Abstract
Background
Klebsiella pneumoniae is a prominent nosocomial pathogen that accounts for up to 10 % of all hospital-acquired infections. It is a frequent cause of ventilator-associated pneumonia (VAP). The purpose of this study was to investigate the clinical characteristics of K. pneumoniae-associated VAP and the molecular characteristics of K. pneumoniae strains.
Methods
We retrospectively reviewed 70 mechanically ventilated patients with K. pneumoniae isolated. All K. pneumoniae strains were examined to determine hypermucoviscosity (HV) phenotype, capsular serotypes, virulence genes, multilocus sequence typing and antimicrobial susceptibility.
Results
Hypermucoviscosity was found in 14 of 70 (20 %) isolates of K. pneumoniae. Among the 70 patients, 43 cases (61.4 %) developed VAP. Furthermore, VAP was more frequently induced by HV-positive K. pneumoniae (14/14, 100 %) than by HV-negative strains (29/56, 51.7 %). HV-positive K. pneumoniae-associated VAP patients were more inclined to develop bacteremia and had a higher mortality rate than HV-negative strains VAP patients. Antibiotic resistance was more frequent in HV- negative strains- than in HV- positive strains-infected patients. The prevalence of rmpA and aerobactin genes were 85.7 % and 85.7 % respectively, and serotypes K1 and K2 accounted for 14.3 % and 28.6 % of the hypermucoviscosity strains, respectively. Strains carrying rmpA and aerobactin genes were significantly associated with HV-phenotype, and rmpA and aerobactin coexisted in HV-positive strains. Multilocus sequence typing analysis identified 24 different sequence types from K. pneumoniae VAP samples.
Conclusions
HV-phenotype is the major virulence determinant for mechanically ventilated patients. There was a specific sequence typing (ST) distribution between HV-positive and HV-negative strains.
Keywords: Klebsiella pneumoniae, Ventilator-associated pneumonia, Hypermucoviscosity, Virulence determinant
Background
Ventilator-associated pneumonia (VAP) is defined as nosocomial pneumonia occurring in a patient after 48 h of mechanical ventilation. The occurrence rate of VAP is reportedly 9–27 %, and mortality reaches 20–50 % [1–3]. Common causative pathogens of VAP include gram-negative bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli and gram-positive bacteria such as Staphylococcus aureus [4–9]. K. pneumoniae is a common pathogen responsible for both community-acquired and nosocomial infections [10]. It also causes miscellaneous infections such as meningitis, septicemia, purulent abscesses, and pneumonia. Previous investigations have implicated K. pneumoniae in 7–12 % of nosocomial pneumonia in intensive care units in the United States [11, 12].
It has been reported that the HV-positive phenotype, certain serotypes, and the presence of rmpA and aerobactin genes are virulence determinants in K. pneumoniae infection [13–17]. However, there have been few reports on the specific roles of these factors in VAP in mainland China. In the present study, we used isolates collected from mechanically ventilated patients to delineate the clinical characteristics of K. pneumoniae-associated VAP and molecular characteristics of K. pneumoniae strains observed over a 2-year period.
Methods
Hospital setting and study population
The Henan Provincial People’s Hospital is a 3900 bed tertiary care hospital with 6 ICU wards with an approximate annual admission of 1600 ICU inpatients. K. pneumoniae strains were collected via endotracheal aspiration from mechanically ventilated patients with suspected pneumonia and stored at −80 °C before use. Medical records of patients from whom the collected strains were isolated were reviewed between January 2012 and August 2014. VAP was diagnosed in these patients who fulfilled both the clinical and microbiological criteria. The clinical criteria for the diagnosis of VAP are the presence of a new pulmonary infiltration on chest radiography plus at least two of the following: fever above 38 °C, purulent secretions, and leukocytosis or leucopenia [18]. The microbiological criteria are quantitative tracheal aspirate culture with ≥105 CFU/mL and positive gram stain (>10 polymorphonuclear cells/low-power field and ≥1 bacteria/oil immersion field with or without intracellular bacteria) [19–21]. The VAP diagnosis was reconfirmed by two infectious diseases specialists independently. Polymicrobial infections were excluded from the analysis.
