Abstract
Background
In Escherichia coli, nagD, yrfG, yjjG, yieH, yigL, surE, and yfbR encode 5′-nucleotidases that hydrolyze the phosphate group of 5′-nucleotides. In Bacillus subtilis, genes encoding 5′-nucleotidase have remained to be identified.
Results
We found that B. subtilis ycsE, araL, yutF, ysaA, and yqeG show suggestive similarities to nagD. Here, we expressed them in E. coli to purify the respective His6-tagged proteins. YcsE exhibited significant 5′-nucleotidase activity with a broader specificity, whereas the other four enzymes had rather weak but suggestive activities with various capacities and substrate specificities. In contrast, B. subtilis yktC shares high similarity with E. coli suhB encoding an inositol monophosphatase. YktC exhibited inositol monophosphatase activity as well as 5′-nucleotidase activity preferential for GMP and IMP. The ycsE, yktC, and yqeG genes are induced by oxidative stress and were dispensable, although yqeG was required to maintain normal growth on solid medium. In the presence of diamide, only mutants lacking yktC exhibited enhanced growth defects, whereas the other mutants without ycsE or yqeG did not.
Conclusions
Accordingly, in B. subtilis, at least YcsE and YktC acted as major 5′-nucleotidases and the four minor enzymes might function when the intracellular concentrations of substrates are sufficiently high. In addition, YktC is involved in resistance to oxidative stress caused by diamide, while YqeG is necessary for normal colony formation on solid medium.
Electronic supplementary material
The online version of this article (doi:10.1186/s12866-016-0866-5) contains supplementary material, which is available to authorized users.
Keywords: Bacillus subtilis, Haloacid dehalogenase superfamily, Inositol monophosphatase, Inositol phosphate, Nucleoside/nucleotide metabolism, 5′-nucleotidase, Oxidative stress, Phosphatase, Protein motif
Background
The pool sizes of nucleotides and nucleosides are balanced to enable the efficient synthesis of DNA and RNA [1]. Numerous enzymes involved in the biosynthesis and catabolism of nucleic acids are controlled to regulate the appropriate pool size of each compound. For example, 5′-nucleotidases hydrolyze 5′-nucleotides to generate nucleosides and inorganic phosphate, which participate in the regulatory mechanism that opposes the generation of nucleotides with the phosphorylation of nucleosides catalyzed by kinases.
5′-Nucleotidases are ubiquitous among species and reside in different subcellular locations. Extracellular 5′-nucleotidases are produced by certain bacteria. For example, Vibrio parahaemolyticus NutA is a 5′-nucleotidase bound to the membrane by a lipid anchor [2]. Escherichia coli UshA is a periplasmic enzyme with 5′-nucleotidase and UDP-sugar hydrolase activities [3]. These 5′-nucleotidases degrade extracellular nucleotides to satisfy the cell’s nutritional requirements.
In contrast, bacteria produce numerous intracellular 5′-nucleotidases that belong to various enzyme families. In E. coli, the substrates of NagD are UMP, GMP, AMP, and CMP [4]. NagD belongs to the haloacid dehalogenase superfamily (HADSF) characterized by a specific protein motif [5]. The HADSF family comprises numerous proteins in organisms ranging from prokaryotes to higher eukaryotes, including humans. The vast majority of enzymes of the HADSF family are phosphoryl transferases, although the superfamily was named after 2-haloacid dehalogenase, because it is the first structurally characterized member. When the amino acid sequences of their entire coding regions are compared, similarities among the HADSF members are not usually very high (15–30 % identical), although their central regions involved in catalytic activity are relatively conserved. Further, correlation between structure and catalytic activity is frequently observed and interrelates with similarities among the structures of substrates [6–8]. E. coli produces other HADSF-family enzymes such as YrfG, YjjG, YieH, and YigL, and each exhibits 5′-nucleotide phosphatase activity [9, 10].
In addition, E. coli produces SurE and YfbR, which exhibit 5′-nucleotidase activity and do not belong to the HADSF family [10]. SurE shows broad substrate specificity, by dephosphorylating 5′-nucleotides as well as 3′-nucleotides, with highest affinity for 3′-AMP. SurE hydrolyzes polyphosphate with preference for short-chain substrates. Homologs of E. coli surE are present in numerous eubacteria and archaea, and SurE represents a family of metal-dependent phosphatases [11]. YfbR belongs to the HD domain superfamily of metal-dependent phosphatases and phosphodiesterases, and the HD domain was named after a protein motif with predicted catalytic residues containing the conserved doublet His–Asp [12]. YfbR specifically hydrolyzes 5′-deoxyribonucleotides.
Inositol monophosphatase denotes phosphatases that liberate inorganic phosphate from myo-inositol 1-monophosphate (MIMP). The mammalian and plant enzymes are involved in the metabolism of inositol phospholipids, including the generation and degradation of inositol phosphates and phosphatidylinositols that mediate cellular signal transduction [13]. Inositol monophosphatases are present in diverse organisms such as bacteria and higher eukaryotes, and evolved from a common ancestral gene [14, 15]. Mammalian inositol monophosphatases exhibit relatively broad substrate specificity for phosphate-containing compounds [16, 17]. For example, the enzyme isolated from rat testis hydrolyzes adenosine 2′-monophosphate [16] and the bovine brain enzyme hydrolyzes β-glycerophosphate and adenosine 2′-monophosphate [17]. E. coli suhB encodes a protein homologous to eukaryotic inositol monophosphatases with equivalent activities [18].
However, phosphatidylinositol is not present in E. coli usually [19], and its physiological function in cells is unknown. In contrast, E. coli SuhB exhibits wider substrate specificity and hydrolyzes β-glycerophosphate and adenosine 2′-monophosphate as well [18]. Thus, we hypothesized that bacterial inositol monophosphatases might also function as 5′-nucleotidase. Moreover, various E. coli enzymes likely dephosphorylate 5′-nucleotides with different specificities and functions and may therefore act together to maintain the sizes of the intracellular pools of nucleotides and nucleosides.
In Bacillus subtilis, the partial nucleotide limitation induces 5′-nucleotidase activity [20], and B. subtilis is used for fermentative inosine production, which may involve the dephosphorylation of IMP catalyzed by an unidentified 5′-nucleotidase [21]. However, B. subtilis genes encoding 5′-nucleotidase have remained to be identified.
Here we selected B. subtilis genes potentially encoding 5′-nucleotidases [22] and expressed them in E. coli to characterize their function. We identified two major and four minor genes encoding 5′-nucleotidases, with various functions and substrate specificities. A major 5′-nucleotidase was involved in resistance to oxidative stress, and a minor enzyme was required for normal growth on solid medium.
Results
Selection of B. subtilis genes similar to E. coli genes encoding 5′-nucleotidase
In E. coli, at least nagD, yrfG, yjjG, yieH, yigL, surE, and yfbR encode 5′-nucleotidases, and their gene products are classified in the families as follows: HADSF (NagD, YrfG, YjjG, YieH, and YigL), SurE, and HD domain (YfbR). In the B. subtilis genome, no gene shares significant homology with surE or yfbR. However, gene products of araL, yutF, yqeG, ysaA, ycsE, gapB, ftsA, and hprP share some suggestive similarities with that of E. coli nagD (Fig. 1); AraL (65.8 % similarity/272 aa overlap), YutF (73.0 %/256 aa), YqeG (15.7 %/172 aa), YsaA (16.9 %/260 aa), YcsE (51.4 %/249 aa), GapB (6.8 %/340 aa), FtsA (9.5 %/440 aa), and HprP (15.7 %/216 aa). Among them, AraL, YutF, YqeG, YsaA, YcsE, and HprP were supposed to belong to HADSF, since their amino acid sequences contain the HADSF motif (http://pfam.xfam.org/family/PF00702.24).
Fig. 1.
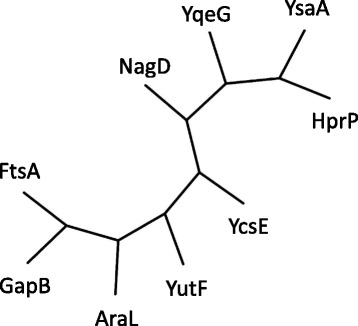
Similarities among the amino acid sequences of the proteins encoded by E. coli nagD homologs in the B. subtilis genome. The amino acid sequences of the proteins encoded by B. subtilis araL, yutF, yqeG, ysaA, ycsE, gapB, ftsA, and hprP, and E. coli nagD were analyzed using CLUSTALW (default fast/approximate settings [http://www.genome.jp/tools/clustalw/]) to generate the phylogenic tree
The araL gene resides within the L-arabinose operon, which encodes enzymes required for the degradation of L-arabinose and is under the control of AraR repressor to be induced in the presence of L-arabinose [23]. AraL was predicted to be a phosphatase that hydrolyzes certain sugar-phosphates [23].
