Abstract
Purpose
REVEL demonstrated improved overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) with docetaxel+ramucirumab versus docetaxel+placebo in 1,253 intent-to-treat (ITT) stage IV non-small cell lung cancer patients with disease progression following platinum-based chemotherapy. Results from the East Asian subgroup analysis are reported.
Materials and Methods
Subgroup analyses were performed in the East Asian ITT population (n=89). Kaplan-Meier analysis and Cox proportional hazards regression were performed for OS and PFS, and the Cochran-Mantel-Haenszel test was performed for response rate.
Results
In docetaxel+ramucirumab (n=43) versus docetaxel+placebo (n=46), median OS was 15.44 months versus 10.17 months (hazard ratio [HR], 0.762; 95% confidence interval [CI], 0.444 to 1.307), median PFS was 4.88 months versus 2.79 months (HR, 0.658; 95% CI, 0.408 to 1.060), and ORR was 25.6% (95% CI, 13.5 to 41.2) versus 8.7% (95% CI, 2.4 to 20.8). Due to increased incidence of neutropenia and febrile neutropenia in East Asian patients, starting dose of docetaxel was reduced for newly enrolled East Asian patients (75 to 60 mg/m2, n=24). In docetaxel+ramucirumab versus docetaxel+placebo, incidence of neutropenia was 84.4% versus 72.7% (75 mg/m2) and 54.5% versus 38.5% (60 mg/m2). Incidence of febrile neutropenia was 43.8% versus 12.1% (75 mg/m2) and 0% versus 7.7% (60 mg/m2).
Conclusion
Results of this subgroup analysis showed a trend favoring ramucirumab+docetaxel for median OS, PFS, and improved ORR in East Asian patients, consistent with ITT population results. Reduction of starting dose of docetaxel in East Asian patients was associated with improved safety.
Keywords: Ramucirumab, Docetaxel, Non-small-cell lung carcinoma, East Asia
Introduction
Lung cancer is the leading cause of cancer-related death in both men and women in East Asia [1]. Lung cancer is classified as either small cell or non-small cell lung cancer (NSCLC) [2], and the latter group is histologically subdivided into squamous-cell carcinomas (25%) and non-squamous cell carcinomas (adenocarcinomas [55%] and large-cell carcinomas [5%]). The remaining NSCLC tumors are classified as “other” (5%) and “not otherwise specified” (10%) [2]. Initial therapy for NSCLC generally consists of four to six cycles of platinum-based chemotherapy [3], and some patients subsequently receive maintenance therapy [4]. Current clinically approved second-line therapies for non-squamous NSCLC include docetaxel, erlotinib, pemetrexed [5-7], and, very recently in some countries, nintedanib and nivolumab.
Treatment with docetaxel has resulted in improved overall survival (OS) compared with best supportive care [5]. Treatment with erlotinib has been shown to result in improved OS [6] over placebo. Pemetrexed provided overall efficacy similar to that of docetaxel [6] but had a greatly improved safety profile and is only approved and recommended for non-squamous NSCLC [7].
Vascular endothelial growth factors (VEGFs) have emerged as key regulators of angiogenesis, and the expression of VEGFs has been correlated with poor prognosis in several solid tumor types, including NSCLC [8]. Both VEGF and VEGF receptor-2 (VEGFR-2)–mediated signaling have an important role in angiogenesis and tumor growth [9]. Blockade of VEGFR-2 signaling inhibits formation, proliferation, and migration of new blood vessels [10].
The addition of bevacizumab, a recombinant humanized monoclonal antibody against VEGF, to carboplatin-paclitaxel first-line chemotherapy resulted in a significant improvement in OS in eligible patients with non-squamous NSCLC [11]. However, the addition of bevacizumab to first-line cisplatin-gemcitabine did not improve OS, albeit an improvement in progression-free survival (PFS) was observed [12].
Ramucirumab (IMC-1121B, ImClone Systems, Bridgewater, NJ) is a human recombinant IgG1 monoclonal antibody that specifically binds to the extracellular domain of VEGFR-2 with high affinity, preventing binding of VEGF ligands and receptor activation [13]. Two positive phase 3 studies investigating second-line treatment of advanced gastric cancer [14,15] showed that ramucirumab significantly improved survival as a single agent and in combination with paclitaxel. In addition, a positive phase 3 study investigating second-line treatment of metastatic colorectal carcinoma [16] showed that ramucirumab significantly improved survival in combination with folinic acid, 5-fluorouracil, and irinotecan (FOLFIRI).