Data collection and microbiologic analysis
The following clinical information was collected for each patient: demographic characteristics, VAP diagnosis, reasons for mechanical ventilation, laboratory data, and chest radiograph reports. Bacteremic VAP was diagnosed when blood and respiratory samples yielded the same microorganism and other sources of infection that could account for the bacteremia were absent. Furthermore, blood and respiratory cultures were performed within 48 h.
Outcome was defined as in-hospital mortality measured 30 days after the onset of VAP. Trauma was defined as the presence of injury in more than one body area or system or the presence of major cranial trauma alone. Chronic lung diseases included bronchiectasis, chronic obstructive pulmonary disease. The initial laboratory value was defined as that measured within 48 h of the onset of VAP.
The BD Phoenix system (Becton Dickinson, USA) was used to confirm bacterial identification. Antibiotic susceptibility was tested with the disk diffusion method, and interpretations were made according to the guidelines of the Clinical and Laboratory Standards Institute [22]. All of the K. pneumoniae isolates were screened and confirmed by a double-disk synergy test for produced extended-spectrum β-lactamase (ESBL).
Detection of HV-phenotype
For HV-phenotype determination, a standard bacteriologic loop was used to stretch a mucoviscous string vertically from a colony. The formation of a viscous string of >5 mm confirmed the HV-positive phenotype.
Serotyping, rmpA and aerobactin gene detection with PCR
PCR was performed to amplify genes specific for serotypes K1/K2 and the rmpA and aerobactin genes as described previously [17, 23]. K. pneumoniae ATCC9997 (K2) was used as a control strain. A bacterial colony from an overnight culture was added to 500 μL water and boiled for 15 min to release the DNA template. PCR was performed with the following conditions: 95 °C initial denaturation for 5 min followed by 30 cycles at 95 °C for 30 s, 55 °C for 30 s, 72 °C for 90 s, and a final extension at 72 °C for 5 min.
Multilocus sequence typing (MLST)
MLST was performed to determine the diversity of clinical K. pneumoniae in patients with VAP. Seven housekeeping genes (rpoB, gapA, mdh, pgi, phoE, infB, and tonB) were amplified via PCR with conditions and primers designated by the Pasteur Institute Klebsiella pneumonia MLST Database (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
Statistical analysis
SPSS 17.0 was used for statistical analysis. The chi-square or Fisher’s exact test was used to analyze contingency data, and continuous data were analyzed with the Student’s t test. A P value of <0.05 was considered significant, and all probabilities were two-tailed.
Results
Clinical characteristics of K. pneumoniae-associated VAP patients
The retrospective study was conducted in 70 mechanically ventilated patients with K. pneumoniae isolated. Among these patients, 43 cases (61.4 %) developed VAP during their ICU stay. The patients had mean ± standard deviation age of 57.0 ± 15.0 years. Thirty-two (74.4 %) were male and 11(25.6 %) were female. Fourteen (32.5 %) HV-positive strains and 29 (67.4 %) HV-negative strains were isolated from the 43 VAP patients. None of the VAP patients had concurrent live abscess or other metastatic infection. All VAP patients received concordant antibiotic treatment according to the results of susceptibility testing. The clinical characteristics of HV-positive and -negative K. pneumoniae VAP patients are compared in Table 1. Compared with patients with HV-negative K. pneumoniae, those with HV-positive strains had a significantly higher prevalence of cardiac-cerebrovascular disease (55.1 % vs 85.7 %, respectively, P = 0.049), a significantly higher prevalence of bacteremic K. pneumoniae (3.4 % vs 35.7 %, respectively, P = 0.017) and a significantly higher mortality rate (57.1 % vs 13.8 %, respectively, P = 0.009). The laboratory data of the K. pneumoniae VAP patients is presented in Table 2. Compared with patients with HV-negative K. pneumoniae, those with HV-positive strains had a significantly higher C-reactive protein (CRP) levels (100.16 ± 77.62 vs 156 ± 53.89, respectively, P = 0.03), lower albumin levels (34.007 ± 6.49 vs 29.68 ± 5.31, respectively, P = 0.038).