The yutF gene encodes a protein that was predicted to be involved in N-acetyl-glucosamine catabolism and similar to 4-nitrophenyl phosphatase [24]. The condition-dependent transcriptome analysis revealed that yutF was almost constitutively expressed at low levels [25].
The yqeG, ysaA, and ycsE genes encode a putative HADSF-family enzyme of unknown function. The yqeG gene is the first gene of the long operon comprising yqeG, yqeH, aroD, yqeI, nadD, yqeK, yqeL, and yqeM. This operon encodes essential genes [26], which are expressed constitutively at significant levels that increase during germination and under conditions of oxidative stress [25]. The ysaA and ycsE genes are constitutively expressed and are induced, respectively, during the nutritional shift from malate to malate plus glucose and in the presence of ethanol or diamide [25].
The products of gapB, ftsA, and hprP show relatively lower similarities to NagD (Fig. 1). GapB is NADP-dependent glyceraldehyde-3-phosphate dehydrogenase, which is involved in gluconeogenesis [27]. FtsA is an actin-like ATPase involved in cell division [28]. And HprP is a P-Ser-HPr phosphatase involved in carbon catabolite repression [29].
5′-Nucleotidase activity of B. subtilis enzymes sharing some similarities with NagD
Of the eight B. subtilis genes that share some similarities with nagD as described above, gapB, ftsA, and hprP have respective and specific functions other than as 5′-nucleotidases [27–29]. Therefore, these genes were excluded from the present study. We cloned and expressed araL, yutF, yqeG, ysaA, and ycsE in E. coli as His6-tag fusion proteins.
The His6-tag fusion proteins were purified to form respective single bands in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, data not shown), and were subjected to phosphatase assays using the substrates as follows: AMP, CMP, GMP, IMP, UMP, and glucose 6-phosphate (G6P) (Fig. 2). G6P was included because it is the substrate of certain HADSF enzymes [9]. YcsE appeared to be the most efficient phosphatase acting on all six substrates at a lower concentration (2 mM), indicating its broad substrate specificity with preference for IMP, CMP, and G6P. Its activity increased in the presence of 10 mM substrates, particularly AMP, GMP, and UMP. The other four enzymes were relatively less active even at the higher substrate concentration. All 5′-nucleotides served almost equally as substrates for AraL at lower and higher concentrations, and AraL activity was enhanced particularly against G6P at the higher concentration. YutF exhibited purine 5′-nucleotidase activity with the substrates GMP and IMP, but only at the higher concentration, and did not hydrolyze pyrimidine nucleotides and G6P. YqeG hydrolyzed GMP and G6P only at the higher concentration. YsaA activity was weakest with purine-nucleotide substrates at the higher concentration.
Fig. 2.
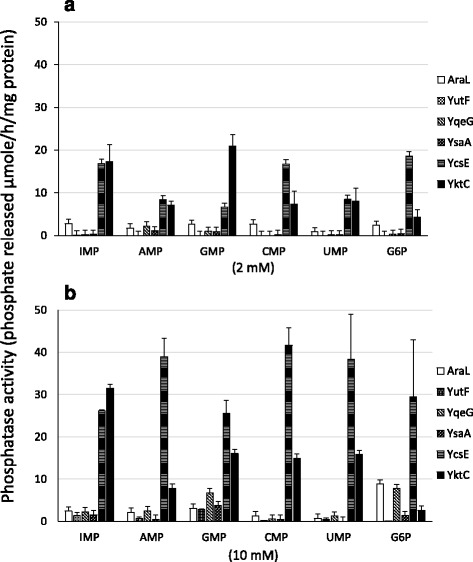
Phosphatase activities of B. subtilis proteins homologous to E. coli NagD. Each purified protein (0.25 mg/ml, indicated on the right) was incubated with 2 mM (a) or 10 mM (b) of various substrates for 12 h and assayed for phosphatase activity as described in Methods. All presented data are the mean values of three independent experiments ± SD
The catalytic properties of YcsE are shown in Table 1. The substrate specificity of YcsE was broad with higher K M values, suggesting that it efficiently dephosphorylated these substrates at higher concentrations. In contrast, the properties of the other four enzymes were not determined because of their lower activities. Nevertheless, the data do not exclude their physiological function as 5′-nucleotidases, because the activities of two of the 5′-nucleotidases YieH and YigL identified previously in E. coli were too low to determine their enzymatic properties [9].
Table 1.
Kinetic constants of YcsE
| Substrate | K M (mM)a | V max (μmol min−1 mg protein−1)a | k cat (s−1)a |
|---|---|---|---|
| IMP | 3.2 ± 0.036 | 0.13 ± 0.060 | 3.7 ± 1.4 × 102 |
| AMP | 8.6 ± 0.80 | 0.50 ± 0.029 | 2.3 ± 0.13 × 103 |
| GMP | 10.3 ± 0.12 | 0.45 ± 0.12 | 2.0 ± 0.55 × 103 |
| CMP | 4.7 ± 0.23 | 0.53 ± 0.18 | 3.4 ± 0.011 × 103 |
| UMP | 8.5 ± 0.69 | 0.20 ± 0.072 | 9.6 ± 3.5 × 102 |
| G6P | 22.2 ± 0.96 | 2.3 ± 0.48 | 1.06 ± 0.22 × 104 |
aAll presented data are the mean values of three independent experiments ± SD
5′-Nucleotidase activity of SuhB homolog in B. subtilis
As described above, E. coli SuhB is an inositol monophosphatase, which exhibits wider substrate specificity, as it hydrolyzes β-glycerophosphate and adenosine 2′-monophosphate as well [18]. Thus, we hypothesized that bacterial inositol monophosphatases might also function as 5′-nucleotidase.
The B. subtilis yktC gene, which is the homolog of E. coli suhB (31.7 % similarity of 265 overlapping amino acid residues), was cloned and expressed as a His6-tagged protein in E. coli. His6-tagged YktC was purified to form a single band in SDS-PAGE (data not shown) and was subjected to phosphatase assays in the presence of various substrates (Fig. 2, Table 2). The inositol monophosphatase activity of YktC was indeed demonstrated by its efficient ability to hydrolyze MIMP, and it also turned out to exhibit 5′-nucleotidase activity preferentially against IMP and GMP as substrates. To our knowledge, this is the first identification of an inositol monophosphatase with 5′-nucleotidase activity. Compared with E. coli SuhB, YktC hydrolyzed G6P less efficiently, while β-glycerophosphate more efficiently [18]. Expression of yktC is mainly constitutive and is markedly enhanced by stressors such as diamide, ethanol, or high salt as well as at higher and lower temperatures [25].
Table 2.
Kinetic constants of YktC
| Substrate | K M (mM)a | V max (μmol min−1 mg protein−1)a | k cat (s−1)a |
|---|---|---|---|
| MIMP | 0.076 ± 0.006 | 0.82 ± 0.16 | 2.9 ± 0.33 × 103 |
| IMP | 1.1 ± 0.083 | 0.078 ± 0.0084 | 1.8 ± 0.59 × 102 |
| AMP | 1.8 ± 0.13 | 0.030 ± 0.00022 | 5.3 ± 0.039 × 101 |
| GMP | 1.9 ± 0.026 | 0.169 ± 0.028 | 3.0 ± 0.34 × 102 |
| CMP | 1.6 ± 0.017 | 0.049 ± 0.014 | 1.5 ± 0.52 × 102 |
| UMP | 2.4 ± 0.052 | 0.11 ± 0.0099 | 9.8 ± 4.6 × 102 |
| G6P | 2.6 ± 0.51 | 0.021 ± 0.010 | 3.7 ± 1.3 × 101 |
| β-glycerophosphate | 0.49 ± 0.035 | 1.2 ± 0.32 | 5.7 ± 1.2 × 102 |
aAll presented data are the mean values of three independent experiments ± SD
Physiological functions of YqeG, YcsE, and YktC
As mentioned above, yqeG expression is enhanced by oxidative stress, ycsE is induced in the presence of diamide or ethanol, and yktC is markedly enhanced by stressors such as diamide, ethanol, or high salt, as well as at higher and lower temperatures [25]. These findings inspired us to investigate the three genes encoding 5′-nucleotidase for their possible functional association with the response to oxidative stress.
