Abstract
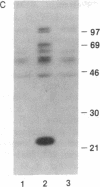
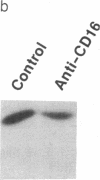
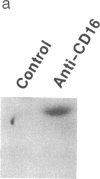
The zeta chain has emerged to be a key subunit of the T-cell antigen receptor with central roles not only in intracellular assembly of the multimeric receptor but also in mediating signal transduction events. This subunit is present in natural killer (NK) cells that lack the other subunits of the T-cell antigen receptor. In NK cells, the zeta chain appears to be associated with the NK Fc receptor [type 3 receptor for the Fc portion of IgG (Fc gamma RIII or CD16)] and may be necessary for efficient cell surface expression of this receptor complex. In T cells, the zeta chain is a prominent substrate that becomes phosphorylated on tyrosine residues after occupancy of the TCR; zeta chain phosphorylation was in fact the first evidence that the TCR was coupled to a protein-tyrosine kinase as well as to inositol phospholipid hydrolysis. To determine if Fc gamma RIII is coupled to a protein-tyrosine kinase in a manner analogous to the T-cell antigen receptor, we investigated ligand-dependent zeta-chain phosphorylation in NK cells. We observed that activation of NK cells with an anti-Fc gamma RIII monoclonal antibody induced tyrosine phosphorylation of the zeta chain whereas other activating stimuli, such as the combination of phorbol ester and ionomycin or a lymphokine, interleukin 2, did not result in phosphorylation of this protein. Perturbation of Fc gamma RIII by the more physiological stimulus, incubation of NK cells with antibody-coated target cells, also induced zeta-chain phosphorylation. Previous data have indicated that the NK-cell Fc gamma RIII is coupled to inositol phospholipid hydrolysis. This present finding that Fc gamma RIII is coupled to a protein-tyrosine kinase illustrates that there are significant similarities in the signaling pathways activated by Fc gamma RIII in NK cells and the T-cell antigen receptor in T cells; the zeta chain is a common element that may serve as a coupling protein for both of these receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Caligiuri M., O'Brien C., Manley T., Ritz J., Schlossman S. F. Fc gamma receptor type III (CD16) is included in the zeta NK receptor complex expressed by human natural killer cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2274–2278. doi: 10.1073/pnas.87.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Caligiuri M., Ritz J., Schlossman S. F. CD3-negative natural killer cells express zeta TCR as part of a novel molecular complex. Nature. 1989 Sep 14;341(6238):159–162. doi: 10.1038/341159a0. [DOI] [PubMed] [Google Scholar]
- Ashwell J. D., Klusner R. D. Genetic and mutational analysis of the T-cell antigen receptor. Annu Rev Immunol. 1990;8:139–167. doi: 10.1146/annurev.iy.08.040190.001035. [DOI] [PubMed] [Google Scholar]
- Baniyash M., Garcia-Morales P., Luong E., Samelson L. E., Klausner R. D. The T cell antigen receptor zeta chain is tyrosine phosphorylated upon activation. J Biol Chem. 1988 Dec 5;263(34):18225–18230. [PubMed] [Google Scholar]
- Blank U., Ra C., Miller L., White K., Metzger H., Kinet J. P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989 Jan 12;337(6203):187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- Cassatella M. A., Anegón I., Cuturi M. C., Griskey P., Trinchieri G., Perussia B. Fc gamma R(CD16) interaction with ligand induces Ca2+ mobilization and phosphoinositide turnover in human natural killer cells. Role of Ca2+ in Fc gamma R(CD16)-induced transcription and expression of lymphokine genes. J Exp Med. 1989 Feb 1;169(2):549–567. doi: 10.1084/jem.169.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. src-related tyrosine protein kinases as signaling components in hematopoietic cells. Cancer Cells. 1990 Oct;2(10):303–310. [PubMed] [Google Scholar]
- Ferris D. K., Willette-Brown J., Ortaldo J. R., Farrar W. L. IL-2 regulation of tyrosine kinase activity is mediated through the p70-75 beta-subunit of the IL-2 receptor. J Immunol. 1989 Aug 1;143(3):870–876. [PubMed] [Google Scholar]
- Frank S. J., Niklinska B. B., Orloff D. G., Merćep M., Ashwell J. D., Klausner R. D. Structural mutations of the T cell receptor zeta chain and its role in T cell activation. Science. 1990 Jul 13;249(4965):174–177. doi: 10.1126/science.2371564. [DOI] [PubMed] [Google Scholar]
- Hibbs M. L., Selvaraj P., Carpén O., Springer T. A., Kuster H., Jouvin M. H., Kinet J. P. Mechanisms for regulating expression of membrane isoforms of Fc gamma RIII (CD16). Science. 1989 Dec 22;246(4937):1608–1611. doi: 10.1126/science.2531918. [DOI] [PubMed] [Google Scholar]
- Hsi E. D., Siegel J. N., Minami Y., Luong E. T., Klausner R. D., Samelson L. E. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J Biol Chem. 1989 Jun 25;264(18):10836–10842. [PubMed] [Google Scholar]