Table 1.
Relationship between hypermucoviscosity phenotype of K. pneumoniae and clinical characteristics
| Hypermucoviscosity | |||
|---|---|---|---|
| Characteristic | Positive | Negative | P value |
| n = 14 | n = 29 | ||
| No. (%) | No. (%) | ||
| Age mean ± SD | 64 ± 14 | 53 ± 15 | 0.613 |
| Male sex | 11 (78.6) | 21 (72.4) | 0.665 |
| Underlying disease | |||
| Diabetes mellitus | 3 (21.4) | 2 (6.8) | 0.376 |
| Malignancy | 1 (7.1) | 1 (3.4) | 1.000 |
| Neurologic disorders | 6 (42.9) | 13 (44.8) | 0.903 |
| Trauma | 1 (7.1) | 5 (17.2) | 0.670 |
| Chronic lung disease | 1 (7.1) | 1 (3.4) | 1.000 |
| Cardiac-cerebrovascular disease | 12 (85.7) | 16 (55.1) | 0.049* |
| ICU stay, days | 14 ± 6 | 13 ± 5 | 0.452 |
| Mechanical ventilation, days | 8 ± 4 | 6 ± 3 | 0.141 |
| Bacteremia | 5(35.7) | 1 (3.4) | 0.017* |
| Mortality | 8 (57.1) | 4 (13.8) | 0.009* |
Values given as means ± SD or No. (%) of patients
* P < 0.05 was considered to be statistically significant
Table 2.
Relationship between hypermucoviscosity phenotype of K. pneumoniae and laboratory data
| Hypermucoviscosity | |||
|---|---|---|---|
| Characteristic | Positive | Negative | P value |
| n = 14 | n = 29 | ||
| No. (%) | No. (%) | ||
| Chest radiography | |||
| Unilateral involvement | 3 (21.7) | 6 (20.6) | 1.000 |
| Bilateral involvement | 11 (78.6) | 23 (79.3) | 1.000 |
| Initial laboratory value | |||
| Leukocyte count, ×109/l | 14.56 ± 7.21 | 15.65 ± 6.21 | 0.613 |
| Platelet, ×109/l | 139.71 ± 82.27 | 192.10 ± 130.13 | 0.177 |
| Albumin, g/l | 29.68 ± 5.31 | 34.007 ± 6.49 | 0.038* |
| C-reactive protein, mg/l | 156.79 ± 53.89 | 100.16 ± 77.62 | 0.03* |
| Glucose, mmol/l | 9.85 ± 3.92 | 8.12 ± 2.94 | 0.115 |
Values given as No. (%) of patients
* P < 0.05 was considered to be statistically significant
Microbiological characteristics of K. pneumoniae
Among 70 K. pneumoniae isolates analyzed, 14 (20 %) of them were found to be HV-positive strains and 56 (80 %) of them were HV-negative strains. We further observed that the frequency of VAP was significantly higher in patients with HV-positive strains (100 %; 14/14) than in patients with HV-negative strains (51.8 %; 29/56). The results showed that HV-phenotype was highly correlated with the presence of the rmpA and aerobactin genes. Of 14 HV-positive isolates, 85.7 % (12/14) were rmpA and aerobactin positive, and 14.3 % (2/14) were rmpA and aerobactin negative. None of HV-negative isolates was rmpA or aerobactin positive. Serotypes K1 and K2 accounted for 14.3 % (2/14) and 28.6 % (4/14), respectively, of the HV-positive isolates. Serotypes K1 and K2 were not found among HV-negative isolates. All 12 rmpA-positive isolates carried the aerobactin gene. All K1/K2 isolates (n = 6) were positive for HV-phenotype and the rmpA and aerobactin genes (Table 3).