We used conventional marker replacement to inactivate ycsE and yktC. The yqeG gene is the first of the long operon comprising yqeG, yqeH, aroD, yqeI, nadD, yqeK, yqeL, and yqeM [26]. At least yqeH, yqeI, and nadD are essential genes [26]. Therefore, we tried to introduce an in-frame deletion of yqeG to maintain the expression of the downstream essential genes. However, numerous attempts failed. Therefore, once an additional copy of yqeG was introduced into the amyE locus under the control of spac promoter, which is conditionally induced by IPTG. And then, in the presence of IPTG, the existing yqeG gene was deleted using marker replacement so that the genes downstream were constitutively expressed by read-through from the kanamycin-resistance cassette (strain NON05). Regardless of the presence and absence of IPTG, strain NON05 grew in liquid medium (Fig. 3), suggesting that yqeG might be dispensable in planktonic growth. However, the spac promoter is somewhat leaky; a significant basal level of expression still exists in the absence of IPTG [30]. Therefore, in the absence of IPTG, there might be some production of YqeG, and this might be sufficient for planktonic growth. Nevertheless, NON05 formed fewer colonies in the absence of IPTG (Fig. 4), suggesting that yqeG was required to maintain normal growth particularly on solid medium. Similarly, less efficient colony-formation was exhibited by the triple mutant NON06 in the absence of IPTG, and colony formation was slightly decreased when ycsE and yktC were inactivated additionally.
Fig. 3.
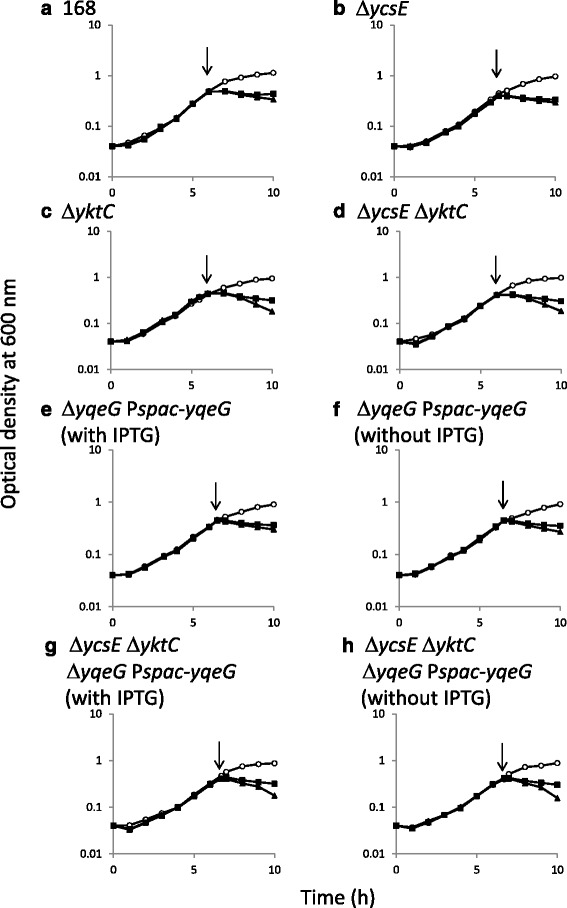
Growth curves of B. subtilis strains. Strains 168 (a), NON01 (b ΔycsE), NON02 (c ΔyktC), NON03 (d ΔycsE ΔyktC), NON05 (e and f, ΔyqeG Pspac-yqeG), and NON06 (g and h, ΔycsE ΔyktC ΔyqeG Pspac-yqeG) were inoculated into liquid medium and their growth was monitored. At the times indicated by the arrowheads, diamide was added to final concentrations of 0 mM (open circle), 1 mM (solid square), and 4 mM (solid triangle). Strains NON05 and NON06 were grown in the presence (e and g) and absence (f and h) of 1 mM IPTG. All experiments were repeated more than three times and similar results were observed
Fig. 4.
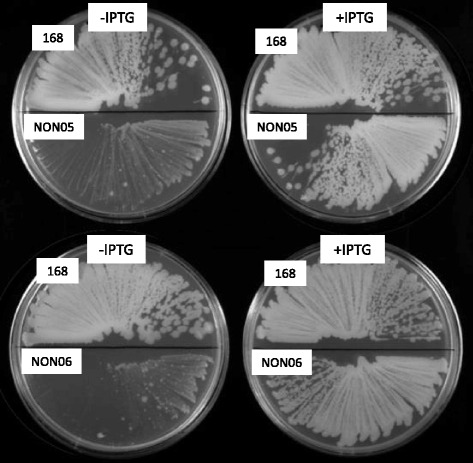
Colony formation by B. subtilis strains. Suspensions of strains 168, NON05, and NON06 were streaked on LB plates with (right) or without (left) 1 mM IPTG and incubated overnight at 37 °C. All experiments were repeated more than three times and similar results were observed
The addition of diamide to liquid medium arrested the growth of the parental strain 168 (Fig. 3). The growth characteristics of the mutant NON01 lacking ycsE were comparable to those of the parental strain in the presence of diamide. Conversely, the mutants NON02 and NON03 lacking yktC exhibited the enhanced growth defect in the presence of the higher concentration of diamide (4 mM), as the optical density for cells decreased significantly possibly because of cell lysis. The results suggest that yktC, but not ycsE, contributed to resistance to oxidative stress induced by diamide. In contrast, NON05 did not exhibit significantly increased sensitivity to diamide, depending on the repression of yqeG in the absence of IPTG (Fig. 3). The triple-mutant NON06 exhibited the growth defect similar to that of NON02 or NON03 in the presence of diamide (Fig. 3), and the growth of NON06 did not depend on IPTG. Therefore, the conditional expression of yqeG was not associated with the susceptibility to diamide. Taken together, the data indicate that only YktC was involved in the resistance to oxidative stress.
Discussion
In eukaryotes, multiple nucleotidases and nucleoside kinases control the intracellular concentration of nucleotides [31–33]. Nucleotidases play a role in the maintenance of a constant nucleoside supply, which is required to maintain RNA metabolism and biosynthesis of cell wall [1]. In bacteria, numerous nucleotidases with different substrate specificities control the intracellular nucleotide pool [34], and it is believed that the accumulation of intracellular nucleotides is potentially toxic and therefore must be prevented [31–33].
Here we show that B. subtilis YktC and YcsE functioned as 5′-nucleotidases, suggesting that they may represent two of the main enzymes involved in intracellular nucleoside/nucleotide metabolism. The other enzymes similar to E. coli NagD, which belong to the HADSF family (YutF, YsaA, YqeG, and AlaL), exhibited lower activity, although the data suggest they may function as minor 5′-nucleotidases. HADSF phosphatases often have broader substrate specificity and hydrolyze various phosphorylated compounds [5]. Thus, 5′-nucleotidases with lower substrate affinity might contribute to the balance of intracellular nucleotide concentrations as well as that of other phosphorylated metabolites. The K M values of E. coli NagD for UMP, CMP, GMP, AMP, and G6P are 0.16 ± 0.038, 1.47 ± 0.044, 0.40 ± 0.130, 0.84 ± 0.250, and 5.90 ± 0.750 mM, respectively [4], which are smaller than those of YcsE and YktC (Tables 1 and 2, respectively). Therefore, B. subtilis YktC and YcsE might be less efficient as 5′-nucleotidase than NagD, but it is still suggestive that they may be able to function as two of the 5′-nucleotidases that are the first to respond to the accumulation of nucleotides, and the other enzymes may function when the concentration of nucleotides is in far excess.
Some of the genes encoding 5′-nucleotidases might be induced upon partial nucleotide limitation as previously reported [20]. Interestingly, the expression of yqeG is enhanced under oxidative stress, that of ycsE is induced by diamide or ethanol, and that of yktC is markedly enhanced in the presence of the various stressors, including diamide, ethanol, and high salt as well as at higher and lower temperatures [25]. These findings indicate that the intracellular levels of phosphorylated metabolites and nucleotides including 5′-nucleotides may be altered in the presence of these stressors, in particular oxidative stress. Our results show that inactivation of yktC led to increased sensitivity to diamide (Fig. 4). When the expression of yqeG was artificially modified, the cells grew normally in liquid medium and did not exhibit increased sensitivity to diamide, depending on repression of yqeG in the absence of IPTG (Fig. 4). Further, in the presence of diamide, the triple mutant NON06, lacking ycsE and yktC and with repressed yqeG, exhibited a growth defect similar to that of NON02 or NON03, which lacks yktC (Fig. 4). Therefore, we conclude that only YktC was involved in resistance to diamide-induced oxidative stress. However, further studies are required to elucidate how its enzymatic functions, including its 5′-nucleotidase activity, are related to resistance to the oxidative stress.
