Abstract
Objectives
Banha-sasim-tang (BST), which consists of seven different herbs, is one of the most popular herbal formulae for treating gastrointestinal disorders in Eastern Asia. The commonly used herbal medicine is often co-administered with other therapeutic drugs, which raises the possibility of herb–drug interactions and may modify the clinical safety profile of therapeutic drugs.
Methods
We investigated the potential herb–drug interactions between BST extract and midazolam (MDZ) in mice. The area under the plasma concentration-time curve (AUC) of MDZ and 1ʹ-hydroxymidazolam (1ʹ-OH-MDZ) was evaluated for both oral and intraperitoneal administration of MDZ, following oral administration of BST (0.5 and 1 g/kg).
Results
It was found that the AUC of MDZ and 1ʹ-OH-MDZ was lower in case of oral administration of MDZ. Administration of BST extract was not associated with hepatic cytochrome P450 activity. BST extract induced a strong reduction in pH and it has been reported that oral mucosal absorption of MDZ is lower at low pH. The decreased absorption rate of MDZ might be caused by the ingredients of BST and may not be related to other factors such as increased excretion of MDZ by P-glycoprotein.
Conclusions
The altered pharmacokinetics of midazolam caused by co-administration with BST in vivo could be attributed to a decrease in pH and subsequent reduction of MDZ absorption rate.
Keywords: Herb-drug interaction, Pharmacokinetics, Banha-sasim-tang, Midazolam
Introduction
Herbal medicines are commonly used to treat various diseases worldwide and are often co-administered with therapeutic drugs. However, the co-administration of herbs and drugs raises the potential of herb–drug interactions, which may affect the clinical safety of therapeutic drugs. Herb–drug interactions can be predicted by evaluating pharmacokinetic parameters, including absorption, metabolism, distribution, and excretion of drugs. Several drugs or herbs may affect the activity of drug-metabolizing enzymes, especially cytochrome P450 (CYP), resulting in herb–drug or drug–drug interactions [1]. Among the CYP subfamily, CYP3A is one of the most important drug-metabolizing enzymes in humans. It metabolizes more than 50% of clinically administered drugs [2]. Midazolam (MDZ), a short-acting, water-soluble benzodiazepine, is widely used as a probe substrate for CYP3A in humans and mice [3,4]. MDZ is mainly metabolized to 1′-hydroxymidazolam (1′-OH-MDZ) by the CYP3A subfamily [5]. Therefore, MDZ and 1′-OH-MDZ are typical probes used to measure the activity of CYP3A both in vivo and in vitro [6,7].
Commonly known as Banha-sasim-tang (BST) in Korea, or Ban-xia-xie-xin tang in China, the herb under study is one of the most popular herbal formulae mentioned in old herbal prescription literature [8]. BST consists of seven different herbs, including Pinellia ternata, Zingiber officinale, Glycyrrhizae uralensis, Coptis japonica, Scutellaria baicalensis, Panax ginseng, and Zizyphus jujuba. In East Asia, BST has been used to treat gastrointestinal disorders [8]. In addition, BST has displayed preventive action against diarrhea induced by irinotecan [9]. BST has been used to treat chronic hypofunction of the gastrointestinal tract (GI) and functional abnormalities of the upper and lower GI system by positively improving GI hormone levels [10]. Recently in China, modified BST was shown to cause symptomatic improvement in patients with functional dyspepsia [11].
To prevent or minimize adverse herb–drug interactions, herbal medicine that interacts with drugs should be investigated in both in vivo and in vitro systems. Despite extensive clinical research conducted on BST, its potential drug interactions remain to be elucidated [8,12]. BST has been more frequently prescribed in combination therapy with other herbs or drugs than on its own. Moreover, BST can be obtained easily without a prescription in Korea and patients sometimes self-prescribe medicines, which can lead to the co-administration of several different kinds of drugs. Here, a potential herb–drug interaction in BST therapy was investigated by evaluating the pharmacokinetics of model drug MDZ when simultaneously administered with BST on mouse plasma parameters, using an liquid chromatography-tandem mass spectrometry (LC-MS/MS) system.