Table 3.
Microbiological characteristics of K. pneumoniae from mechanically ventilated patients
| Variable | No. of isolates n = 70 | Hypermucoviscosity | P value | |
|---|---|---|---|---|
| Positive | Negative | |||
| n = 14 | n = 56 | |||
| No. (%) | No. (%) | |||
| Capsular serotype | ||||
| K1 | 2 | 2 (14.3) | 0 (0) | 0.038* |
| K2 | 4 | 4 (28.6) | 0 (0) | 0.001* |
| Virulence factors | ||||
| rmpA | 12 | 12 (85.7) | 0 (0) | <0.001* |
| aerobactin | 12 | 12 (85.7) | 0 (0) | <0.001* |
| VAP due to K.pneumoniae | 43 | 14 (100) | 29 (51.7) | 0.001* |
Values given as No. (%) of patients
* P < 0.05 was considered to be statistically significant
Antimicrobial susceptibility test
The prevalence of HV-negative isolates exhibiting resistance to the tested antimicrobials was higher than that of the HV-positive isolates (Table 4). The detection rates of ESBL-producing K. pneumoniae isolates were 46.5 % (20/43). The percentage of ESBL-producing HV-negative isolates was significantly higher than that of ESBL-producing HV-positive isolates (62.1 % compared to 14.3 %, P < 0.05). Among all of the isolates, one of them (1/43, 2.3 %) was resistant to imipenem and meropenem.
Table 4.
Difference of the antimicrobial susceptibility between HV-positive and- negative of K. pneumoniae
| No. (%) of patients susceptible | |||
|---|---|---|---|
| Antimicrobial agent | HV-Positive isolates | HV-Negative isolates | P value |
| n = 14 | n = 29 | ||
| piperacillin | 8 (57.1) | 9 (31.0) | 0.101 |
| Cefazolin | 5 (35.7) | 10 (34.4) | 1.000 |
| Cefoxitin | 10 (71.4) | 14 (48.3) | 0.152 |
| Cefuroxime | 11 (78.6) | 11(37.9) | 0.012* |
| Ceftriaxone | 12 (85.7) | 12 (41.4) | 0.006* |
| Ceftazidime | 12 (85.7) | 12 (41.4) | 0.006* |
| Cefotaxime | 12 (85.7) | 13 (44.8) | 0.011* |
| Cefepime | 13 (92.9) | 23 (79.3) | 0.492 |
| Imipenem | 14 (100) | 28 (96.6) | 1.000 |
| Meropenem | 14 (100) | 28 (96.6) | 1.000 |
| Ciprofloxacin | 12 (85.7) | 15 (51.7) | 0.031* |
| Levofloxacin | 12 (85.7) | 16 (55.2) | 0.104 |
| Gentamicin | 14 (100) | 17(58.6) | 0.013* |
| Amikacin | 14 (100) | 18 (62.1) | 0.022* |
| Trimethoprim-sulfamethoxazole | 12 (85.7) | 17 (58.6) | 0.013* |
| ESBLs | 2 (14.3) | 18 (62.1) | 0.003* |
Values given as No. (%) of patients
ESBL, extended-spectrum β-lactamase
* P < 0.05 was considered to be statistically significant
MLST profiles of isolates from patients with VAP
Fourteen HV-positive strains and 29 HV-negative strains were isolated from VAP patients. Eleven MLSTs—including sequence types (STs) 23, 36, 65, 86, 218, 225, 374, 412, 499, 592, and 660—were identified among the HV-positive strains. Two major MLST groups, ST218-like and ST23-like, were obtained based on minimum-spanning tree analysis (Fig. 1a). Two serotype K1 isolates belonged to ST23. In addition, three MLSTs—STs 374, 86, and 65—were identified among serotype K2 isolates. All of the remaining HV-positive strains except STs 225 and 499 were positive for rmpA and aerobactin. On the contrary, 13 MLSTs—including STs 1, 11, 15, 17, 35, 37, 107, 147, 515, 716, 988, 1269, and 1419—were identified among HV-negative isolates. Minimum-spanning tree analysis showed that all of these isolates belonged to two major MLST groups, ST1419-like and ST515-like (Fig. 1b). ST11 (n = 7) and ST15 (n = 7) were the prevalent STs in HV-negative isolates.