It is important to note that studies from East Asia have reported higher rates of epidermal growth factor receptor (EGFR) mutations in NSCLC patients [17]. The superior response of EGFR-mutated NSCLC patients to therapy may impact the analysis of various treatment types, indicating the need to include screening for EGFR mutations in routine diagnostics and to analyze the response of East Asian NSCLC patients to therapy as a separate subgroup of the intent-to-treat (ITT) population.
Recognition of differences in OS and toxicity between East Asian and Caucasian patients with NSCLC, with longer survival [18], higher response rates, and greater toxicity to chemotherapy and targeted therapy reported in East Asian patients is increasing [19]. Therefore, subanalyses are now often conducted in East Asian NSCLC patients to establish the dosage in this ethnic group of NSCLC patients.
The REVEL study was a global, randomized, placebo-controlled, double-blind, multi-center phase 3 study comparing docetaxel+ramucirumab combination treatment with docetaxel treatment (docetaxel+placebo) in patients with stage IV NSCLC who showed disease progression after platinumbased therapy. This study showed that docetaxel+ramucirumab combination treatment improves survival as second-line treatment of patients with stage IV NSCLC. The aim of our analysis was to assess the efficacy and safety of docetaxel+ramucirumab combination treatment versus docetaxel treatment in the East Asian versus the non–East Asian subgroups from the REVEL study.
Materials and Methods
1. Study design and patients
The study design and patient eligibility for REVEL has been previously published [20]. Each center’s institutional review board or independent ethics committee approved this study. The study followed the guiding principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonisation. All patients provided written informed consent before enrollment. The key endpoints evaluated in the East Asian subgroup included OS, PFS, objective response rate (ORR), and safety.
2. Randomization
Randomization and procedures have also been published previously [20]. Patients were randomly assigned on a 1:1 basis to receive either docetaxel (75 mg/m2, 60-minute intravenous infusion)+ramucirumab (10 mg/kg, 60-minute intravenous infusion) combination treatment administered on day 1 of a 21-day (3-week) cycle or docetaxel (75 mg/m2, 60-minute intravenous infusion)+placebo (60-minute intravenous infusion) administered on day 1 of a 3-week cycle.
Randomization was stratified according to Eastern Cooperative Oncology Group performance status (0 vs. 1), sex (female vs. male), prior maintenance therapy (yes vs. no), and geographic region (East Asia vs. non-East Asia). Randomization was performed separately within each of the 16 strata (or cells) defined by all combinations of these four variables.
In May 2012, based on a higher rate of neutropenia and febrile neutropenia in East Asian patients compared to non-East Asian patients, the independent data monitoring committee recommended amending the protocol to reduce the starting dose of docetaxel for newly enrolled patients in East Asia from 75 to 60 mg/m2. All East Asian patients enrolled at an earlier stage of the study and receiving treatment at the time of this decision remained at the original dose of docetaxel and continued to receive a docetaxel dose of 75 mg/m2 for the remainder of the study.
3. Statistical analysis
Detailed statistical methods have been previously published [20]. The East Asian population (Korea and Taiwan) used for the subgroup analyses was defined as patients enrolled at study sites in Korea and Taiwan; patients of Korean and Taiwanese ethnicity enrolled at sites in countries other than Korea or Taiwan were not included in the East Asian subgroup.
The non–East Asian population used in the subgroup analyses was defined as patients enrolled at study sites throughout the world outside of Korea and Taiwan.
The primary and secondary endpoints evaluated were OS, PFS, ORR, and safety. Kaplan-Meier analysis and Cox proportional hazards regression were performed for OS and PFS and the Cochran-Mantel-Haenszel test was performed for ORR. This study is registered with http://www.ClinicalTrials.gov (No. NCT01168973).
Results
1. Patients
Baseline demographics, disease characteristics, NSCLC histology, and prior therapy were balanced between treatment arms and the two populations of patients (Table 1).
Table 1.