Materials and Methods
Chemicals and Reagents
BST solid extract was obtained from Hanzung Pharmaceutical (Daejeon, Korea) and prepared in accordance with the Korean Pharmacopoeia (KP), 10th edition. The preparation contained the following ingredients: water extract of P. ternate (1.67 g, KP), Zingiber officinale (0.83 g, KP), Glycyrrhizae uralensis (1.0 g, KP), Coptis japonica (0.33 g, KP), Scutellaria baicalensis (1.0 g, KP), Panax ginseng (1.0 g, KP), and Zizyphus jujuba (1.0 g, KP). MDZ and 1′-OH-MDZ were obtained from Bukwang Pharmaceutical (Seoul, Korea) and Cayman Chemical (Ann Arbor, MI, USA), respectively.
Animals
Specific pathogen-free, 5-week-old male ICR mice (24 to 26 g) were obtained from Orient Bio (Seongnam, Korea) and acclimated for at least seven days before the experiment. Upon arrival, the animals were randomly housed in cages, with four or five per cage. The animal quarters were strictly maintained at 23 ± 3°C and 50 ± 10% relative humidity with a 12 hours light/dark cycle (intensity: 150–300 Lux). All animal procedures were performed in accordance with the Society of Toxicology guidelines of 1989. The study was approved by the institutional review board of the Kyungpook National University (2015-0099).
Pharmacokinetics Study
A total of 18 male ICR mice (30 ± 2 g) were randomly divided into a vehicle-treated, and two MDZ-treated groups, which also received BST extract dissolved in saline. In the first group, MDZ (2.0 mg/kg) was orally administered to nine mice after 5 minutes of oral administration of BST extract (0, 0.5 or 1.0 g/kg, n = 3). In the second group, nine mice were intraperitoneally treated with MDZ (2.0 mg/kg) after 5 minutes of oral administration of BST extract (0, 0.5 or 1.0 g/kg, n = 3). After the mice received MDZ, blood samples were collected from the tail vein into heparinized capillary tubes at 0.08-, 0.167-, 0.25-, 0.5-, 1-, and 2 hour time-points. The collected blood was immediately centrifuged at 4000 g for 10 minutes and 10 μL of plasma was obtained for each sample. The samples were stored at −20°C until analysis.
Plasma samples (10 μL) were added to 90 μL of acetonitrile (ACN) with 0.1% formic acid and 5 μM reserpine solution (internal standard [IS], 97.5:2.5, v/v) was added. The solution was mixed and centrifuged at 13000 rpm for 10 minutes at 4°C, and 90 μL of the supernatant was obtained. The supernatants were transferred to autosampler vials, and 5 μL aliquots were injected into the LC system.
Liquid Chromatography-tandem Mass Spectrometry Analysis System
All measurements were performed using an LC-MS/MS system in the selective reaction monitoring (SRM) mode. A Triple Stage Quadrupole Vantage mass spectrometer an HESI-II spray source coupled to an Accela™ LC system (Thermo Fisher Scientific, Waltham, MA, USA) was employed. The vaporizer and capillary temperatures were set to 150°C and 300°C, respectively. Electrospray ionization was performed in the positive mode at a spray voltage of 3500 V. Nitrogen was used as the sheath and auxiliary gas, and was set to 45 and 20 (arbitrary units), respectively. Data were analyzed using the Xcalibur software (Thermo Fisher Scientific). An ACE® 5C18 column (5 μm, 50 mm × 2.1 mm, Advanced Chromatography Technologies, Scotland, UK) and a guard C18 column (2 μm, 2.1 mm ID, Phenomenex, Torrance, CA, USA) were employed for LC separation. The mobile phase consisted of ACN with 0.1% FA (mobile phase A) and water (mobile phase B) at a flow rate of 220 μL/min. The gradient was as follows: 0 minute 5% A, 0.5 minutes 5% A, 1.5 minutes 95% A, 3.0 minutes 95% A, 3.5 minutes 5% A, 5.0 minutes 5% A. Ions monitored in the SRM mode were m/z 326.0→291.0 for MDZ, 342.0→203.0 for 1’-OH-MDZ m/z, and 609.4→174.1 for the IS, respectively, at an SRM collision energy of 29 eV for MDZ, 15 eV for 1ʹ-OH-MDZ and 42 eV for IS. Data procurement was controlled using the Xcalibur software (Thermo Fisher Scientific).
