Abstract
BACKGROUND: Preoperative nutritional deficiency (ND) has been shown to be a valuable prognostic factor in urologic malignancies. We aimed to investigate the prognostic value of ND in patients with gastric cancer (GC). METHODS: A single-center cohort of 1026 GC patients undergoing curative resection between 2003 and 2012 was categorized to ND and nutritionally replete (NR) groups. Patients with body mass index <18.5 kg/m2, preoperative albumin <35 g/l, or preoperative weight loss ≥5% of body weight were defined as ND. RESULTS: Of the 1026 patients included in the study, 585 (57.0%) were categorized as ND. Overall survival (OS) at 5 years was 68.5% for ND patients and 44.0% for NR patients (P < .001). Multivariate analysis revealed that ND was a significant predictor of OS (hazard ratio: 1.954; 95% confidence interval: 1.552-2.460; P < .001). In stage-stratified analysis, it was still independently associated with OS in tumor-nodes-metastasis stage II and III (P = .004 and P < .001, respectively). Of note, the prognostic significance of ND was still maintained when stratified by age, sex, anemia, and adjuvant chemotherapy (all Ps < .05). CONCLUSION: Preoperative ND is a novel predictor of outcome in GC, especially in stage II to III GC, and may help clinicians identify high-risk patients for proactive nutritional interventions.
Introduction
Over the past decades, the incidence and mortality rate of gastric cancer (GC) have been steadily decreased. However, GC is still one of the most common malignancies nowadays, with a high incidence of recurrence and metastasis even after curative resection [1], [2]. In China, GC is the second leading cause of cancer death among both men and women in 2015 [3]. Despite advances in surgical techniques, the long-term postoperative survival of GC patients is still poor with the relatively late stage of diagnosis [4].
In many cancers, independent prognostic factors are useful for selecting high-risk patients and tailoring treatment. Currently, pathologic stage and lymph nodes status, which determine the GC American Joint Committee on Cancer stages, represent the gold standard for assessing GC prognosis after radical surgery [5]. However, many other tumor features have also been validated to play an important role in predicting the postoperative survival in GC. For example, systemic inflammatory response has been consistently recognized to confer poorer outcome in patients with various cancers and become the hot topic for clinicians and researchers [6], [7], [8]. Moreover, cancer-associated malnutrition is also increasingly appreciated to have a major role [9], [10]. Malnutrition, defined as a nutritional status in which there is a deficiency of energy, protein, and other nutrients, can negatively influence the defensive system in our body and may cause adverse clinical outcomes [11], [12]. However, up to now, no ideal indices exist to evaluate patients for preoperative nutritional risk [13]. In recent years, nutritional deficiency (ND), a nutritional-based index, has been demonstrated as a strong predictor of postoperative outcomes in urologic malignancies [14], [15]. With regard to GC, the clinical significance and prognostic value of this index remain uncertain.
In the present study, we aimed to investigate the prognostic utility of preoperative ND, as measured by body mass index (BMI), serum albumin, and preoperative weight loss, in patients undergoing curative resection for GC.
Material and Methods
Ethics Statement
The study complied with the standards of the Declaration of Helsinki and was approved by the Research Ethics Committee at the Cancer Center of Sun Yat-sen University. Written informed consent was obtained from each patient.
Study Population
This study reviewed 1026 patients undergoing curative resection for GC at Cancer Center of Sun Yat-sen University between January 2003 and December 2012. All patients were histologically confirmed as having stage I to III gastric adenocarcinoma, with stage determined according to the 7th edition of the American Joint Committee on Cancer tumor-nodes-metastasis (TNM) classification [5]. According to current guidelines, patients with stage II or stage III GC and no significant comorbidities precluding chemotherapy use were offered primarily 5-fluorouracil–based adjuvant chemotherapy after surgery [16], [17], [18]. The exclusion criteria were as follows: 1) no entire set of clinicopathological and laboratory data, 2) neoadjuvant chemotherapy or radiotherapy, 3) preoperative nutritional intervention (e.g., albumin) within 1 month before surgery, and 4) clinical evidence of non–cancer-related malnutrition.