B. subtilis induces S-cysteinylation to protect protein thiols after exposure to diamide, because this bacterium does not produce thiols such as glutathione [35]. To our knowledge, enzymes with 5′-nucleotidase activity have not been demonstrated to be involved in resistance to oxidative stress. In response to oxidative stress, Pseudomonas fluorescens evokes a metabolic adaptation to increase NADPH synthesis and to decrease NADH production, accompanied by the induction and repression, respectively, of NAD+ kinase and NADP+ phosphatase, which regulate the levels of NAD+ and NADP+ [36]. Namely, oxidative stress represses the NADP+ phosphatase in P. fluorescens [36], whereas it induces yktC in B. subtilis [25]. If YktC might function as NADP+ phosphatase, it could decrease NADPH synthesis under oxidative stress, which is contradictory to the physiological demand. Therefore, YktC is unlikely to act as a NADP+ phosphatase, and we did not determine if NADP+ was a substrate of the phosphatase activity of YktC. On the other hand, yqeG is the first gene of a long operon comprising essential genes such as nadD that encodes nicotinamide-nucleotide adenylyltransferase required for de novo biosynthesis of NAD+ and NADP+ [37]. The induction of yqeG by oxidative stress, together with the other members of the operon, might be relevant to the enhanced NADPH synthesis as found in P. fluorescens. However, our results indicate that yqeG was dispensable, except for normal colony formation on solid medium (Fig. 4). We were therefore unable to determine the physiological function of yqeG.
In mammalian and plant cells, inositol monophosphatases play important roles in the metabolism of inositol polyphosphates and phosphatidylinositols [13, 38]. De novo phosphatidylinositol synthesis in mammals and Saccharomyces cerevisiae involves a reaction that combines CDP-diacylglycerol with myo-inositol. myo-Inositol is produced through the isomerization of glucose 6-phosphate to form MIMP [39] and the subsequent dephosphorylation of MIMP by inositol monophosphatase [16]. Further, MIMP is produced by the dephosphorylation of the second messenger inositol polyphosphates [40]. In E. coli and B. subtilis, however, phosphatidylinositols are not components of phospholipids usually present in the cell membrane [41, 42], and inositol 1-phosphate synthase has not been identified. Therefore, it is unlikely that MIMP occurs naturally in these bacteria and might serve as the physiological substrate of E. coli SuhB and B. subtilis YktC. In E. coli, a decrease in intracellular SuhB levels compensated the defects in secY and rpoH [43, 44]. The secY gene encodes a membrane component involved in protein secretion [45], and rpoH encodes σ32, a component of the RNA polymerase involved in heat-shock induction [46]. Therefore, defects in secY and rpoH impair secretion and the heat-shock response, respectively, which are involved in posttranslational quality control of proteins [18]. Therefore, SuhB may control the rates of peptide chain elongation and protein folding [42]. In contrast, the SuhB homolog of P. aeruginosa plays an important role in pathogenesis to control the genes required for acute and chronic infection [47]. Burkholderia cepacia SuhB is required for the secretion of proteins associated with motility and biofilm formation [48], suggesting that the physiological functions of SuhB homologs are complex, which may be true for B. subtilis YktC. Nevertheless, it is intriguing that MIMP is the best substrate for these enzymes. Kozloff and the colleagues detected a small amount of phosphatidylinositol from E. coli cells, implying the possibility of a weak phosphatidylinositol biosynthesis under some specific conditions [49]. E. coli SuhB and B. subtilis YktC might be involved in bacterial metabolism of phosphatidylinositol, which has not been characterized yet.
The broad substrate specificity of YktC revealed here implies the association of this enzyme with sugar-phosphate stress. Although sugars serve as energy and carbon sources, sugar phosphates are produced during their metabolism. Excess accumulation of sugar phosphates impairs cell growth [50] and may trigger cell death [51, 52] through an unknown mechanism. Similarly, accumulation of nucleotides is detrimental to the cell [34]. Therefore, by hydrolyzing sugar phosphates as well as nucleotides, YktC may play a role in correcting imbalances of metabolites. For example, we found that the K M value of YktC for G6P was 2.6 ± 0.51 mM, which is greater than the reported intracellular concentrations of glucose 6-phosphate in E. coli (0.8–2.0 mM) [53]. However, under certain conditions, the intracellular concentration of G6P in Lactococcus lactis reaches 20–50 mM [54], indicating that G6P accumulates to higher levels in bacterial cells. Accordingly, in the presence of excess intracellular concentrations of G6P, YktC may hydrolyze it to intervene in glucose metabolism. This may be true as well for YcsE, because it hydrolyzed G6P in vitro more efficiently than YktC (Fig. 2). Similarly, YktC may hydrolyze β-glycerophosphate, which is a biosynthetic precursor of phospholipids [55], to interfere with the maintenance of the cell membrane. Therefore, their phosphatase activities with broad-specificity may exert pleiotropic effects on metabolism and other cellular functions. It would be worthwhile to perform metabolomic analyses on the mutants lacking yktC and ycsE to determine concentrations of the various metabolites, including 5′-nucleotides and other sugar phosphates, which would reinforce the hypothesis concerning the role of these enzymes.
Together with sodium glutamate, IMP and GMP are generally used as food additives to enhance taste. Industrial production of IMP is achieved using enzymatic conversion of inosine into IMP. B. subtilis strains producing large amounts of inosine in the fermentation medium were generated [56–60], and 5′-nucleotidase activity of these strains is remarkably increased, which may convert IMP into inosine [59]. Thus, optimizing the expression of genes encoding 5′-nucleotidases such as those studied here may further improve the fermentation of inosine production.
Conclusions
B. subtilis ycsE, araL, yutF, ysaA, and yqeG show suggestive similarities to E. coli nagD encoding 5′-nucleotidase. Among the five, only YcsE exhibited significant 5′-nucleotidase activity with a broader specificity, whereas the other four enzymes had rather weak but suggestive activities with various capacities and substrate specificities. Interestingly, YqeG was required to maintain normal growth on solid medium. On the other hand, B. subtilis yktC shares high similarity with E. coli suhB encoding inositol monophosphatase, the gene product of which had 5′-nucleotidase activity preferential for GMP and IMP, and turned out to be involved in resistance to oxidative stress.
Methods
Bacterial strains, plasmids, oligonucleotide primers, and culture conditions
Bacterial strains and plasmids are listed in Table 3. Bacterial strains were usually grown aerobically at 37 °C and maintained in Luria-Bertani (LB) medium (Becton Dickinson, NJ, USA) containing 50 mg/l ampicillin and 50 mg/l kanamycin as required. Oligonucleotide primers are listed in Table 4. To assess the resistance to oxidative stress of B. subtilis strains, cells were inoculated into LB and allowed to grow. The oxidizing agent diamide was added to the exponentially growing culture at various concentrations, and growth was continuously monitored.
Table 3.
Bacterial strains and plasmids
| Strain and plasmid | Description | Source or reference |
|---|---|---|
| B. subtilis | ||
| 168 | trpC2 | Laboratory stock |
| NON01 | trpC2 ycsE::spc | This study |
| NON02 | trpC2 yktC::cat | This study |
| NON03 | trpC2 ycsE::spc yktC::cat | This study |
| NON04 | trpC2 amyE::(Pspac-yqeG erm) | This study |
| NON05 | trpC2 amyE::(Pspac-yqeG erm) ΔyqeG::kan | This study |
| NON06 | trpC2 ycsE::spc yktC::cat amyE::(Pspac-yqeG lacI erm) ΔyqeG::kan | This study |
| TMO310 | trpC2 aprE::(spec lacI Pspac-mazF) | [62] |
| TMO311 | trpC2 aprE::(kan lacI Pspac-mazF) | [62] |
| E. coli | ||
| DH5α | F− Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17(rK −, mK +) phoA supE44 λ− thi-1 gyrA96 relA1 | Laboratory stock |
| BL21(DE3) | F− ompT gal dcm lon hsdS B(r B −, m B −) λ(DE3 [lacI lacUV5-T7p07 ind1 sam7 nin5]) [malB +]K-12(λS) | Laboratory stock |
| Plasmids | ||
| pCRE-test | cat | [63] |
| pET28b | kan | Takara Bio |
| pET28b-araL | kan, araL | This work |
| pET28b-yutF | kan, yutF | This work |
| pET28b-yqeG | kan, yqeG | This work |
| pET28b-ysaA | kan, ysaA | This work |
| pET28b-ycsE | kan, ycsE | This work |
| pET28b-yktC | kan, yktC | This work |
| pMD20-araL | amp | This work |
| pMD20-yutF | amp | This work |
| pMD20-yqeG | amp | This work |
| pMD20-ysaA | amp | This work |
| pMD20-ycsE | amp | This work |
| pMD20-yktC | amp | This work |
| pMutin2 | erm lacI | [64] |
Table 4.