Method Validation
The calibration curves of MDZ and 1′-OH-MDZ ranged from 0.05 to 2.0 μg/mL, respectively. Calibration curves were constructed by plotting the peak-area ratios of analyte or IS vs. the concentrations of MDZ and 1’-OH-MDZ in mouse plasma. The equations for the calibration curves of MDZ and 1ʹ-OHMDZ were y = 25114 x–13.85 (r2 = 0.999) and y = 19380x – 9.12 (r2= 0.998), respectively. The lower limits of quantification values of MDZ and 1ʹ-OH-MDZ were 50 and 50 ng/mL, respectively. The quality control samples of the methodology were evaluated by analyzing five replicates of mouse plasma spiked with known concentrations of MDZ (0.05, 0.5, 1.0, and 2.0 μg/mL) or 1’-OH-MDZ (0.05, 0.5, 1.0, and 2.0 μg/mL) (Table S1).
PH Determination of the Mixture Containing Banha-sasim-tang Extract
The pH of BST extract was measured using a Metter Toledo S220 SevenCompact™ pH/Ion pH meter (Metter-Toledo International Inc., Columbus, OH, USA). The pH of each concentration of BST extract in the mixture was measured immediately after it dissolved.
Pharmacokinetic Parameters
A non-compartmental model was used to calculate the pharmacokinetic parameters, using WinNonlin version 2.1 (Scientific Consulting Inc., Cary, NC, USA), and includes maximum plasma concentration (Cmax), time to reach maximum plasma concentration (Tmax), area under the plasma concentration-time curve (AUC), and half-life.
Statistics
The mean value ± standard error was determined for each treatment group of a given experiment. Dunnett’s t-test was performed to compare the statistical significance of the data. The p-values < 0.05 were considered statistically significant. These are represented by asterisks.
Results
To investigate the potential herb–drug interactions observed during co-administration of BST with MDZ, two different administration methods were evaluated (Figure 1). Firstly, MDZ (2.0 mg/kg) was orally administered after treatment with BST extract (0, 0.5, or 1.0 g/kg) by oral gavage (Figure 1A). By monitoring the plasma concentration of MDZ and 1ʹ-OH-MDZ, first-pass metabolism of BST can be determined. In the other group, MDZ was intraperitoneally administered following oral administration of BST, to focus on the effects of BST in the metabolic system and eliminate the absorption step (Figure 1B).
Figure 1.
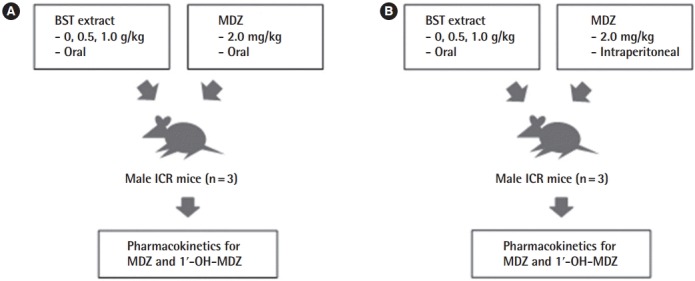
Experimental design. (A) Male ICR mice were orally administered MDZ (2.0 mg/kg) following administration of BST extract (0, 0.5, or 1.0 g/kg) or vehicle (saline) by oral gavage. (B) Male ICR mice were intraperitoneally treated with MDZ (2.0 mg/kg) following administration of BST extract (0, 0.5, or 1.0 g/kg) or vehicle (saline) by oral gavage. The blood samples were obtained and the concentrations of MDZ and 1ʹ-OH-MDZ (metabolite) were determined. MDZ, midazolam; BST, Banha-sasim-tang; 1ʹ-OH-MDZ, 1ʹ-hydroxymidazolam.
Mean plasma concentration–time profiles of MDZ and 1′-OH-MDZ were obtained (Figure 2) after simultaneous administration of a single-dose of BST (0, 0.5, or 1.0 g/kg, peroral [PO]) or vehicle control and a single dose of MDZ (2 mg/kg, PO) in mice. The pharmacokinetic parameters of MDZ and 1′-OH-MDZ were compared between the vehicle- and BST-treated groups (Table 1). Following oral co-administration of BST and MDZ, the mean Cmax and AUC values were significantly lower than those observed in the vehicle-treated group. The mean AUC decreased by 76% in the BST co-treated group (1.0 g/kg, PO). This corresponds to the plasma concentrationtime profile of 1′-OH-MDZ, in which Cmax and AUC were significantly decreased by 51% and 53%, respectively in the BST co-treated group (1.0 g/kg, PO). Here, both the AUC and Cmax of MDZ and its metabolite, 1′-OH-MDZ, decreased in the BST co-administered group, indicating decreased absorption of the parent drug MDZ.