Data Acquisition
Clinicopathological and outcome data were collected by review of the medical records. Routine laboratory measurements, including the serum levels of carcinoembryonic antigen (CEA) and albumin, were carried out within 1 week before surgery. Unintentional preoperative weight loss within 6 months was recorded at the time of diagnosis. Regarding the histological grade, patients with papillary and well- or moderately differentiated GC were categorized as the well-differentiated histology group, and those with undifferentiated, signet ring cell, and mucinous GC were categorized as the poorly differentiated histology group [19].
Nutritional-Based Indices
The BMI was calculated as previously described (<18.5 kg/m2, ≥18.5 to <25.0 kg/m2, ≥25.0 kg/m2) [20]. Based on previous studies, preoperative weight loss was divided into three groups: <5% weight loss, 5% to 10% weight loss and >10% weight loss [15].
Patients were categorized into two groups: nutritionally replete (NR) and ND. ND was defined as meeting one or more of the following criteria: BMI <18.5 kg/m2, preoperative albumin <35 g/l, or preoperative weight loss ≥5% of body weight [14], [15].
Follow-Up
Patients were routinely followed up every 3 months during the first 2 years and every 6 months thereafter, including the laboratory testing, dynamic abdominal computed tomography, and gastroscope examination. The latest follow-up was June, 2015. Overall survival (OS) was defined as the duration from the date of surgery until death or last follow-up.
Statistical Analysis