Baseline characteristics
| Variable | Docetaxel+ramucirumab combination treatment |
Docetaxel (docetaxel+placebo) |
||
|---|---|---|---|---|
| East Asian (n=43) | Non–East Asian (n=585) | East Asian (n=46) | Non–East Asian (n=579) | |
| Age (yr) | ||||
| Median (range) | 62 (35-81) | 62 (21-85) | 57.5 (25-78) | 61 (26-86) |
| < 65 | 27 (62.8) | 364 (62.2) | 33 (71.7) | 374 (64.6) |
| ≥ 65 | 16 (37.2) | 221 (37.8) | 13 (28.3) | 205 (35.4) |
| Sex | ||||
| Male | 36 (83.7) | 383 (65.5) | 37 (80.4) | 378 (65.3) |
| Female | 7 (16.3) | 202 (34.5) | 9 (19.6) | 201 (34.7) |
| Racea),b) | ||||
| White | 0 | 526 (89.9) | 0 | 503 (86.9) |
| Asian | 43 (100) | 31 (5.3) | 46 (100) | 40 (6.9) |
| Black | 0 | 17 (2.9) | 0 | 16 (2.8) |
| Other | 0 | 11 (1.9) | 0 | 20 (3.5) |
| Geographic region of origin | 43 (100) | 585 (100) | 46 (100) | 579 (100) |
| ECOG performance statusc),d) | ||||
| 0 | 12 (27.9) | 195 (33.3) | 11 (23.9) | 188 (32.5) |
| 1 | 31 (72.1) | 389 (66.5) | 35 (76.1) | 390 (67.4) |
| Missing | 0 | 1 (0.2) | 0 | 1 (0.2) |
| Disease | ||||
| Measurable | 42 (97.7) | 564 (96.4) | 45 (97.8) | 558 (96.4) |
| Non-measurable | 1 (2.3) | 21 (3.6) | 1 (2.2) | 21 (3.6) |
| Smoking history | ||||
| Ever | 34 (79.1) | 484 (82.7) | 32 (69.6) | 451 (77.9) |
| Never | 9 (20.9) | 100 (17.1) | 14 (30.4) | 127 (21.9) |
| Unknown | 0 | 1 (0.2) | 0 | 1 (0.2) |
| Histological subtype | ||||
| Non-squamous | 28 (65.1) | 437 (74.7) | 30 (65.2) | 417 (72.0) |
| Squamous | 15 (34.9) | 142 (24.3) | 16 (34.8) | 155 (26.8) |
| Unknown | 0 | 6 (1.0) | 0 | 7 (1.2) |
| EGFR status | ||||
| Wild type | 18 (41.9) | 189 (32.3) | 22 (47.8) | 175 (30.2) |
| Mutant | 3 (7.0) | 12 (2.1) | 1 (2.2) | 17 (2.9) |
| Unknown | 22 (51.2) | 380 (65.0) | 23 (50.0) | 383 (66.1) |
| Missing | 0 | 4 (0.7) | 0 | 4 (0.7) |
| Best response to platinum based chemotherapy | ||||
| CR, PR, or SD | 32 (74.4) | 388 (66.3) | 38 (82.6) | 379 (65.5) |
| PD | 8 (18.6) | 170 (29.1) | 5 (10.9) | 177 (30.6) |
| Missing | 3 (7.0) | 27 (4.6) | 3 (6.5) | 23 (4.0) |
| Previous maintenance treatment | ||||
| No | 38 (88.4) | 455 (77.8) | 34 (73.9) | 448 (77.4) |
| Yes | 5 (11.6) | 130 (22.2) | 12 (26.1) | 131 (22.6) |
| Previous taxane | ||||
| No | 37 (86.0) | 438 (74.9) | 42 (91.3) | 434 (75.0) |
| Yes | 6 (14.0) | 147 (25.1) | 4 (8.7) | 145 (25.0) |
| Previous bevacizumab | ||||
| No | 41 (95.3) | 499 (85.3) | 44 (95.7) | 489 (84.5) |
| Yes | 2 (4.7) | 86 (14.7) | 2 (4.3) | 90 (15.5) |
| Time since previous therapy (mo) | ||||
| < 9 | 28 (65.1) | 372 (63.6) | 31 (67.4) | 343 (59.2) |
| ≥ 9 | 14 (32.6) | 212 (36.2) | 15 (32.6) | 236 (40.8) |
| Missing | 1 (2.3) | 1 (0.2) | 0 | 0 |
Values are presented as median interquartile range or number (%). ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
As established by self-report,
Data not available for one patient in the ramucirumab group,
Assessed according to ECOG guidelines, with 0 as asymptomatic, 1 as restricted in strenuous activity but ambulatory and able to do light work, or 2 as ambulatory and capable of all self-care but unable to work,
Data not available for one patient in each group.