Figure 2.
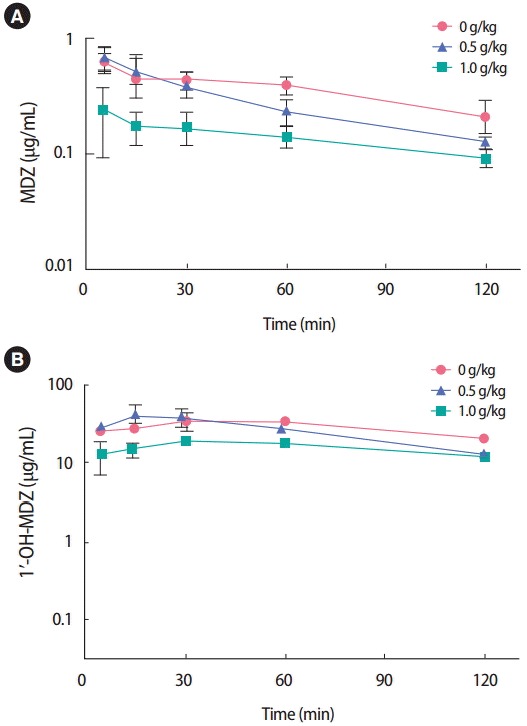
Mean plasma concentration-time profiles of MDZ and 1ʹ-OHMDZ in mice simultaneously treated with BST (0, 0.5, or 1.0 g/kg, PO) and MDZ (2.0 mg/kg, PO). Data are presented as mean±standard error (n=3). MDZ, midazolam; 1ʹ-OH-MDZ, 1ʹ-hydroxymidazolam; BST, Banha-sasimtang; PO, peroral.
Table 1.
Effects of co-administration of BST extract with MDZ on the pharmacokinetic parameters of MDZ and 1ʹ-OH-MDZ (n=3)
| Parameters | BST extract (g/kg) | Oral administration of MDZ |
Intraperitoneal administration of MDZ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC (mg-min/mL) | Cmax (mg/mL) | Half-life (min) | Tmax (min) | AUC (mg-min/mL) | Cmax (mg/mL) | Half-life (min) | Tmax (min) | ||
| MDZ | 0.0 | 45.7±2.9 | 0.7±0.1 | 167.3±116.8 | 5.0±0.0 | 234.5±9.5 | 4.5±0.1 | 23.1±1.5 | 11.7±1.7 |
| 0.5 | 33.9±6.5 | 0.7±0.1 | 59.3±15.3 | 8.3±3.3 | 220.1±26.2 | 4.5±0.5 | 25.0±3.1 | 10.0±0.0 | |
| 1.0 | 16.9±4.1** | 0.3±0.1 | 79.1±1.2 | 26.7±16.9 | 197.9±7.8 | 3.9±0.2 | 23.1±0.8 | 9.0±1.3 | |
| 1'-OH MDZ | 0.0 | 3540.8±312.7 | 43.1±8.5 | 55.3±0.0 | 50.0±10.0 | 18.9±0.8 | 0.2±0.01 | 159.8±11.2 | 20.0±5.0 |
| 0.5 | 3189.7±907.0 | 42.9±12.6 | 71.8±12.3 | 16.7±7.3 | 18.2±0.3 | 0.1±0.01 | 166.0±6.1 | 13.8±1.3 | |
| 1.0 | 1901.0±194.2* | 21.4±2.5 | 101.1±1.7 | 31.7±15.9 | 18.2±0.4 | 0.1±0.0 | 169.0±11.3 | 21.0±3.8 | |
Data are presents as mean±standard errors.
BST, Banha-sasim-tang; MDZ, midazolam; 1ʹ-OH-MDZ, 1ʹ-hydroxymidazolam; AUC, area under the plasma concentration-time curve; Cmax, maximum plasma concentration; Tmax, maximum plasma concentration
p<0.05
p<0.01 compared to the vehicle-treated group.