- Inoue K., Yamamoto T., Toyoshima K. Specific expression of human c-fgr in natural immunity effector cells. Mol Cell Biol. 1990 Apr;10(4):1789–1792. doi: 10.1128/mcb.10.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D. Architectural editing: determining the fate of newly synthesized membrane proteins. New Biol. 1989 Oct;1(1):3–8. [PubMed] [Google Scholar]
- Klausner R. D., O'Shea J. J., Luong H., Ross P., Bluestone J. A., Samelson L. E. T cell receptor tyrosine phosphorylation. Variable coupling for different activating ligands. J Biol Chem. 1987 Sep 15;262(26):12654–12659. [PubMed] [Google Scholar]
- Kurosaki T., Ravetch J. V. A single amino acid in the glycosyl phosphatidylinositol attachment domain determines the membrane topology of Fc gamma RIII. Nature. 1989 Dec 14;342(6251):805–807. doi: 10.1038/342805a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Yu G., Phillips J. H. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989 Dec 14;342(6251):803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Merćep M., Weissman A. M., Frank S. J., Klausner R. D., Ashwell J. D. Activation-driven programmed cell death and T cell receptor zeta eta expression. Science. 1989 Dec 1;246(4934):1162–1165. doi: 10.1126/science.2531464. [DOI] [PubMed] [Google Scholar]
- Orloff D. G., Frank S. J., Robey F. A., Weissman A. M., Klausner R. D. Biochemical characterization of the eta chain of the T-cell receptor. A unique subunit related to zeta. J Biol Chem. 1989 Sep 5;264(25):14812–14817. [PubMed] [Google Scholar]
- Orloff D. G., Ra C. S., Frank S. J., Klausner R. D., Kinet J. P. Family of disulphide-linked dimers containing the zeta and eta chains of the T-cell receptor and the gamma chain of Fc receptors. Nature. 1990 Sep 13;347(6289):189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A., Overton R. Lymphokine-activated killer cells. Analysis of progenitors and effectors. J Exp Med. 1986 Oct 1;164(4):1193–1205. doi: 10.1084/jem.164.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo J. R., Young H. A., Varesio L. Modulation of CD3- large granular lymphocyte functions by agonist and antagonists of protein kinase C: effects on NK and lymphokine-activated killer activity and production of IFN-gamma. J Immunol. 1989 Jul 1;143(1):366–371. [PubMed] [Google Scholar]
- Oshimi K., Oshimi Y., Yamada O., Wada M., Hara T., Mizoguchi H. Cytotoxic T lymphocyte triggering via CD16 is regulated by CD3 and CD8 antigens. Studies with T cell receptor (TCR)-alpha beta+/CD3+16+ and TCR-gamma delta+/CD3+16+ granular lymphocytes. J Immunol. 1990 May 1;144(9):3312–3317. [PubMed] [Google Scholar]
- Patel M. D., Samelson L. E., Klausner R. D. Multiple kinases and signal transduction. Phosphorylation of the T cell antigen receptor complex. J Biol Chem. 1987 Apr 25;262(12):5831–5838. [PubMed] [Google Scholar]
- Ravetch J. V., Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989 Aug 1;170(2):481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman E. M., Thom R. R., Casnellie J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [PubMed] [Google Scholar]
- Samelson L. E., Harford J. B., Klausner R. D. Identification of the components of the murine T cell antigen receptor complex. Cell. 1985 Nov;43(1):223–231. doi: 10.1016/0092-8674(85)90027-3. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., O'Shea J. J., Luong H., Ross P., Urdahl K. B., Klausner R. D., Bluestone J. T cell antigen receptor phosphorylation induced by an anti-receptor antibody. J Immunol. 1987 Oct 15;139(8):2708–2714. [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. J., Bonifacino J. S., Lippincott-Schwartz J., Weissman A. M., Saito T., Klausner R. D., Ashwell J. D. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 1988 Jan 15;52(1):85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984 May;50(5):489–513. [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Baniyash M., Hou D., Samelson L. E., Burgess W. H., Klausner R. D. Molecular cloning of the zeta chain of the T cell antigen receptor. Science. 1988 Feb 26;239(4843):1018–1021. doi: 10.1126/science.3278377. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Bonifacino J. S., Klausner R. D., Samelson L. E., O'Shea J. J. T cell antigen receptor: structure, assembly and function. Year Immunol. 1989;4:74–93. [PubMed] [Google Scholar]
- Weissman A. M., Ross P., Luong E. T., Garcia-Morales P., Jelachich M. L., Biddison W. E., Klausner R. D., Samelson L. E. Tyrosine phosphorylation of the human T cell antigen receptor zeta-chain: activation via CD3 but not CD2. J Immunol. 1988 Nov 15;141(10):3532–3536. [PubMed] [Google Scholar]
- Weissman A. M., Samelson L. E., Klausner R. D. A new subunit of the human T-cell antigen receptor complex. Nature. 1986 Dec 4;324(6096):480–482. doi: 10.1038/324480a0. [DOI] [PubMed] [Google Scholar]
- van de Griend R. J., Tax W. J., van Krimpen B. A., Vreugdenhil R. J., Ronteltap C. P., Bolhuis R. L. Lysis of tumor cells by CD3+4-8-16+ T cell receptor alpha beta- clones, regulated via CD3 and CD16 activation sites, recombinant interleukin 2, and interferon beta 1. J Immunol. 1987 Mar 1;138(5):1627–1633. [PubMed] [Google Scholar]