Oligonucleotide primers
| Name | Sequence (5′–3')a | Restriction site |
|---|---|---|
| araL-F | gggaattccatatgcgtattatggccagtcatgat | NdeI |
| araL-R | ccgctcgagtatcagaatcccctcctcagc | XhoI |
| yutF-F | cgcggatccatgaaaacatataaagggtattta | BamHI |
| yutF-R | cccaagcttaatgtatggaatccattcagt | HindIII |
| ysaA-F | cgcggatccatgaaagccgtattttttgattta | BamHI |
| ysaA-R | ccgctcgagtttctctaatataggaaacaa | XhoI |
| YqeG-F | gggaatccatatgttaaaaaagtttttt | NdeI |
| YqeG-R | cccaagcttttactcctcccactgaat | HindIII |
| ycsE-F | gggaattccatatgtctgtccaaagagaagatgta | NdeI |
| ycsE-R | ccgctcgagtagtacccaatggcgaatcgcttta | XhoI |
| yktC-F | cgcggatccatgacaaattggacggaaatcgat | BamHI |
| yktC-R | cccaagcttcttccgagcatgaagatactc | HindIII |
| ycsE-1 | gtttattgatggcgtgac | |
| ycsE-2 | ctgtgtagccttgagagtgatgaacagacatatatgtacctctc | |
| ycsE-3 | tcactctcaaggctacacag | |
| ycsE-4 | cgcaagcttcaaaaattatatggag | |
| ycsE-5 | ctccatataatttttgaagcttgcggggtactataaaaaaagagagtcc | |
| ycsE-6 | aacacttcatttgcggtc | |
| yktC-1 | gttcgcctagagctgtaagcttc | |
| yktC −2 | gccgatgataagctgtcaaagcaatctcatcgatttccgtcc | |
| yktC −3 | tttgacagcttatcatcggc | |
| yktC −4 | ttataaaagccagtcattaggc | |
| yktC −5 | gcctaatgactggcttttataagtgctagccggaaacccatc | |
| yktC −6 | gaacatctatcaccagacgacatc | |
| yqeG-d1 | gacataatgcgccatttgatggttg | |
| yqeG-d2 | gcttgagtcaattccgctgtcgctcaaacgaaaagggcacattcag | |
| yqeG-d3 | cgacagcggaattgactcaagc | |
| yqeG-d4 | cgcaagcttacgataaacccagc | |
| yqeG-d5 | gctgggtttatcgtaagcttgcgcaaaacccgcacccttccc | |
| yqeG-d6 | caggcagagcatatggatacg | |
| yqeG-s1 | ccttccagggtatgtttctc | |
| yqeG-s2 | gatactgcactatcaacacactctttcgacatggatgagcgatgatg | |
| yqeG-s3 | aagagtgtgttgatagtgcagtatc | |
| yqeG-s4 | caacaagctggggatccg | |
| yqeG-s5 | cggatccccagcttgttgcgacaatcattttgaaagaaagaaaaaggg | |
| yqeG-s6 | gatgacctcgtttccaccggcaaccttttccatttcttactcctcc | |
| yqeG-s7 | ccggtggaaacgaggtcatc | |
| yqeG-s8 | cacaaattaaaaactggtctgatcggcctaactcacattaattgcgttg | |
| yqeG-s9 | cgatcagaccagtttttaatttgtg | |
| yqeG-s10 | ttaacaaaattctccagtcttcacatcg |
aRestriction enzyme cleavage sites are underlined
Construction of bacterial strains
E. coli strains expressing B. subtilis genes were constructed as follows: DNA fragments corresponding to the open reading frames of genes of interest were PCR-amplified using B. subtilis strain 168 chromosomal DNA (Table 3) as template and pairs of respective oligonucleotide primers with generation of unique restriction sites at their 5′-termini (araL-F/araL-R for araL, ycsE-F/ycsE-R for ycsE, yktC-F/yktC-R for yktC, yqeG-F/yqeG-R for yqeG, ysaA-F/ysaA-R for ysaA, and yutF-F/yutF-R for yutF) (Table 4). Each fragment was cloned into the pMD20-T plasmid using the Mighty TA-cloning kit (Takara Bio, Otsu, Japan) to yield pMD20-araL, pMD20-ycsE, pMD20-yktC, pMD20-yqeG, pMD20-ysaA, and pMD20-yutF, which were used to transform E. coli strain DH5α. After performing nucleotide sequencing to confirm the constructs, each recombinant plasmid was digested with the appropriate restriction enzyme(s) to prepare a DNA fragment corresponding to its respective open reading frame and then ligated to the arms of pET28b (Takara Bio) digested with the same enzyme(s). The plasmids pET28b-araL, pET28B-ycsE, pET28B-yktC, pET28B-yqeG, pET28B-ysaA, and pET28B-yutF were constructed to express each protein as a C-terminal His6-fusion, and expression was controlled by the T7 promoter in E. coli strain BL21(DE3) (Takara Bio).
Mutant strains of B. subtilis lacking ycsE and yktC were constructed using conventional marker replacement as follows (Additional file 1: Figure S1): To delete ycsE, two PCR fragments (each approximately 700-bp) corresponding to the upstream and downstream regions of ycsE were amplified from the DNA of B. subtilis strain 168 using the primer pairs ycsE-1/ycsE-2 and ycsE-5/ycsE-6, respectively (Table 4). A PCR fragment containing the spectinomycin-resistance gene cassette was amplified from the DNA of strain TMO310 (Table 3) using the primer pair ycsE-3/ycsE-4 (Table 4). The three PCR fragments were ligated using recombinant PCR with the primer pair ycsE-1/ycsE-6 to generate a single PCR fragment comprising the spectinomycin-resistance gene cassette flanked by the upstream and downstream regions of ycsE. B. subtilis strain 168 was transformed with the recombinant PCR fragment to confer resistance to spectinomycin, and a transformant with correct marker replacement was selected and designated strain NON01 (Table 3). To delete yktC, upstream and downstream regions of yktC were amplified using the primer pairs yktC-1/yktC-2 and yktC-5/yktC-6, respectively (Table 4). The chloramphenicol-resistance cassette was amplified from pCRE-test using the primer pair yktC-3/yktC-4 (Table 4). The three PCR fragments were ligated using recombinant PCR with the primer pair of yktC-1/yktC-6 to generate a single PCR fragment, which was integrated into chromosome of B. subtilis strain 168 to yield strain NON02 (Table 3). Strain NON01 was further transformed with NON02 DNA to confer resistance to chloramphenicol and generate strain NON03 lacking both ycsE and yktC (Table 3).
Another mutant that conditionally expressed yqeG was constructed as follows (Additional file 1: Figure S1). Five DNA fragments, corresponding to the C-terminal region of amyE, the stretch containing the erythromycin-resistance cassette and the spac promoter of pMutin2 (Table 3), the coding region of yqeG, the lacI gene of pMutin2, and the N-terminal region of amyE, were PCR-amplified using the primer pairs yqeG-s1/yqeG-s2, yqeG-s3/yqeG-s4, yqeG-s5/yqeG-s6, yqeG-s7/yqeG-s8, and yqeG-s9/yqeG-s10, respectively (Table 4). Using recombinant PCR with the primer pair yqeG-s1/yqeG-s10, all these five fragments were ligated in the order as described above and then integrated into the amyE locus of strain 168 to generate the erythromycin-resistant strain NON04 harboring an additional IPTG-inducible copy of yqeG.
Next, the endogenous yqeG gene of NON04 was deleted using marker replacement. Two DNA fragments comprising the upstream and downstream regions of yqeG (approximately 700 bp each) were amplified using the primer pairs yqeG-d1/yqeG-d2 and yqeG-d5/yqeG-d6, respectively (Table 4). The kanamycin-resistance cassette was amplified from the DNA of strain TMO311 using the primer pair yqeG-d3/yqeG-d4 (Table 4). Recombinant PCR was used to ligate together the three fragments using the primer pair of yqeG-d1/yqeG-d6t, and the ligation product was integrated into the chromosome of NON04 to yield strain NON05 in which expression of the additional yqeG was induced with 1 mM IPTG (Table 3). In NON05, yqeG was replaced with the kanamycin-resistance cassette so that the rest of operon comprising yqeH, aroD, yqeI, nadD, yqeK, yqeL, and yqeM, which reside downstream of yqeG, are constitutively expressed by read-through from the kanamycin-resistance gene. NON05 was transformed further with NON03 DNA to confer resistance to both chloramphenicol and spectinomycin inactivating yktC and ycsE, respectively (strain NON06).