Fig. 1.
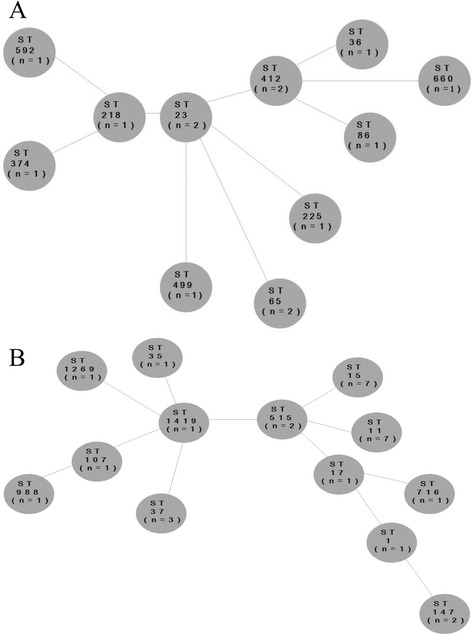
Minimum-spanning tree of 14 isolates of hypermucoviscosity-positive strains (a) and 29 hypermucoviscosity-negative strains (b) generated with multilocus sequence typing (MLST) allelic data (analysis at MLST website)
Discussion
In the retrospective study, we analyzed 70 isolates of K. pneumoniae from mechanically ventilated patients between January 2012 and August 2014. We observed that HV-positive strains accounted for 20 % (14/70) K. pneumoniae isolates. The genotypes of K1, K2, rmpA and aerobactin were only positive for HV-positive strains. The medical records showed that 43 strains accounted for 61.4 % (43/70) induced VAP in mechanically ventilated patients. There was difference in ST distribution between HV-positive and HV-negative stains in VAP patients.
We further observed that the occurrence rate of VAP was significantly higher for HV-positive strains infection than HV-negative strains infection (100 % vs 51.7 %, P < 0.05). Furthermore, the results showed that bacteremic VAP occurred more frequently in HV-positive strains than in HV-negative strains (35.7 % vs 3.4 %, respectively, P = 0.017). The mortality rate was higher in the HV-positive group (57.1 %) than in the HV-negative group (13.8 %). Laboratory data also showed that HV-positive strains VAP patients had increased frequency of higher C-reactive protein (CRP) and lower albumin levels. CRP is an acute-phase protein that has been evaluated in the critical care setting [24]. Moreover, CRP values correlated well with the severity of the infection. Hillas et al. [25] observed that a rise in CRP between days 1 and 7 increases the risk of septic shock. Albumin level reportedly decreases as part of negative acute-phase protein. Previous studies have indicated that the degree of hypoalbuminemia in critically ill patients correlates with the intensity of the inflammatory response caused by infection [26, 27]. These findings indicated that compared with HV-negative strains, HV-positive strains are more virulent in the development of VAP.