ICR male mice were simultaneously treated with MDZ (2.0 mg/kg, intraperitoneal [IP]) following oral administration of BST (0.5 or 1.0 g/kg), to investigate the inhibitory effect of BST on CYP3A4 activity. The concentration-time profiles of MDZ and 1′-OH-MDZ in plasma are shown in Figure 3. The pharmacokinetics parameters of MDZ and 1′-OH-MDZ in the BST-treated group did not differ significantly from those seen in the vehicle-treated group (Table 1). In addition, BST did not exert an inhibitory effect on CYP1A, 2B, 2C, 2D, or 3A4 at concentrations of 0 to 0.5 g/mL in human liver microsomes, based on the use of a cocktail of probe substrate and LC-MS/MS analysis (data not shown) [7].
Figure 3.
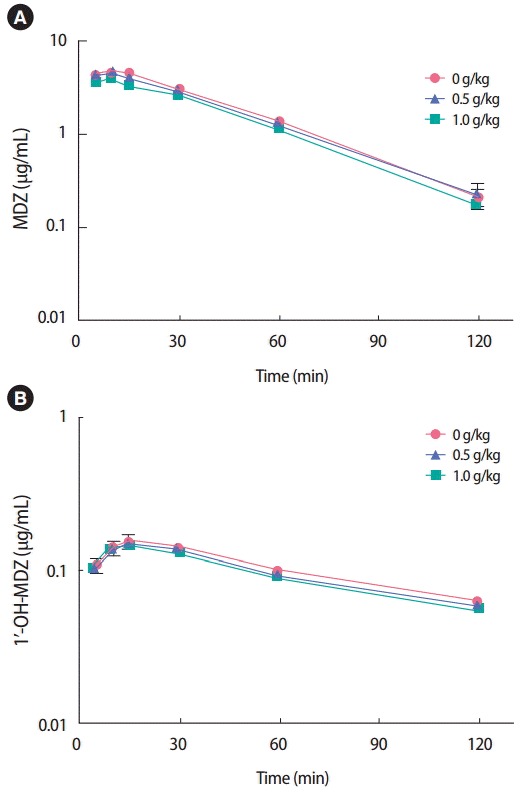
Mean plasma concentration-time profiles of MDZ and 1ʹ-OH-MDZ in mice simultaneously treated with BST (0, 0.5, or 1.0 g/kg, PO) and MDZ (2.0 mg/kg, IP). Data are presented as mean ± standard error (n=3). MDZ, midazolam; 1ʹ-OH-MDZ, 1ʹ-hydroxymidazolam; BST, Banha-sasimtang; PO, peroral; IP, intraperitoneal.
Discussion
Our study showed that the pharmacokinetic parameters of MDZ and 1′-OH-MDZ decreased following oral co-administration with BST, but not in case of IP administration. When MDZ was orally administrated, the plasma concentration of MDZ was regulated by two mechanisms: absorption rate and metabolic stability in the liver. The plasma concentration of MDZ was found to be mainly affected by a mechanism involving metabolic conversion of MDZ in the liver. The plasma concentration of MDZ and its metabolites was not changed by BST IP co-administration, which indicates that BST does not have a strong inhibitory action on CYP activities in the liver. Moreover, a decrease in plasma concentration of MDZ and 1’-OH-MDZ is observed following oral co-administration with BST. The findings therefore indicate that BST affects the absorption of MDZ, but not its metabolism.
The reduction in plasma concentration of MDZ and 1′-OH-MDZ in animals co-administered with MDZ and BST (1.0 g/kg, PO) indicates that the absorption of MDZ might be inhibited by BST, and that this inhibition might not be associated with metabolic activity or CYP3A4 enzyme. The reduced absorption of MDZ may be attributed to several phenomena. One is the formation of insoluble particles from the components of BST extract interacting with MDZ at physiological pH conditions (pH 1-2 in the stomach, or pH 5-6 in the intestines). However, the concentrations of MDZ in the supernatants of the BST-MDZ mixture in artificial gastric juice or intestinal juice was not significantly altered (Figure S1). In this way, the ingredients in the extract were found not to form insoluble particles that could reduce the absorption of MDZ in the stomach and intestines.
