core (CL) and K12-HD055929 (BKS) and Eli Lilly company. The OAIC is supported by a Grant from the National Institutes of Health/National Institute on Aging (1P30AG028740).
Abbreviations
- MV
mechanical ventilation
- VIDD
ventilator induced diaphragm dysfunction
- NYHA
New York Heart Association
- FEV-1
forced expiratory volume 1
- ROS
reactive oxygen species
- IRB
Institutional Review Board
- SDS
sodium dodecyl sulfate
- PVDF
polyvinylidene difluoride
- WB
western blot
- 4-HNE
4-Hydroxynonenal
- ECL
enhanced chemiluminescence
- LC3
microtubule-associated protein light chain 3
- CuZnSOD
copper-zinc superoxide dismutase
- MnSOD
manganese superoxide dismutase
Contributor Information
Robert T. Mankowski, Email: r.mankowski@ufl.edu
Shakeel Ahmed, Email: shakeel81@ufl.edu.
Thomas Beaver, Email: thomas.beaver@surgery.ufl.edu.
Marvin Dirain, Email: mdirain@ufl.edu.
Chul Han, Email: cinbaram@ufl.edu.
Phillip Hess, Email: phess2@iuhealth.org.
Tomas Martin, Email: tdmartin2000@gmail.com.
Barbara K. Smith, Email: bksmith@phhp.ufl.edu
Shinichi Someya, Email: someya@ufl.edu.
Christiaan Leeuwenburgh, Phone: 352-294-5859, Email: cleeuwen@ufl.edu.
A. Daniel Martin, Phone: (352) 273-6105, Email: DMartin@phhp.ufl.edu.
References
- 1.Li LF, Chang YL, Chen NH, Wang CY, Chang GJ, Lin MC, Chang CH, Huang CC, Chuang JH, Yang YP, Chiou SH, Liu YY. Inhibition of Src and forkhead box O1 signaling by induced pluripotent stem-cell therapy attenuates hyperoxia-augmented ventilator-induced diaphragm dysfunction. Transl Res. 2016;173:131–147e1. doi: 10.1016/j.trsl.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Hussain SN, Cornachione AS, Guichon C, Khunaizi AL, de Souza Leite F, et al. Prolonged controlled mechanical ventilation in humans triggers myofibrillar contractile dysfunction and myofilament protein loss in the diaphragm. Thorax. 2016;71(5):436–445. doi: 10.1136/thoraxjnl-2015-207559. [DOI] [PubMed] [Google Scholar]
- 3.Onders RP, Khansarinia S, Weiser T, Chin C, Hungness E, Soper N, DeHoyos A, Cole T, Ducko C. Multicenter analysis of diaphragm pacing in tetraplegics with cardiac pacemakers: positive implications for ventilator weaning in intensive care units. Surgery. 2010;148(4):893–898. doi: 10.1016/j.surg.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Onders RP, Elmo M, Kaplan C, Katirji B, Schilz R. Identification of unexpected respiratory abnormalities in patients with amyotrophic lateral sclerosis through electromyographic analysis using intramuscular electrodes implanted for therapeutic diaphragmatic pacing. Am J Surg. 2015;209(3):451–456. doi: 10.1016/j.amjsurg.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Ahn B, Beaver T, Martin T, Hess P, Brumback BA, Ahmed S, Smith BK, Leeuwenburgh C, Martin AD, Ferreira LF. Phrenic nerve stimulation increases human diaphragm fiber force after cardiothoracic surgery. Am J Respir Crit Care Med. 2014;190(7):837–839. doi: 10.1164/rccm.201405-0993LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin AD, Joseph AM, Beaver TM, Smith BK, Martin TD, Berg K, Hess PJ, Deoghare HV, Leeuwenburgh C. Effect of intermittent phrenic nerve stimulation during cardiothoracic surgery on mitochondrial respiration in the human diaphragm. Crit Care Med. 2014;42(2):e152–e156. doi: 10.1097/CCM.0b013e3182a63fdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med. 2009;46(6):842–850. doi: 10.1016/j.freeradbiomed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith IJ, Godinez GL, Singh BK, McCaughey KM, Alcantara RR, Gururaja T, Ho MS, Nguyen HN, Friera AM, White KA, McLaughlin JR, Hansen D, Romero JM, Baltgalvis KA, Claypool MD, et al. Inhibition of Janus kinase signaling during controlled mechanical ventilation prevents ventilation-induced diaphragm dysfunction. FASEB J. 2014;28(7):2790–2803. doi: 10.1096/fj.13-244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, Godin R, Danialou G, Chaturvedi R, Rygiel K, Matecki S, Jaber S, Des Rosiers C, Karpati G, Ferri L, et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med. 2012;186(11):1140–1149. doi: 10.1164/rccm.201206-0982OC. [DOI] [PubMed] [Google Scholar]