2. Efficacy
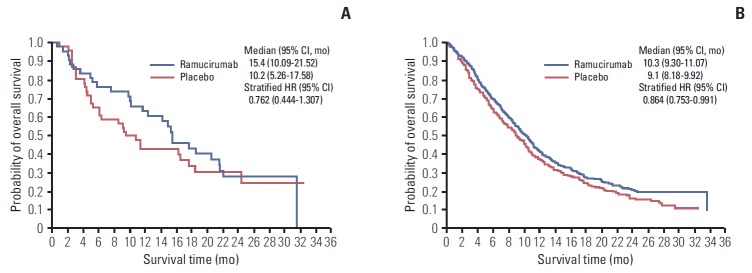
In the 89 East Asian patients, median OS was 15.4 months (95% confidence interval [CI], 10.09 to 21.52) for the docetaxel+ ramucirumab combination treatment arm (n=43) and 10.2 months (95% CI, 5.26 to 17.58) for the docetaxel treatment arm (n=46) (stratified hazard ratio [HR], 0.762; 95% CI, 0.444 to 1.307) (Fig. 1A).
Fig. 1.
Kaplan-Meier estimates of overall survival for East Asian patients (A) and non–East Asian patients (B). CI, confidence interval; HR, hazard ratio.
In the 1,164 non–East Asian patients, median OS was 10.3 months (95% CI, 9.30 to 11.07) for the docetaxel+ramucirumab combination treatment arm (n=585) and 9.1 months (95% CI, 8.18 to 9.92) for the docetaxel treatment arm (n=579) (stratified HR, 0.864; 95% CI, 0.753 to 0.991) (Fig. 1B).
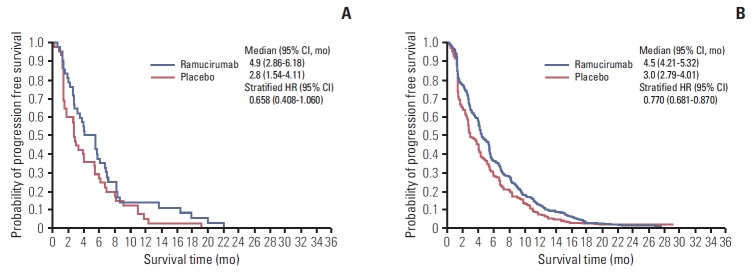
In East Asian patients, median PFS was 4.9 months (95% CI, 2.86 to 6.18) for the docetaxel+ramucirumab combination treatment arm and 2.8 months (95% CI, 1.54 to 4.11) for the docetaxel treatment arm (stratified HR, 0.658; 95% CI, 0.408 to 1.060) (Fig. 2A). The ORR was 25.6% (95% CI, 13.5 to 41.2) in the docetaxel+ramucirumab combination treatment arm and 8.7% (95% CI, 2.4 to 20.8) in the docetaxel treatment arm (Table 2).
Fig. 2.
Kaplan-Meier estimates of progression-free survival for East Asian patients (A) and non–East Asian patients (B). CI, confidence interval; HR, hazard ratio.
Table 2.