Another hypothesis is that the reduced pH induced by BST extract in the stomach or intestines affects the oral mucosal absorption of MDZ, since it has been reported that MDZ absorption is strongly inhibited under low pH conditions [13]. Furthermore, changes in local pH affect intestinal absorption of water-soluble, weakly acidic compounds. The bioavailability of MDZ at pH 2.8, 3.2, and 3.9 solutions was 6.2, 18.7, and 22.6% respectively [14]. As shown in Figure 4, pH significantly reduced with increasing concentrations of BST extract (1-100 mg/mL). The pH value of normal saline was 5.9, whereas that of 1 and 10 mg/mL BST extract was 5.0 and 4.7, respectively. Since BST consists of seven herbs, it exhibits varying concentration ranges for each of its diverse ingredients, and thus might contain acidic compounds that decrease pH.
Figure 4.
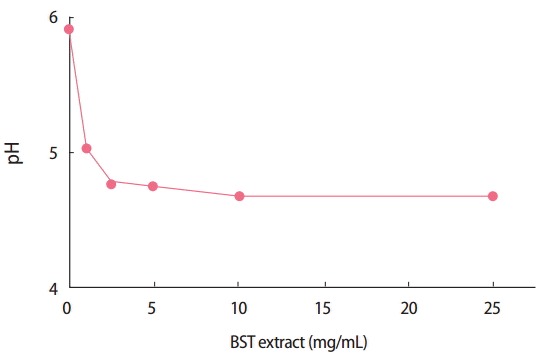
PH values of the Banha-sasim-tang (BST) extract mixtures (0–100 mg/mL).
The other possible mechanism involved in the phenomena under investigation is that the ingredients in BST inhibits the absorption of MDZ in the stomach or intestine. The complex ingredients in BST might compete with the absorption of MDZ. Since MDZ is not a substrate of P-glycoprotein (P-gp), the reduced plasma concentration cannot be related to increased excretion by P-gp [13]. Further, following co-administration with BST (1.0 g/kg, PO), the absorption of caffeine was found to be inhibited, since the bioavailability of caffeine has been shown to be almost 100% in vivo [14]. Moreover, we evaluated the change in the pharmacokinetic parameters of caffeine and paraxanthine after BST administration Table S2. The plasma concentration of caffeine and its metabolite, paraxanthine, decreased following the administration of BST (0.5 and 1.0 g/kg) by oral gavage, as was seen in case of MDZ-BST co-administration. In a previous study, Kampo extract used in traditional Japanese medicines, originated from Glycyrrhiza uralensis, reduced the Cmax and AUC of talinolol, a P-gp substrate drug [15]. Decreased intestinal talinolol absorption induced by Kampo extract might be caused by the inhibition of organic anion transporting peptides, and not by inhibition of P-gp. The low plasma concentration of MDZ or caffeine following co-administration with BST indicated that the absorption rate was inhibited by components of the BST extract.
Here, we investigated the herb–drug interactions between BST extract and MDZ in vivo, following oral administration of BST extract (1.0 g/kg). BST extract did not exert effects on the activity of an enzyme crucial to drug interactions, CYP, in the liver of the mouse model studied. Nevertheless, the pharmacokinetic parameters of MDZ were altered when it was orally administered following BST extract treatment (1.0 g/kg), as evidenced by the observed reduction in the total absorption of MDZ and 1′-OH-MDZ. The reduction of pH induced by the administration of BST extract indicates mechanisms behind the decreased rate of mucosal absorption of MDZ. In conclusion, the oral administration of BST extract together with MDZ might cause potential herb–drug interactions in vivo.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Export Promotion Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant no. 316017-3).
Footnotes
The authors have no conflicts of interest associated with material presented in this paper.
Supplementary Material
The accuracy and RSD of quality control samples of MDZ and 1’-OH MDZ
Effects of co-administration of BST extract on the pharmacokinetic parameters of caffeine and paraxanthine
The concentration of MDZ in the supernatant of artificial gastric juice (A) and intestinal juice (B) with MDZ and BST. The data shown are the means of duplicates. MDZ, midazolam; BST, Banha-sasim-tang.