Comparisons between groups were performed using the χ2 test for categorical variables. Cumulative survival was estimated using the Kaplan-Meier method, and differences in survival rates between the groups were assessed by the log-rank test. Variables that proved to be significant (P < .05) in the univariate analysis and not significantly associated with others were subsequently entered into a multivariate Cox proportional hazards model. All statistical analyses were performed using the SPSS statistical software package, version 19.0 (IBM Corporation, Armonk, NY). A two-sided P value < .05 was considered significant.
Results
A total of 1026 GC patients were enrolled; 700 (68.2%) patients were males, and 326 (31.8%) were females. The median age was 59 years, with an age range from 19 to 89 years. A total of 177 patients were in stage I, 257 were in stage II, and 592 were in stage III (Table 1). Overall, 585 (57.0%) met the criteria for ND with one or more of the following: BMI <18.5 kg/m2 (39.1%), preoperative albumin <35 g/l (8.3%), and preoperative weight loss ≥5% (32.4%). One hundred seventy patients (16.6%) had 2 ND factors, and 28 patients (2.7%) had 3. The median follow-up period was 34 months (range 1-136). During the follow-up period, 397 (38.7%) patients died, and 629 (61.3%) were alive at last follow-up.
Table 1.
General Characteristics of 1026 GC Patients
| No. of Patients (%) | |
|---|---|
| Age (y) | |
| < 60 | 544 (53.0%) |
| ≥ 60 | 482 (47.0%) |
| Sex | |
| Female | 326 (31.8%) |
| Male | 700 (68.2%) |
| Tumor size (cm) | |
| <5 | 583 (56.8%) |
| ≥5 | 443 (43.2%) |
| Tumor location | |
| Upper third | 411 (40.1%) |
| Middle third | 202 (19.7%) |
| Lower third | 413 (40.3%) |
| Histological grade | |
| Well differentiated | 175 (17.1%) |
| Poorly differentiated | 851 (82.9%) |
| Anemia | |
| No | 737 (71.8%) |
| Yes | 289 (28.2%) |
| BMI (kg/m2) | |
| <18.5 | 401 (39.1%) |
| ≥18.5 to <25.0 | 351 (34.2%) |
| ≥25.0 | 274 (26.7%) |
| Serum albumin (g/l) | |
| ≥35 | 941 (91.7%) |
| <35 | 85 (8.3%) |