- 10.Laghi F, Shaikh H. Preventing ventilator-induced diaphragmatic dysfunction with phrenic nerve stimulation. Crit Care Med. 2014;42(2):492–494. doi: 10.1097/CCM.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 11.Tang H, Lee M, Budak MT, Pietras N, Hittinger S, Vu M, Khuong A, Hoang CD, Hussain SN, Levine S, Shrager JB. Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3-Bim axis. FASEB J. 2011;25(9):2921–2936. doi: 10.1096/fj.11-183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wohlgemuth SE, Lees HA, Marzetti E, Manini TM, Aranda JM, Daniels MJ, Pahor M, Perri MG, Leeuwenburgh C, Anton SD. An exploratory analysis of the effects of a weight loss plus exercise program on cellular quality control mechanisms in older overweight women. Rejuvenation Res. 2011;14(3):315–324. doi: 10.1089/rej.2010.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picca A, Fracasso F, Pesce V, Cantatore P, Joseph AM, Leeuwenburgh C, Gadaleta MN, Lezza AM. Age- and calorie restriction-related changes in rat brain mitochondrial DNA and TFAM binding. Age (Dordr) 2013;35(5):1607–1620. doi: 10.1007/s11357-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta D, Xu J, Dirain ML, Leeuwenburgh C. Calorie restriction combined with resveratrol induces autophagy and protects 26-month-old rat hearts from doxorubicin-induced toxicity. Free Radic Biol Med. 2014;74:252–262. doi: 10.1016/j.freeradbiomed.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5(1):51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2011;39(7):1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45(10):2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26(7):1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshihara T, Ichinoseki-Sekine N, Kakigi R, Tsuzuki T, Sugiura T, Powers SK, Naito H. Repeated exposure to heat stress results in a diaphragm phenotype that resists ventilator-induced diaphragm dysfunction. J Appl Physiol. 2015;119(9):1023–1031. doi: 10.1152/japplphysiol.00438.2015. [DOI] [PubMed] [Google Scholar]
- 21.Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, Danialou G, Matecki S, Jaber S, Petrof BJ, Goldberg P. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med. 2010;182(11):1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- 22.Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E, Blaauw B, Urciuolo A, Tiepolo T, Merlini L, Maraldi NM, Bernardi P, Sandri M, Bonaldo P. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med. 2010;16(11):1313–1320. doi: 10.1038/nm.2247. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Munoz-Canoves P. Autophagy maintains stemness by preventing senescence. Nature. 2016;529(7584):37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441(2):523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodson M, Darley-Usmar V, Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med. 2013;63:207–221. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zampieri S, Pietrangelo L, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Pond A, Grim-Stieger M, Cvecka J, Sedliak M, Tirpakova V, Mayr W, Sarabon N, Rossini K, Barberi L, et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J Gerontol A Biol Sci Med Sci. 2015;70(2):163–173. doi: 10.1093/gerona/glu006. [DOI] [PubMed] [Google Scholar]
- 27.Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008;410(3):525–534. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- 28.Haberzettl P, Hill BG. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013;1:56–64. doi: 10.1016/j.redox.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study could be available from the corresponding author on reasonable request.