References
- 1.Chen XW, Sneed KB, Pan SY, Cao C, Kanwar JR, Chew H, et al. Herb-drug interactions and mechanistic and clinical considerations. Curr Drug Metab. 2012;13(5):640–651. doi: 10.2174/1389200211209050640. [DOI] [PubMed] [Google Scholar]
- 2.Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13(3):129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 3.Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. J Emerg Med. 1997;15(3):357–365. doi: 10.1016/s0736-4679(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 4.Perloff MD, von Moltke LL, Court MH, Kotegawa T, Shader RI, Greenblatt DJ. Midazolam and triazolam biotransformation in mouse and human liver microsomes: relative contribution of CYP3A and CYP2C isoforms. J Pharmacol Exp Ther. 2000;292(2):618–628. [PubMed] [Google Scholar]
- 5.Kronbach T, Mathys D, Umeno M, Gonzalez FJ, Meyer UA. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol. 1989;36(1):89–96. [PubMed] [Google Scholar]
- 6.Kato R, Yamashita S, Moriguchi J, Nakagawa M, Tsukura Y, Uchida K, et al. Changes of midazolam pharmacokinetics in Wistar rats treated with lipopolysaccharide: relationship between total CYP and CYP3A2. Innate Immun. 2008;14(5):291–297. doi: 10.1177/1753425908095956. [DOI] [PubMed] [Google Scholar]
- 7.Song M, Hong M, Lee MY, Jee JG, Lee YM, Bae JS, et al. Selective inhibition of the cytochrome P450 isoform by hyperoside and its potent inhibition of CYP2D6. Food Chem Toxicol. 2013;59:549–553. doi: 10.1016/j.fct.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Park JW, Ko SJ, Han G, Yeo I, Ryu B, Kim J. The effects of Banhasasim-tang on dyspeptic symptoms and gastric motility in cases of functional dyspepsia: a randomized, double-blind, placebo-controlled, and two-center trial. Evid Based Complement Alternat Med. 2013;2013:265035. doi: 10.1155/2013/265035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori K, Kondo T, Kamiyama Y, Kano Y, Tominaga K. Preventive effect of Kampo medicine (Hangeshashin-to) against irinotecan-induced diarrhea in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2003;51(5):403–406. doi: 10.1007/s00280-003-0585-0. [DOI] [PubMed] [Google Scholar]
- 10.Naito T, Itoh H, Yasunaga F, Takeyama M. Hange-shashin-to raises levels of somatostatin, motilin, and gastrin in the plasma of healthy subjects. Biol Pharm Bull. 2002;25(3):327–331. doi: 10.1248/bpb.25.327. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Zhang S, Wang Z, Wang C, Huang S, Shen H, et al. Efficacy of modified ban xia xie xin decoction on functional dyspepsia of cold and heat in complexity syndrome: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:812143. doi: 10.1155/2013/812143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JW, Ryu B, Yeo I, Jerng UM, Han G, Oh S, et al. Banha-sasimtang as an herbal formula for the treatment of functional dyspepsia: a randomized, double-blind, placebo-controlled, two-center trial. Trials. 2010;11:83. doi: 10.1186/1745-6215-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirby B, Kharasch ED, Thummel KT, Narang VS, Hoffer CJ, Unadkat JD. Simultaneous measurement of in vivo P-glycoprotein and cytochrome P450 3A activities. J Clin Pharmacol. 2006;46(11):1313–1319. doi: 10.1177/0091270006292625. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard J, Sawers SJ. The absolute bioavailability of caffeine in man. Eur J Clin Pharmacol. 1983;24(1):93–98. doi: 10.1007/BF00613933. [DOI] [PubMed] [Google Scholar]
- 15.Iwanaga K, Arimune K, Miyazaki M, Shibano M, Taniguchi M, Baba K, et al. Effects of furanocoumarins in Kampo extract-based medicines on rat intestinal absorption of CYP3A and P-glycoprotein substrate drugs in vivo. Arch Pharm Res. 2012;35(6):1055–1064. doi: 10.1007/s12272-012-0613-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The accuracy and RSD of quality control samples of MDZ and 1’-OH MDZ
Effects of co-administration of BST extract on the pharmacokinetic parameters of caffeine and paraxanthine
The concentration of MDZ in the supernatant of artificial gastric juice (A) and intestinal juice (B) with MDZ and BST. The data shown are the means of duplicates. MDZ, midazolam; BST, Banha-sasim-tang.