Capsular serotypes, especially serotypes K1 and K2, are important virulence factors for K. pneumoniae [28, 29]. Yu et al. reported that the capsular serotypes K1 and K2 have particular virulence and were more common in patients with community-acquired pneumonia (23/49 isolates, 47 %) than in those with hospital-acquired pneumonia (2/18 isolates, 11 %) [30]. Our results were similar to the study. We also found that capsular serotypes K1 and K2 comprised only 4.7 % (2/43) and 9.3 % (4/43), respectively, of all K. pneumoniae isolated from VAP patients. In addition, the HV-positive strains accounted for 32.6 % (14/43) K. pneumoniae in VAP patients. These results suggest that hypermucoviscosity rather than serotype K1 or K2 has become a common pathogen of VAP in mechanically ventilated patients.
Previous study has shown that K1 serotype and rmpA are associated with HV-phenotype in K. pneumoniae [17]. In this study, we found that HV-phenotype frequently coexists with the rmpA gene. In addition, the prevalence of the K1 serotype in HV-positive strains was only 14.3 % (2/14). Chen et al. further confirmed that the rmpA gene is a regulator of HV-phenotype [31]. Loss of this gene leads to the loss of HV-phenotype. These data showed that the rmpA gene rather than the K1 serotype correlated with HV-phenotype in K. pneumoniae. Meanwhile, the results also showed that two HV-positive strains which did not possessed rmpA gene. This indicated that there may be other regulatory mechanisms for expression of HV-phenotype.
In addition, we found that rmpA gene coexists with the aerobactin gene in HV-positive K. pneumoniae. This result was in line with the observations of Yu et al. [30]. Further investigation showed that rmpA is located on a 180-kb virulence plasmid, which also contains many virulence-associated genes, including aerobactin for iron acquisition [15, 32]. However, we did not determine which of the two virulence factors (rmpA or aerobactin) of HV-positive strains is more critical in VAP.
Among these VAP patients, the HV-positive isolates were significantly more susceptible to the antimicrobial agents that were tested, when compared with the HV-negative isolates. However, we found two ESBL-producing HV-positive isolates. Previous study also showed that most HV-positive strains were very susceptible to antimicrobials [33]. Nonetheless, some cases of infection due to multidrug resistant HV-positive K. pneumoniae have already been described [34]. Therefore, management of VAP due to HV-positive isolates will become extremely challenging.
In the present study, 24 STs were observed in the 43 K. pneumoniae isolates from VAP patients. Eleven MLSTs were identified in 14 HV-positive strains, suggesting a polyclonal origin. Thirteen STs were observed in 29 HV-negative strains, among which ST11 (n = 7) and ST15 (n = 7) were the most prevalent. We noticed that a specific ST distribution occurred between HV-positive and HV-negative strains, suggesting the difference genetic background existed among the 2 phenotype isolates.
This study had several limitations. First, it was a retrospective study from a single hospital and the small sample size may have had selection bias. Second, the diagnosis of VAP in mechanically ventilated patients is difficult, and still there is no “gold-standard” diagnostic method. Third, the pathogenic mechanism of HV-positive K. pneumoniae in VAP is unclear and requires further investigation in future studies.
Conclusion
We have shown that HV-phenotype, rather than serotype K1 or K2, was the major virulence determinant for mechanically ventilated patients. Patients infected with HV-positive strains were more likely to develop VAP and bacteremic VAP. Furthermore, HV-positive K. pneumoniae VAP had a higher mortality than HV-negative strains VAP. We hope our results will draw attention from physicians, which may lead to prompt recognition and successful management of HV-positive K. pneumoniae VAP.
Acknowledgments
No one other the authors contributed substantially to the study or the manuscript.
Funding
This study was supported by the Henan Province Medical Science and Technique Foundation (No. 201203094), the Joint Funds of National Natural Science Foundation of China (No. U1304804).
Availability of data and materials
The dataset supporting the conclusion of this article is included within the article.
Authors’ contributions
SG and JJX conceived the study, and participated in its design and coordination. SG, JJX, YSW performed the experiments. JJX, SG, YSW and JHX reviewed the medical records. SG, JJX, RX and YL analyzed and interpreted the data. JJX, RX and YL drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
No applicable.