| Preoperative weight loss | |
| <5% | 694 (67.6%) |
| ≥5% to ≤10% | 221 (21.5%) |
| >10% | 111 (10.8%) |
| Nutrition status | |
| NR | 441 (43.0%) |
| ND | 585 (57.0%) |
| CEA | |
| Normal | 742 (77.6%) |
| Elevated | 214 (22.4%) |
| TNM stage | |
| I | 177 (17.3%) |
| II | 257 (25.0%) |
| III | 592 (57.7%) |
| Adjuvant chemotherapy | |
| No | 393 (38.3%) |
| Yes | 633 (61.7%) |
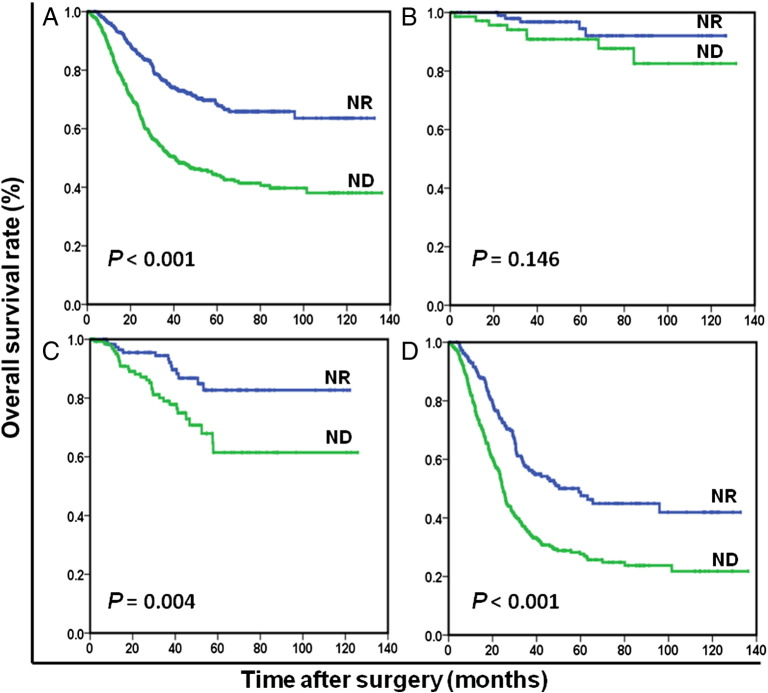
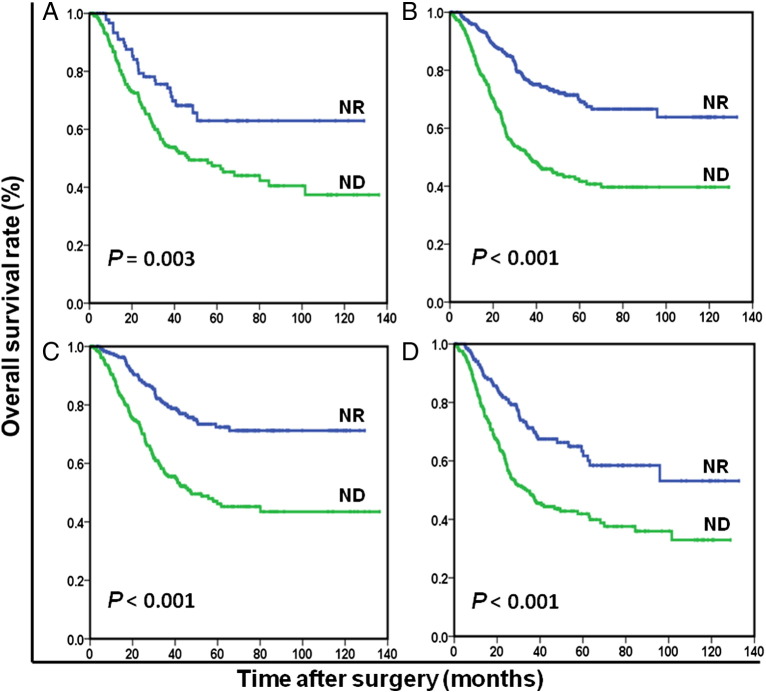
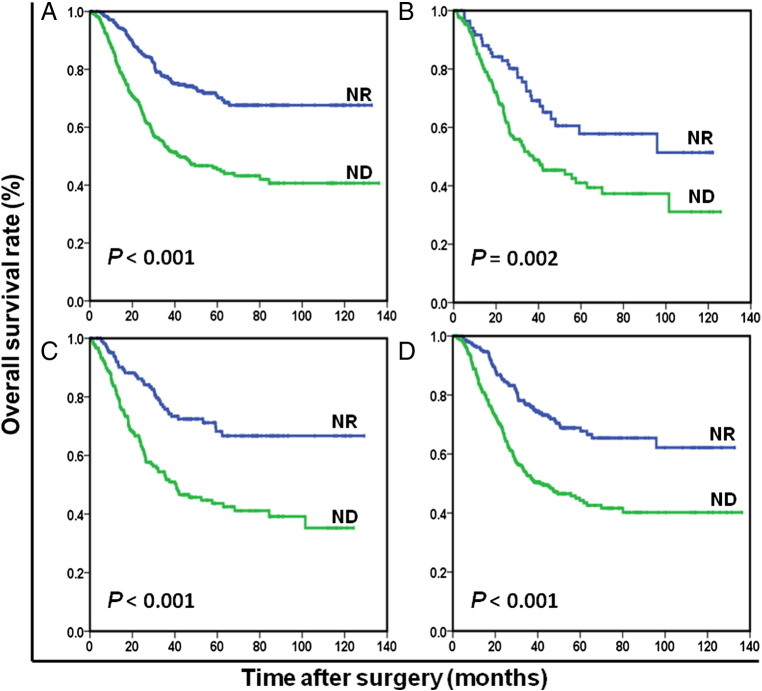
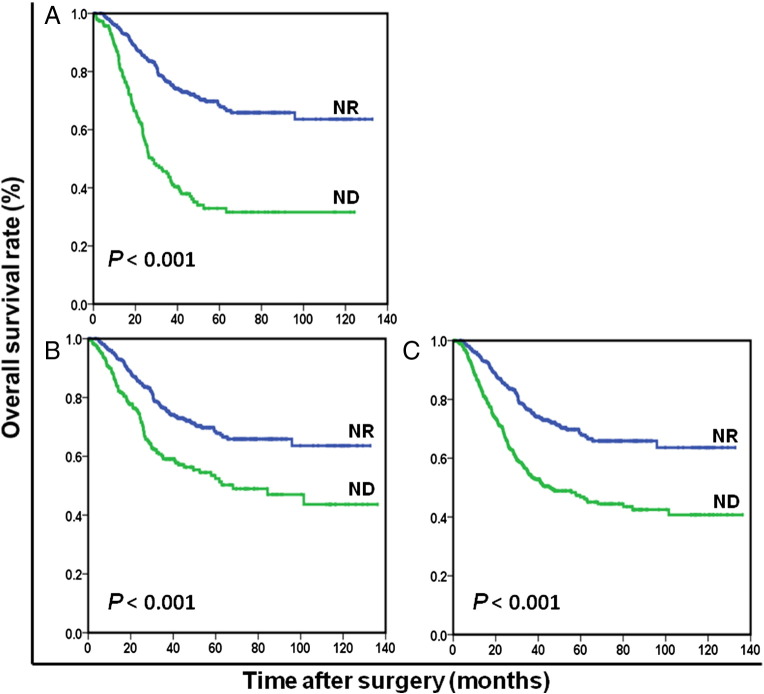
OS at 5 years was 68.5% for ND patients and 44.0% for NR patients (P < .001). The NR patients (96.8 months) had significantly longer mean survival compared with ND patients (68.8 months) (P < .001). The results of the univariate analysis were shown in Table 2. Because of correlations among the BMI, serum albumin, preoperative weight loss, and nutrition status, the variables (age, tumor size, tumor location, histological grade, anemia, nutrition status, CEA, and TNM stage) were tested in a multivariate analysis. Multivariate analysis revealed that ND was a strong predictor of OS (hazard ratio [HR]: 1.954; 95% confidence interval (CI): 1.552-2.460; P < .001; Table 2). In stage-stratified analysis, it was still independently associated with OS in TNM stage II and III (P = .004 and P < .001, respectively). However, its prognostic value was limited in TNM stage I (P = .146; Figure 1). Furthermore, the prognostic significance of ND was still maintained when stratified by age, sex, anemia, and adjuvant chemotherapy (all Ps < .05; Figures 2 and 3). It should be noted that ND was still associated with OS in patients with BMI ≥18.5 kg/m2, preoperative weight loss <5% of body weight, or serum albumin ≥35 g/l, respectively (all Ps < .001; Figure 4).