Chromosomal DNAs of mutant strains of B. subtilis were subjected to diagnostic PCR experiments with the respective primer pairs used for recombinant PCR, and their correct construction was confirmed by appearance of the respective PCR fragments with altered length (Additional file 1: Figure S1).
Purification of gene products produced in E. coli
E. coli strain BL21(DE3) bearing the pET28b derivatives were inoculated into 600 ml of LB medium containing kanamycin and were cultured at 37 °C with shaking at 180 rpm. When the optical density of the cultures reached 0.4–0.6, IPTG was added to a final concentration of 1 mM, the cells were cultivated further for 5 h, harvested using centrifugation, and resuspended into 10.8 ml of NP buffer (50 mM NaH2PO4, pH 8.0, and 300 mM NaCl). The suspension was chilled in an ice bath, mixed with 1.2 ml of 10 mg/ml lysozyme in NP buffer, incubated for 30 min, and sonicated to disrupt the cells. After centrifugation, the supernatant was subjected to His6-tag affinity purification using TALON Metal Affinity Resins (Takara Bio). The His6-tagged proteins were purified in a reaction buffer containing 1 mM NH4Cl, 250 mM KCl, 10 mM MgCl2, and 50 mM Tris–HCl (pH 7.8), and subjected to SDS-PAGE followed by Coomassie brilliant blue staining.
Enzyme assay
The phosphatase assay was performed as previously described [61]. Each purified enzyme (0.25 mg/ml) was incubated with substrate in 50 μl of reaction buffer containing 1 mM NH4Cl, 250 mM KCl, 10 mM MgCl2, and 50 mM Tris–HCl (pH 7.8) at 37 °C, and the reaction was terminated using 50 μl of 10 % trichloroacetic acid. After centrifugation, the supernatant was mixed with 20 μl of solution A (10 % ascorbic acid in 2.25 M H2SO4) and 20 μl of solution B (2.4 % ammonium molybdate and 1 mg/ml potassium antimonyl tartrate in 2.25 M H2SO4). The mixture was incubated at room temperature for 10 min, and absorbance at 820 nm was measured to calculate the concentration of the released phosphate ion with reference to a standard curve made with various concentrations of inorganic phosphate. Trace of phosphate released in the reaction mixtures in the absence of purified enzyme was subtracted as background.
Acknowledgements
We thank Mr. Kentaro Kojima for his technical contributions in the earlier stage of this work.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan and in part by the Special Coordination Funds for Promoting Science and Technology, Creation of Innovative Centers for Advanced Interdisciplinary Research Areas; the Advanced Low-Carbon Technology Research and Development Program; KAKENHI (26660067); and Grants-in-Aid from the NC-CARP project.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request. The phylogenetic tree, sequence data, and alignments used to produce the results displayed in Fig. 1 are deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S19999).
Authors’ contributions
AT conducted most of the experiments and analyzed the results. The enzymatic experiments were performed under the supervision of KT and ST. KT, AN, AO, SN, and JK conducted experiments with the mutant strains of B. subtilis. KY conceived the idea for the project and wrote the final manuscript with SI. All authors read and approved the final manuscript.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- G6P
Glucose 6-phosphate
- HADSF
Haloalkane acid dehalogenase superfamily
- IPTG
Isopropyl β-D-1-thiogalactopyranoside
- MIMP
myo-Inositol 1-monophosphate
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
Additional file
Genetic organization of the altered chromosomal loci; ΔycsE::spc(A), ΔyktC::cat(B), ΔyqeG::kan(C), and amyE::(Pspac-yqeG lacI erm)(D). Genes and primers are indicated schematically by thick arrows and arrowheads, respectively. (PDF 144 kb)
Contributor Information
Ayako Terakawa, Email: te_ra0826@yahoo.co.jp.
Ayane Natsume, Email: taboomboompow@gmail.com.
Atsushi Okada, Email: atsushi_okada_1711@yahoo.co.jp.
Shogo Nishihata, Email: shogo.nishihata@gmail.com.
Junko Kuse, Email: mikanecoeg6@gmail.com.
Kosei Tanaka, Email: ktanaka@people.kobe-u.ac.jp.
Shinji Takenaka, Email: stakenaka@people.kobe-u.ac.jp.
Shu Ishikawa, Email: shu@people.kobe-u.ac.jp.
Ken-ichi Yoshida, Email: kenyoshi@kobe-u.ac.jp.
References
- 1.Rampazzo C, Ferraro P, Pontarin G, Fabris S, Reichard P, Bianchi V. Mitochondrial deoxyribonucleotides, pool sizes, synthesis, and regulation. J Biol Chem. 2004;279:17019–17026. doi: 10.1074/jbc.M313957200. [DOI] [PubMed] [Google Scholar]
- 2.Tamao Y, Noguchi K, Sakai-Tomita Y, Hama H, Shimamoto T, Kanazawa H, Tsuda M, Tsuchiya T. Sequence analysis of nutA gene encoding membrane-bound Cl(−)-dependent 5′-nucleotidase of Vibrio parahaemolyticus. J Biochem. 1991;109:24–29. doi: 10.1093/oxfordjournals.jbchem.a123345. [DOI] [PubMed] [Google Scholar]
- 3.Burns DM, Beacham IR. Nucleotide sequence and transcriptional analysis of the E. coli ushA gene, encoding periplasmic UDP-sugar hydrolase (5′-nucleotidase): regulation of the ushA gene, and the signal sequence of its encoded protein product. Nucleic Acids Res. 1986;14:4325–4342. doi: 10.1093/nar/14.10.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremblay LW, Dunaway-Mariano D, Allen KN. Structure and activity analyses of Escherichia coli K-12 NagD provide insight into the evolution of biochemical function in the haloalkanoic acid dehalogenase superfamily. Biochemistry. 2006;45:1183–1193. doi: 10.1021/bi051842j. [DOI] [PubMed] [Google Scholar]
- 5.Koonin EV, Tatusov RL. Computer analysis of bacterial haloacid dehalogenases defines a large superfamily of hydrolases with diverse specificity: Application of an iterative approach to database search. J Mol Biol. 1994;244:125–132. doi: 10.1006/jmbi.1994.1711. [DOI] [PubMed] [Google Scholar]
- 6.Allen KN, Dunaway-Mariano D. Phosphoryl group transfer: evolution of a catalytic scaffold. Trends Biochem Sci. 2004;29:495–503. doi: 10.1016/j.tibs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Lu Z, Dunaway-Mariano D, Allen KN. HAD superfamily phosphotransferase substrate diversification: structure and function analysis of HAD subclass IIB sugar phosphatase BT4131. Biochemistry. 2005;44:8684–8696. doi: 10.1021/bi050009j. [DOI] [PubMed] [Google Scholar]
- 8.Shin DH, Roberts A, Jancarik J, Yokota H, Kim R, Wemmer DE, Kim SH. Crystal structure of a phosphatase with a unique substrate binding domain from Thermotoga maritime. Protein Sci. 2003;12:1464–1472. doi: 10.1110/ps.0302703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuznetsova E, Proudfoot M, Gonzalez CF, Brown G, Omelchenko MV, Borozan I, Carmel L, Wolf YI, Mori H, Savchenko AV, Arrowsmith CH, Koonin EV, Edwards AM, Yakunin AF. Genome-wide analysis of substrate specificities of the Escherichia coli haloacid dehalogenase-like phosphatase family. J Biol Chem. 2006;281:36149–36161. doi: 10.1074/jbc.M605449200. [DOI] [PubMed] [Google Scholar]
- 10.Proudfoot M, Kuznetsova E, Brown G, Rao NN, Kitagawa M, Mori H, Savchenko A, Yakunin AF. General enzymatic screens identify three new nucleotidases in Escherichia coli. Biochemical characterization of SurE, YfbR, and YjjG. J Biol Chem. 2004;279:54687–54694. doi: 10.1074/jbc.M411023200. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Kwak JE, Moon J, Eom SH, Liong EC, Pedelacq JD, Berendzen J, Suh SW. Crystal structure and functional analysis of the SurE protein identify a novel phosphatase family. Nat Struct Biol. 2001;8:789–794. doi: 10.1038/nsb0901-789. [DOI] [PubMed] [Google Scholar]