Ethics approval and consent to participate
The study protocol was reviewed and approved by the the Henan Provincial People’s Hospital Medical Ethics Committee. The identities of patients and their data remained anonymous. A written informed consent was not required, because the study was retrospective, presents no more than minimal risk of harm to participants and involves no procedure.
Abbreviations
- ESBL
Extended-spectrum β-lactamase
- HV
Hypermucoviscosity
- ICU
Intensive care units
- MLST
Multilocus sequence typing
- VAP
Ventilator-associated pneumonia.
Contributor Information
Yi Li, Email: hnssmyy@163.com.
Rui Xue, Email: xuerui04301617@126.com.
References
- 1.Cunnion KM, Weber DJ, Broadhead WE, Hanson LC, Pieper CF, Rutala WA. Risk factors for nosocomial pneumonia: comparing adult critical-care populations. Am J Respir Crit Care Med. 1996;153(1):158–62. doi: 10.1164/ajrccm.153.1.8542110. [DOI] [PubMed] [Google Scholar]
- 2.Kirschenbaum L, Azzi E, Sfeir T, Tietjen P, Astiz M. Effect of continuous lateral rotational therapy on the prevalence of ventilator-associated pneumonia in patients requiring long-term ventilator care. Crit Care Med. 2002;30(9):1983–6. doi: 10.1097/00003246-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Baker AM, Meredith JW, Haponik EF. Pneumonia in intubated trauma patients. Microbiology and outcomes. Am J Respir Crit Care Med. 1996;153(1):343–9. doi: 10.1164/ajrccm.153.1.8542141. [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274(8):639–44. doi: 10.1001/jama.1995.03530080055041. [DOI] [PubMed] [Google Scholar]
- 5.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 6.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(Suppl 1):S81–7. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 7.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21(8):510–5. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 8.Alcón A, Fàbregas N, Torres A. Hospital-acquired pneumonia: etiologic considerations. Infect Dis Clin North Am. 2003;17(4):679–95. doi: 10.1016/S0891-5520(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 9.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(6):3854–62. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 10.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996; 24(5):380–8 [PubMed]
- 12.National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1997, issued May 1997. A report from the NNIS system. Am J Infect Control. 1997; 25(6):477–87 [PubMed]
- 13.Wiskur BJ, Hunt JJ, Callegan MC. Hypermucoviscosity as a virulence factor in experimental Klebsiella pneumonia endophthalmitis. Invest Ophthalmol Vis Sci. 2008;49(11):4931–8. doi: 10.1167/iovs.08-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YT, Chen TL, Siu LK, Hsu SF, Fung CP. Clinical and microbiological characteristics of community-acquired thoracic empyema or complicated parapneumonic effusion caused by Klebsiella pneumonia in Taiwan. Eur J Clin Microbiol Infect Dis. 2010;29(8):1003–10. doi: 10.1007/s10096-010-0961-8. [DOI] [PubMed] [Google Scholar]
- 15.Nassif X, Fournier JM, Arondel J, Sansonetti PJ. Mucoid phenotype of Klebsiella pneumonia is a plasmid-encoded virulence factor. Infect Immun. 1989;57(2):546–52. doi: 10.1128/iai.57.2.546-552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS. Endogenous bacterial endophthalmitis: report of a ten-year retrospective study. Ophthalmology. 1994;101(5):832–8. doi: 10.1016/S0161-6420(13)31255-X. [DOI] [PubMed] [Google Scholar]
- 17.Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. Comparison of prevalence of virulence factors for Klebsiella pneumonia liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotype. Diagn Microbiol Infect Dis. 2008;62(1):1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. [DOI] [PubMed]