Table 2.
Univariate and Multivariate Analyses of OS in 1026 Patients Undergoing Curative Resection for GC
| Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age (y) | <.001 | <.001 | ||
| <60 | 1.00 | 1.00 | ||
| ≥60 | 1.462 (1.200-1.781) | 1.556 (1.259-1.923) | ||
| Sex | .188 | |||
| Female | 1.00 | |||
| Male | 0.870 (0.707-1.071) | |||
| Tumor size (cm) | <.001 | .705 | ||
| <5 | 1.00 | 1.00 | ||
| ≥5 | 1.915 (1.571-2.335) | 1.044 (0.837-1.302) | ||
| Tumor location | <.001 | .003 | ||
| Upper third | 1.00 | 1.00 | ||
| Middle third | 0.613 (0.467-0.804) | <.001 | 0.769 (0.578-1.025) | .073 |
| Lower third | 0.481 (0.384-0.602) | <.001 | 0.665 (0.522-0.847) | .001 |
| Histological grade | .014 | .015 | ||
| Well differentiated | 1.00 | 1.00 | ||
| Poorly differentiated | 1.441 (1.077-1.929) | 1.487 (1.081-2.046) | ||
| Anemia | .006 | .702 | ||
| No | 1.00 | 1.00 | ||
| Yes | 1.349 (1.092-1.667) | 1.045 (0.837-1.311) | ||
| BMI (kg/m2) | <.001 | |||
| <18.5 | 1.00 | |||
| ≥18.5 to <25.0 | 0.817 (0.654-1.022) | .077 | ||
| ≥25.0 | 0.593 (0.457-0.768) | <.001 | ||
| Serum albumin (g/l) | <.001 | |||
| <35 | 1.00 | |||
| ≥35 | 2.362 (1.767-3.156) | |||
| Preoperative weight loss | <.001 | |||
| <5% | 1.00 | |||
| ≥5% to ≤10% | 2.156 (1.722-2.698) | <.001 | ||
| >10% | 2.184 (1.635-2.918) | <.001 | ||
| Nutrition status | <.001 | <.001 | ||
| NR | 1.00 | 1.00 | ||
| ND | 2.326 (1.876-2.885) | 1.954 (1.552-2.460) | ||
| CEA | <.001 | .364 | ||
| Normal | 1.00 | 1.00 | ||
| Elevated | 1.665 (1.327-2.090) | 1.114 (0.883-1.405) | ||
| TNM stage | <.001 | <.001 | ||
| I | 1.00 | 1.00 | ||
| II | 2.970 (1.602-5.507) | <.001 | 2.429 (1.265-4.662) | .008 |
| III | 12.573 (7.219-21.896) | <.001 | 9.986 (5.537-18.008) | <.001 |
| Adjuvant chemotherapy | .799 | |||
| No | 1.00 | |||
| Yes | 0.974 (0.794-1.194) | |||
Figure 1.
Overall survival based on nutritional status in patients with stage I to III (A), stage I (B), stage II (C), and stage III (D) GC, respectively.
Figure 2.
Overall survival based on nutritional status in female patients (A), male patients (B), patient <60 years old (C), and patients ≥ 60 years old (D), respectively.
Figure 3.
Overall survival based on nutritional status in nonanemic patients (B), anemic patients (A), patients without adjuvant chemotherapy (C), and patients with adjuvant chemotherapy (D), respectively.
Figure 4.
Overall survival based on nutritional status in patients with BMI ≥18.5 kg/m2 (A), preoperative weight loss <5% of body weight (B), or serum albumin ≥35 g/l (C), respectively.