- 12.Aravind L, Koonin EV. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci. 1998;23:469–472. doi: 10.1016/S0968-0004(98)01293-6. [DOI] [PubMed] [Google Scholar]
- 13.Drøbak BK. The plant phosphoinositide system. Biochem J. 1992;288:697–712. doi: 10.1042/bj2880697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gläser HU, Thomas D, Gaxiola R, Montrichard F, Surdin-Kerjan Y, Serrano R. Salt tolerance and methionine biosynthesis in Saccharomyces cerevisiae involve a putative phosphatase gene. EMBO J. 1993;12:3105–3110. doi: 10.1002/j.1460-2075.1993.tb05979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuwald AF, York JD, Majerus PW. Diverse proteins homologous to inositol monophosphatase. FEBS Lett. 1991;294:16–18. doi: 10.1016/0014-5793(91)81332-3. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg F., Jr D-Myoinositol 1-phosphate as product of cyclization of glucose 6-phosphate and substrate for a specific phosphatase in rat testis. J Biol Chem. 1967;242:1375–1382. [PubMed] [Google Scholar]
- 17.Gee NS, Ragan CI, Watling KJ, Aspley S, Jackson RG, Reid GG, Gani D, Shute JK. The purification and properties of myo-inositol monophosphatase from bovine brain. Biochemistry. 1988;249:883–889. doi: 10.1042/bj2490883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuhisa A, Suzuki N, Noda T, Shiba K. Inositol monophosphatase activity from the Escherichia coli suhB gene product. J Bacteriol. 1995;177:200–205. doi: 10.1128/jb.177.1.200-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. doi: 10.1016/B978-1-4831-9938-2.50008-X. [DOI] [PubMed] [Google Scholar]
- 20.Dhariwal KR, Vasantha N, Freese E. Partial nucleotide limitation induces phosphodiesterase I and 5′-nucleotidase in Bacillus subtilis. J Bacteriol. 1982;149:1146–1149. doi: 10.1128/jb.149.3.1146-1149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asahara T, Mori Y, Zakataeva NP, Livshits VA, Yoshida K, Matsuno K. Accumulation of gene-targeted Bacillus subtilis mutations that enhance fermentative inosine production. Appl Microbiol Biotechnol. 2010;87:2195–2207. doi: 10.1007/s00253-010-2646-8. [DOI] [PubMed] [Google Scholar]
- 22.Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessières P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V, Carter NM, Choi S-K, Codani JJ, Connerton IF, Cummings NJ, Daniel RA, Denizot F, Devine KM, Düsterhöft A, Ehrlich SD, Emmerson PT, Entian KD, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim S-Y, Glaser P, Goffeau A, Golightly EJ, Grandi G, Guiseppi G, Guy BJ, Haga K, Haiech J, Harwood CR, Hénaut A, Hilbert H, Holsappel S, Hosono S, Hullo M-F, Itaya M, Jones L, Joris B, Karamata D, Kasahara Y, Klaerr-Blanchard M, Klein C, Kobayashi Y, Koetter P, Koningstein G, Krogh S, Kumano M, Kurita K, Lapidus A, Lardinois S, Lauber J, Lazarevic V, Lee S-M, Levine A, Liu H, Masuda S, Mauël C, Médigue C, Medina N, Mellado RP, Mizuno M, Moestl D, Nakai S, Noback M, Noone D, O’Reilly M, Ogawa K, Ogiwara A, Oudega B, Park S-H, Parro V, Pohl TM, Portetelle D, Porwollik S, Prescott AM, Presecan E, Pujic P, Purnelle B, Rapoport G, Rey M, Reynolds S, Rieger M, Rivolta C, Rocha E, Roche B, Rose M, Sadaie Y, Sato T, Scanlan E, Schleich S, Schroeter R, Scoffone F, Sekiguchi J, Sekowska A, Seror SJ, Serror P, Shin B-S, Soldo B, Sorokin A, Tacconi E, Takagi T, Takahashi H, Takemaru K, Takeuchi M, Tamakoshi A, Tanaka T, Terpstra P, Tognoni A, Tosato V, Uchiyama S, Vandenbol M, Vannier F, Vassarotti A, Viari A, Wambutt R, Wedler E, Wedler H, Weitzenegger T, Winters P, Wipat A, Yamamoto H, Yamane K, Yasumoto K, Yata K, Yoshida K, Yoshikawa H-F, Zumstein E, Yoshikawa H, Danchin A. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 23.Sa-Nogueira I, Nogueira TV, Soares S, de Lencastre H. The Bacillus subtilis L-arabinose (ara) operon: nucleotide sequence, genetic organization and expression. Microbiology. 1997;143:957–969. doi: 10.1099/00221287-143-3-957. [DOI] [PubMed] [Google Scholar]
- 24.Guo Z, Wang F, Shen T, Huang J, Wang Y, Ji C. Crystal structure of thermostable p-nitrophenylphosphatase from Bacillus Stearothermophilus (Bs-TpNPPase) Protein Pept Lett. 2014;21:483–489. doi: 10.2174/0929866520666131119200255. [DOI] [PubMed] [Google Scholar]
- 25.Nicolas P, Mäder U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, Becher D, Bisicchia P, Botella E, Delumeau O, Doherty G, Denham EL, Fogg MJ, Fromion V, Goelzer A, Hansen A, Härtig E, Harwood CR, Homuth G, Jarmer H, Jules M, Klipp E, Le Chat L, Lecointe F, Lewis P, Liebermeister W, March A, Mars RA, Nannapaneni P, Noone D, Pohl S, Rinn B, Rügheimer F, Sappa PK, Samson F, Schaffer M, Schwikowski B, Steil L, Stülke J, Wiegert T, Devine KM, Wilkinson AJ, van Dijl JM, Hecker M, Völker U, Bessières P, Noirot P. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science. 2012;335:1103–1106. doi: 10.1126/science.1206848. [DOI] [PubMed] [Google Scholar]
- 26.Morimoto T, Loh PC, Hirai T, Asai K, Kobayashi K, Moriya S, Ogasawara N. Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis. Microbiology. 2002;148:3539–3552. doi: 10.1099/00221287-148-11-3539. [DOI] [PubMed] [Google Scholar]
- 27.Fillinger S, Boschi-Muller S, Azza S, Dervyn E, Branlant G, Aymerich S. Two glyceraldehyde-3-phosphate dehydrogenases with opposite physiological roles in a nonphotosynthetic bacterium. J Biol Chem. 2000;275:14031–14037. doi: 10.1074/jbc.275.19.14031. [DOI] [PubMed] [Google Scholar]
- 28.Feucht A, Lucet I, Yudkin MD, Errington J. Cytological and biochemical characterization of the FtsA cell division protein of Bacillus subtilis. Mol Microbiol. 2001;40:115–125. doi: 10.1046/j.1365-2958.2001.02356.x. [DOI] [PubMed] [Google Scholar]
- 29.Galinier A, Kravanja M, Engelmann R, Hengstenberg W, Kilhoffer MC, Deutscher J, Haiech J. New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression. Proc Natl Acad Sci U S A. 1998;95:1823–1828. doi: 10.1073/pnas.95.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yansura DG, Henner DJ. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984;81:439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bianchi V, Spychala J. Mammalian 5′-nucleotidases. J Biol Chem. 2003;278:46195–46198. doi: 10.1074/jbc.R300032200. [DOI] [PubMed] [Google Scholar]
- 32.Koshland DE., Jr Control of enzyme activity and metabolic pathways. Trends Biochem Sci. 1984;9:155–159. doi: 10.1016/0968-0004(84)90125-7. [DOI] [Google Scholar]
- 33.Newsholme EA, Challiss RAJ, Crabtree B. Substrate cycles: their role in improving sensitivity in metabolic control. Trends Biochem Sci. 1984;9:277–280. doi: 10.1016/0968-0004(84)90165-8. [DOI] [Google Scholar]