- 19.Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19(4):637–57. doi: 10.1128/CMR.00051-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porzecanski I, Bowton DL. Diagnosis and treatment of ventilator- associated pneumonia. Chest. 2006;130(2):597–604. doi: 10.1378/chest.130.2.597. [DOI] [PubMed] [Google Scholar]
- 21.Wu CL, Yang DI, Wang NY, Kuo HT, Chen PZ. Quantitative culture of endotracheal aspirates in the diagnosis of ventilator-associated pneumonia in patients with treatment failure. Chest. 2002;122(2):662–8. doi: 10.1378/chest.122.2.662. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standard Institute . Performance Standards for antimicrobial disk susceptibility tests approved standard. Wayne: CLSI document; 2012. pp. M2–49. [Google Scholar]
- 23.Turton JF, Baklan H, Siu LK, Kaufmann ME, Pitt TL. Evaluation of a multiplex PCR for detection of serotypes K1, K2 and K5 in Klebsiella sp. and comparison of isolates within these serotypes. FEMS Microbiol Lett. 2008;284(2):247–52. doi: 10.1111/j.1574-6968.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 24.Póvoa P. C-reactive protein: a valuable marker of sepsis. Intensive Care Med. 2002;28(3):235–43. doi: 10.1007/s00134-002-1209-6. [DOI] [PubMed] [Google Scholar]
- 25.Hillas G, Vassilakopoulos T, Plantza P, Rasidakis A, Bakakos P. C-reactive protein and procalcitonin as predictors of survival and septic shock in ventilator-associated pneumonia. Eur Respir J. 2010;35(4):805–11. doi: 10.1183/09031936.00051309. [DOI] [PubMed] [Google Scholar]
- 26.Al-subaie N, Reynolds T, Myers A, Sunderland R, Rhodes A, Grounds RM, et al. C-reactive protein as a predictor of outcome after discharge from the intensive care: a prospective observational study. Br J Anaesth. 2010;105(3):318–25. doi: 10.1093/bja/aeq171. [DOI] [PubMed] [Google Scholar]
- 27.Domínguez de Villota E, Mosquera JM, Rubio JJ, Galdos P, Díez Balda V, de la Serna JL, et al. Association of a low serum albumin with infection and increased mortality in critically ill patients. Intensive Care Med. 1980;7(1):19–22. doi: 10.1007/BF01692917. [DOI] [PubMed] [Google Scholar]
- 28.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. K. pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45(3):284–93. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 29.Lin JC, Ko WC, Lee N, Fung CP, Chang FY, Tsai YK, et al. Genotypes and virulence in serotype K2 K. pneumoniae from liver abscess and non-infectious carriers in Hong Kong, Singapore and Taiwan. Gut Pathog. 2014;6:21. doi: 10.1186/1757-4749-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, von Gottberg A, et al. Virulence Characteristics of Klebsiella and Clinical Manifestations of K. pneumoniae Bloodstream infections. Emerg Infect Dis. 2007;13(7):986–93. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng HY, Chen YS, Wu CY, Chang HY, Lai YC, Peng HL. RmpA regulation of capsular polysaccharide biodsynthesis in Klebsiella pneumonia CG43. J Bacteriol. 2010;192(12):3144–58. doi: 10.1128/JB.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YT, Chang HY, Lai YC, Pan CC, Tsai SF, Peng HL. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumonia CG43. Gene. 2004;337:189–98. doi: 10.1016/j.gene.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Liu YM, Li BB, Zhang YY, Zhang W, Shen H, Li H, Cao B. Clinical and molecular characteristics of emerging hypervirulent klebsiella pneumoniae bloodstream infections in mainland China. Antimicrob Agents Chemother. 2014;58(9):5379–85. doi: 10.1128/AAC.02523-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng NC, Yu YC, Tai HC, Hsueh PR, Chang SC, Lai SY, et al. Recent trend of necrotizing fasciitis in TaiWan: focus on monomicrobial Klebsiella pneumoniae necrotizing fasciitis. Clin Infect Dis. 2012;55(7):930–9. doi: 10.1093/cid/cis565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusion of this article is included within the article.