The relationship between nutrition status and clinicopathologic characteristics in GC patients was shown in Table 3. Our study showed that ND was associated with age ≥ 60 years (P = .030), female patients (P < .001), larger tumor size (P < .001), higher TNM stage (P < .001), the presence of preoperative anemia (P < .001), and elevated CEA (P = .006).
Table 3.
The Relationships between Nutrition Status and Clinicopathological Characteristics
| NR |
ND |
P Value |
|
|---|---|---|---|
| (n = 441) | (n = 585) | ||
| Age (y) | .030 | ||
| <60 | 251 | 293 | |
| ≥60 | 190 | 292 | |
| Sex | <.001 | ||
| Female | 94 | 232 | |
| Male | 347 | 353 | |
| Tumor size (cm) | <.001 | ||
| <5 | 294 | 289 | |
| ≥5 | 147 | 296 | |
| Tumor location | .103 | ||
| Upper third | 167 | 244 | |
| Middle third | 80 | 122 | |
| Lower third | 194 | 219 | |
| Histological grade | .332 | ||
| Well differentiated | 81 | 94 | |
| Poorly differentiated | 360 | 491 | |
| Anemia | <.001 | ||
| No | 355 | 382 | |
| Yes | 86 | 203 | |
| CEA | .006 | ||
| Normal | 346 | 396 | |
| Elevated | 77 | 137 | |
| TNM stage | <.001 | ||
| I | 104 | 73 | |
| II | 119 | 138 | |
| III | 218 | 374 | |
| Adjuvant chemotherapy | .690 | ||
| No | 172 | 221 | |
| Yes | 269 | 364 |
Discussion
Because of the adverse impact on physical, psychological, and social functions caused by cancer, malnutrition is common in patients with cancer, especially gastrointestinal malignancies [21], [22]. Estimated prevalence rates can range from 9% in urological cancer and up to 85% in pancreatic cancer [23]. Accumulating evidence has indicated that malnutrition is associated with a series of clinical consequences, including poor quality of life, decreased response to adjuvant treatment, increased risk of chemotherapy-induced toxicity, and poor outcome [24], [25]. Therefore, early identification of malnutrition is of vital importance among cancer patients, especially considering that effective nutritional intervention may have an important role in reducing postsurgical morbidity and mortality.
Over the past decades, a number of nutritional-based scores have been proposed for determining the prognostic impact of nutritional status in patients with various cancers. Recently, a prospective study from Minami al, which used pretreatment BMI to evaluate nutritional status, revealed that nutritional status might be a valuable prognostic indicator in older GC patients [26]. A study from our center provided concrete evidence that critical weight loss was a significant and independent predictor of long-term survival in nasopharyngeal carcinoma patients [27]. Lien et al reported that preoperative low serum albumin level was an independent factor correlated with prognosis and that postoperative adjuvant therapy should be given to all GC patients with hypoalbuminemia preoperatively [28]. Additionally, several composite scoring systems, including the Nutritional Risk Index, Nutritional Risk Score, and Geriatric Assessment, also failed to gain widespread consensus for nutrition evaluation in gastrointestinal malignancies [9], [29]. Thus, up to now, no standardized method exists to identify patients at a high nutritional risk preoperatively.
In recent years, a new nutritional-based score, which includes objective and easily measurable criteria, has been increasingly getting attention. ND, as measured by BMI, serum albumin, and preoperative weight loss, has been validated as a strong predictor of clinical outcome in urologic malignancies. One group found that ND was associated with increased 90-day mortality and poor OS in patients undergoing radical cystectomy for bladder cancer [14]. Another study reported that ND was a significant predictor of overall mortality in patients undergoing nephrectomy for renal cell carcinoma, independent of key clinicopathological factors [15]. In fact, our conclusions were in line with the studies.
In the current study, we found that preoperative ND was independently predictive of poor OS after curative resection for GC, especially in TNM stage II and III GC. In subgroup analyses, its prognostic significance was still maintained when stratified by age, sex, anemia, and adjuvant chemotherapy. Furthermore, we found that preoperative ND was associated with larger tumor size and higher TNM stage. The observation was supported by previous studies which indicated that poor nutritional status was significantly parallel to tumor progression [30]. Tokunaga et al recently demonstrated in a cohort of 556 patients with colorectal cancer undergoing surgery that poor nutritional status was correlated with tumors invading muscular or deeper layers, distant metastasis, tumor recurrence, and poor survival [31].