- 34.Breton A, Pinson B, Coulpier F, Giraud MF, Dautant A, Daignan-Fornier B. Lethal Accumulation of Guanylic Nucleotides in Saccharomyces cerevisiae HPT1-Deregulated Mutants. Genetics. 2008;178:815–824. doi: 10.1534/genetics.107.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pöther DC, Liebeke M, Hochgräfe F, Antelmann H, Becher D, Lalk M, Lindequist U, Borovok I, Cohen G, Aharonowitz Y, Hecker M. Diamide triggers mainly S thiolations in the cytoplasmic proteomes of Bacillus subtilis and Staphylococcus aureus. J Bacteriol. 2009;191:7520–7530. doi: 10.1128/JB.00937-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh R, Mailloux RJ, Puiseux-Dao S, Appanna VD. Oxidative stress evokes a metabolic adaptation that favors increased NADPH synthesis and decreased NADH production in Pseudomonas fluorescens. J Bacteriol. 2007;189:6665–6675. doi: 10.1128/JB.00555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, Asai K, Ashikaga S, Aymerich S, Bessieres P, Boland F, Brignell SC, Bron S, Bunai K, Chapuis J, Christiansen LC, Danchin A, Débarbouille M, Dervyn E, Deuerling E, Devine K, Devine SK, Dreesen O, Errington J, Fillinger S, Foster SJ, Fujita Y, Galizzi A, Gardan R, Eschevins C, Fukushima T, Haga K, Harwood CR, Hecker M, Hosoya D, Hullo MF, Kakeshita H, Karamata D, Kasahara Y, Kawamura F, Koga K, Koski P, Kuwana R, Imamura D, Ishimaru M, Ishikawa S, Ishio I, Le Coq D, Masson A, Mauël C, Meima R, Mellado RP, Moir A, Moriya S, Nagakawa E, Nanamiya H, Nakai S, Nygaard P, Ogura M, Ohanan T, O’Reilly M, O’Rourke M, Pragai Z, Pooley HM, Rapoport G, Rawlins JP, Rivas LA, Rivolta C, Sadaie A, Sadaie Y, Sarvas M, Sato T, Saxild HH, Scanlan E, Schumann W, Seegers JF, Sekiguchi J, Sekowska A, Séror SJ, Simon M, Stragier P, Studer R, Takamatsu H, Tanaka T, Takeuchi M, Thomaides HB, Vagner V, van Dijl JM, Watabe K, Wipat A, Yamamoto H, Yamamoto M, Yamamoto Y, Yamane K, Yata K, Yoshida K, Yoshikawa H, Zuber U, Ogasawara N. Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins AR, Lamb HK, Smith M, Keyte JW, Roberts CF. Molecular organisation of the quinic acid utilization (QUT) gene cluster in Aspergillus nidulans. Mol Gen Genet. 1988;214:224–231. doi: 10.1007/BF00337715. [DOI] [PubMed] [Google Scholar]
- 39.Chen IW, Charalampous FC. A soluble enzyme system from yeast which catalyzes the biosynthesis of inositol from glucose. Biochem Biophys Res Commun. 1963;12:62–67. doi: 10.1016/0006-291X(63)90414-5. [DOI] [PubMed] [Google Scholar]
- 40.Majerus PW. Inositol phosphate biochemistry. Annu Rev Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- 41.Cronan JE. Bacterial membrane lipids: where do we stand? Annu Rev Microbiol. 2003;57:203–224. doi: 10.1146/annurev.micro.57.030502.090851. [DOI] [PubMed] [Google Scholar]
- 42.den Kamp JA, Redai I, van Deenen LL. Phospholipid composition of Bacillus subtilis. J Bacteriol. 1969;99:298–303. doi: 10.1128/jb.99.1.298-303.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiba K, Ito K, Yura T. Mutation that suppresses the protein export defect of the secY mutation and causes cold-sensitive growth of Escherichia coli. J Bacteriol. 1984;160:696–701. doi: 10.1128/jb.160.2.696-701.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yano R, Nagai H, Shiba K, Yura T. A mutation that enhances synthesis of sigma 32 and suppresses temperature-sensitive growth of the rpoH15 mutant of Escherichia coli. J Bacteriol. 1990;172:2124–2130. doi: 10.1128/jb.172.4.2124-2130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito K, Wittekind M, Nomura M, Shiba K, Yura T, Miura A, Nashimoto H. A temperature-sensitive mutant of E. coli exhibiting slow processing of exported proteins. Cell. 1983;32:789–797. doi: 10.1016/0092-8674(83)90065-X. [DOI] [PubMed] [Google Scholar]
- 46.Grossman AD, Erickson JW, Gross CA. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984;38:383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- 47.Li K, Xu C, Jin Y, Sun Z, Liu C, Shi J, Chen G, Chen R, Jin S, Wu W. SuhB is a regulator of multiple virulence genes and essential for pathogenesis of Pseudomonas aeruginosa. MBio. 2013;4:e00419–13. doi: 10.1128/mBio.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosales-Reyes R, Saldías MS, Aubert DF, El-Halfawy OM, Valvano MA. The suhB gene of Burkholderia cenocepacia is required for protein secretion, biofilm formation, motility and polymyxin B resistance. Microbiology. 2012;158:2315–2324. doi: 10.1099/mic.0.060988-0. [DOI] [PubMed] [Google Scholar]
- 49.Kozloff LM, Turner MA, Arellano F, Lute M. Phosphatidylinositol, a phospholipid of ice-nucleating bacteria. J Bacteriol. 1991;173:2053–2060. doi: 10.1128/jb.173.6.2053-2060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Englesberg E, Anderson RL, Weinberg R, Lee N, Hoffee P, Huttenhauer G, Boyer H. L-Arabinose-sensitive, L-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol. 1962;84:137–146. doi: 10.1128/jb.84.1.137-146.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irani MH, Maitra PK. Properties of Escherichia coli mutants deficient in enzymes of glycolysis. J Bacteriol. 1977;132:398–410. doi: 10.1128/jb.132.2.398-410.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yarmolinsky MB, Wiesmeyer H, Kalckar HM, Jordan E. Hereditary defects in galactose metabolism in Escherichia coli mutants. II. Galactose-induced sensitivity. Proc Natl Acad Sci U S A. 1959;45:1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lowry OH, Carter J, Ward JB, Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971;246:6511–6521. [PubMed] [Google Scholar]
- 54.Papagianni M, Avramidis N, Filiousis G. Glycolysis and the regulation of glucose transport in Lactococcus lactis spp. lactis in batch and fed-batch culture. Microb Cell Fact. 2007;6:16. doi: 10.1186/1475-2859-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paoletti L, Lu YJ, Schujman GE, de Mendoza D, Rock CO. Coupling of fatty acid and phospholipid synthesis in Bacillus subtilis. J Bacteriol. 2007;189:5816–5824. doi: 10.1128/JB.00602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishii K, Shiio I. Improved inosine production and derepression of purine nucleotide biosynthetic enzymes in 8-azaguanine resistant mutants of Bacillus subtilis. Agric Biol Chem. 1972;36:1511–1522. doi: 10.1080/00021369.1972.10860448. [DOI] [Google Scholar]
- 57.Matsui H, Sato K, Enei H, Hirose Y. Mutation of an inosine producing strain of Bacillus subtilis to DL-methionine sulfoxide resistant for guanosine production. Appl Environ Microbiol. 1977;34:337–341. doi: 10.1128/aem.34.4.337-341.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotani Y, Yamaguchi K, Kato F, Furuya A. Inosine accumulation by mutants of Brevibacterium ammoniagenes: strain improvement and culture conditions. Agric Biol Chem. 1978;42:399–405. [Google Scholar]
- 59.Matsui H, Sato K, Enei H, Takinami K. 5′-Nucleotidase activity in improved inosine-producing mutants of Bacillus subtilis. Agric Biol Chem. 1982;46:2347–2352. [Google Scholar]
- 60.Chen S, Chu J, Zhuang Y, Zhang S. Enhancement of inosine production by Bacillus subtilis through suppression of carbon overflow by sodium citrate. Biotechnol Lett. 2005;27:689–692. doi: 10.1007/s10529-005-4686-1. [DOI] [PubMed] [Google Scholar]
- 61.Barnett JE, Brice RE, Corina DL. A colorimetric determination of inositol monophosphates as an assay for D-glucose 6-phosphate-1 L-myoinositol 1-phosphate cyclase. Biochem J. 1970;119:183–186. doi: 10.1042/bj1190183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morimoto T, Ara K, Ozaki K, Ogasawara N. A new simple method to introduce marker-free deletions in the Bacillus subtilis genome. Genes Genet Syst. 2009;84:315–318. doi: 10.1266/ggs.84.315. [DOI] [PubMed] [Google Scholar]
- 63.Miwa Y, Nakata A, Ogiwara A, Yamamoto M, Fujita Y. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 2000;28:1206–1210. doi: 10.1093/nar/28.5.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vagner V, Dervyn E, Ehrlich SD. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request. The phylogenetic tree, sequence data, and alignments used to produce the results displayed in Fig. 1 are deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S19999).