Of note, ND was still associated with OS in patients with BMI ≥18.5 kg/m2, preoperative weight loss <5% of body weight, or serum albumin ≥35 g/l, respectively. These data suggest, therefore, that ND may identify more patients at high risk of recurrence or mortality than the individual index. Obviously, ND, which includes all three variables, was a more comprehensive and superior predictor to evaluate nutritional status in GC. Furthermore, in stage-stratified analysis, ND may have merit as a gauge of prognosis in patients with GC at stage II and III. We speculated, in the context of stage I GC, that nutritional status did not exert potent prognostic value. Given that studies of Gregg and Morgan did not perform subgroup analysis to support our conclusion, it is worthy of being further verified in future studies [14], [15].
In clinical practice, ND patients may need closer follow-up and more aggressive adjuvant therapy. In addition, patients with ND may benefit from nutritional support [32], [33]. At present, although many studies have reported the promising results of targeted nutritional intervention in GC, no standardized method or strategy exists to select appropriate patients [34], [35]. Therefore, whether ND may aid in the selection of patients with stage II to III GC likely to benefit from nutritional intervention would be of considerable interest.
A potential limitation of the present study is that it was a retrospective single-center study. However, our study is based on a large and representative sample, which provides a valid base to evaluate the prognostic significance of nutritional status. In addition, postoperative treatment heterogeneity was inevitable because of the retrospective design, which might have confounded the results. Finally, we lack complete information on postoperative complications in our medical records. As a result, future studies are needed to further explore the relationship between ND and postoperative complications.
Conclusions
Our study showed that ND was a valuable independent predictor of outcome in patients undergoing curative resection for GC, especially in stage II to III GC. With our results in mind, we encourage prospective randomized multicenter trials determining the clinical utility of nutritional intervention in GC patients with ND.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
L. X. C. and X. P. F. contributed equally to this work; S. X. W. contributed to the conception and design of the study; Q. H. B., X. D. Z., and L. W. performed literature search and data extraction; L. Y. F., C. Y. B., and Z. Y. Q. performed quality assessment and statistical analyses; L. X. C. composed the first draft of the manuscript; X. P. F. and Z. Z. W. read and critically revised the manuscript.
Acknowledgements
The authors thank all of the people who helped with this study.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79(9–10):1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20(16):4483–4490. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 6.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 8.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi WJ, Kim J. Nutritional care of gastric cancer patients with clinical outcomes and complications: a review. 2016;5(2):65–78. doi: 10.7762/cnr.2016.5.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl. 2):S51–S63. doi: 10.1016/j.ejon.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Thoresen L, Frykholm G, Lydersen S, Ulveland H, Baracos V, Prado CM, Birdsell L, Falkmer U. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin Nutr. 2013;32(1):65–72. doi: 10.1016/j.clnu.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. 2012;31(3):345–350. doi: 10.1016/j.clnu.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Khan S, Alibay TA, Merad M, DiPalma M, Raynard B, Antoun S. [Detection and evaluation of malnutrition in oncology: what tools, what type of cancer and for what purposes?] Bull Cancer. 2016 doi: 10.1016/j.bulcan.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Gregg JR, Cookson MS, Phillips S, Salem S, Chang SS, Clark PE, Davis R, Stimson CJ, Jr, Aghazadeh M, Smith JA., Jr Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. 2011;185(1):90–96. doi: 10.1016/j.juro.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan TM, Tang D, Stratton KL, Barocas DA, Anderson CB, Gregg JR, Chang SS, Cookson MS, Herrell SD, Smith JA., Jr Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. 2011;59(6):923–928. doi: 10.1016/j.eururo.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72(1):37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303(17):1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 18.Oh DY, Bang YJ. Adjuvant and neoadjuvant therapy for gastric cancer. Curr Treat Options Oncol. 2013;14(3):311–320. doi: 10.1007/s11864-013-0238-4. [DOI] [PubMed] [Google Scholar]
- 19.Rausei S, Dionigi G, Boni L. Evaluation of the Seventh American Joint Committee on Cancer/International Union Against Cancer classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2011;117(12):2823–2824. doi: 10.1002/cncr.25801. [author reply 4] [DOI] [PubMed] [Google Scholar]
- 20.Chen HN, Chen XZ, Zhang WH, Yang K, Chen XL, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. The impact of body mass index on the surgical outcomes of patients with gastric cancer: a 10-year, single-institution cohort study. Medicine. 2015;94(42):e1769. doi: 10.1097/MD.0000000000001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafsson UO, Ljungqvist O. Perioperative nutritional management in digestive tract surgery. Curr Opin Clin Nutr Metab Care. 2011;14(5):504–509. doi: 10.1097/MCO.0b013e3283499ae1. [DOI] [PubMed] [Google Scholar]
- 22.Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252(2):325–329. doi: 10.1097/SLA.0b013e3181e9819a. [DOI] [PubMed] [Google Scholar]
- 23.von Meyenfeldt M. Cancer-associated malnutrition: an introduction. Eur J Oncol Nurs. 2005;9(Suppl. 2):S35–S38. doi: 10.1016/j.ejon.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Cho YS, Jung S, Kim H. Effect of nutritional risk at admission on the length of hospital stay and mortality in gastrointestinal cancer patients. Clin Nutr Res. 2013;2(1):12–18. doi: 10.7762/cnr.2013.2.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian J, Chen JS. Nutritional status and quality of life of the gastric cancer patients in Changle County of China. World J Gastroenterol. 2005;11(11):1582–1586. doi: 10.3748/wjg.v11.i11.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minami Y, Kawai M, Fujiya T, Suzuki M, Noguchi T, Yamanami H, Kakugawa Y, Nishino Y. Family history, body mass index and survival in Japanese patients with stomach cancer: a prospective study. Int J Cancer. 2015;136(2):411–424. doi: 10.1002/ijc.29001. [DOI] [PubMed] [Google Scholar]
- 27.Zeng Q, Shen LJ, Guo X, Guo XM, Qian CN, Wu PH. Critical weight loss predicts poor prognosis in nasopharyngeal carcinoma. BMC Cancer. 2016;16:169. doi: 10.1186/s12885-016-2214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lien YC, Hsieh CC, YC W, Hsu HS, Hsu WH, Wang LS, Huang MH, Huang BS. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8(8):1041–1048. doi: 10.1016/j.gassur.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 29.Ommundsen N, Wyller TB, Nesbakken A, Jordhoy MS, Bakka A, Skovlund E, Rostoft S. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014;19(12):1268–1275. doi: 10.1634/theoncologist.2014-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, Tanaka H, Muguruma K, Yashiro M, Maeda K. Predictive potential of preoperative nutritional status in long-term outcome projections for patients with gastric cancer. Ann Surg Oncol. 2016;23(2):525–533. doi: 10.1245/s10434-015-4814-7. [DOI] [PubMed] [Google Scholar]
- 31.Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, Watanabe M, Baba H. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum. 2015;58(11):1048–1057. doi: 10.1097/DCR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 32.Rosania R, Chiapponi C, Malfertheiner P, Venerito M. Nutrition in patients with gastric cancer: an update. Gastrointest Tumors. 2016;2(4):178–187. doi: 10.1159/000445188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meijerink WJ, von Meyenfeldt MF, Rouflart MM, Soeters PB. Efficacy of perioperative nutritional support. Lancet. 1992;340(8812):187–188. doi: 10.1016/0140-6736(92)93278-u. [DOI] [PubMed] [Google Scholar]
- 34.Gavazzi C, Colatruglio S, Valoriani F, Mazzaferro V, Sabbatini A, Biffi R, Mariani L, Miceli R. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: a multicentre randomised clinical trial. Eur J Cancer. 2016;64:107–112. doi: 10.1016/j.ejca.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 35.Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001;358(9292):1487–1492. doi: 10.1016/S0140-6736(01)06578-3. [DOI] [PubMed] [Google Scholar]