Objective tumor response
| Best overall response | East Asian |
Non-East Asian |
||
|---|---|---|---|---|
| Docetaxel+ramucirumab combination treatment (n=43) | Docetaxel (docetaxel+placebo) (n=46) | Docetaxel+ramucirumab combination treatment (n=585) | Docetaxel (docetaxel+placebo) (n=579) | |
| Complete response | 0 | 0 | 3 (0.5) | 2 (0.3) |
| Partial response | 11 (25.6) | 4 (8.7) | 130 (22.2) | 79 (13.6) |
| Stable disease | 19 (44.2) | 19 (41.3) | 239 (40.9) | 225 (38.9) |
| Objective response | 11 (25.6) | 4 (8.7) | 133 (22.7) | 81 (14.0) |
| 95% CI | 13.5-41.2 | 2.4-20.8 | 19.4-26.3 | 11.3-17.1 |
| Disease control ratea) | 30 (69.8) | 23 (50.0) | 372 (63.6) | 306 (52.8) |
| 95% CI | 53.9-82.8 | 34.9-65.1 | 59.5-67.5 | 48.7-57.0 |
Values are presented as number (%) unless otherwise indicated. CI, confidence interval.
Denotes best response for complete response, partial response, or stable disease.
In non–East Asian patients, median PFS was 4.5 months (95% CI, 4.21 to 5.32) for the docetaxel+ramucirumab combination treatment arm and 3.0 months (95% CI, 2.79 to 4.01) for the docetaxel treatment arm (stratified HR, 0.770; 95% CI, 0.681 to 0.870) (Fig. 2B). The ORR was 22.7% (95% CI, 19.4 to 26.3) in the docetaxel+ramucirumab combination treatment arm and 14% (95% CI, 11.3 to 17.1) in the docetaxel treatment arm (Table 2).
3. Safety
A summary of the safety evaluation at the 75 mg/m2 dose of docetaxel in East Asian and non–East Asian patients is shown in Table 3. A summary of the safety evaluation for newly enrolled East Asian patients (n=24) who received the reduced starting dose of docetaxel (60 mg/m2) in both the docetaxel+ramucirumab combination and the docetaxel treatment arms is shown in Table 4.
Table 3.
Summary of treatment-emergent adverse events at 75 mg/m2 docetaxel in ≥ 20% East Asian and non–East Asian patients in the docetaxel+ramucirumab combination treatment arm
| Variable | Docetaxel+ramucirumab combination treatment (75 mg/m2) |
Docetaxel (docetaxel+placebo) (75 mg/m2) |
||||||
|---|---|---|---|---|---|---|---|---|
| East Asian (n=32) |
Non–East Asian (n=584) |
East Asian (n=33) |
Non–East Asian (n=572) |
|||||
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| Adverse event | ||||||||
| Decreased appetite | 21 (65.6) | 0 | 158 (27.1) | 14 (2.4) | 12 (36.4) | 0 | 136 (23.8) | 8 (1.4) |
| Stomatitis | 16 (50.0) | 1 (3.1) | 124 (21.2) | 26 (4.5) | 12 (36.4) | 0 | 66 (11.5) | 10 (1.7) |
| Dyspnea | 14 (43.8) | 0 | 122 (20.9) | 23 (3.9) | 8 (24.2) | 1 (3.0) | 140 (24.5) | 50 (8.7) |
| Fatiguea) | 15 (46.9) | 1 (3.1) | 324 (55.5) | 87 (14.9) | 13 (9.4) | 0 | 291 (50.9) | 65 (11.4) |
| Diarrhea | 12 (37.5) | 1 (3.1) | 186 (31.8) | 28 (4.8) | 8 (24.2) | 0 | 160 (28.0) | 19 (3.3) |
| Myalgia | 12 (37.5) | 0 | 64 (11.0) | 4 (0.7) | 12 (36.4) | 0 | 52 (9.1) | 4 (0.7) |
| Productive cough | 12 (37.5) | 0 | 26 (4.5) | 1 (0.2) | 4 (12.1) | 0 | 25 (4.4) | 2 (0.3) |
| Pyrexia | 12 (37.5) | 0 | 92 (15.8) | 3 (0.5) | 5 (15.2) | 0 | 72 (12.6) | 2 (0.3) |
| Alopecia | 11 (34.4) | 0 | 146 (25.0) | 0 | 15 (45.5) | 0 | 138 (24.1) | 0 |
| Anemiaa) | 10 (31.3) | 3 (9.4) | 118 (20.2) | 15 (2.6) | 9 (27.3) | 2 (6.1) | 163 (28.5) | 33 (5.8) |
| Insomnia | 10 (31.3) | 0 | 57 (9.8) | 3 (0.5) | 4 (12.1) | 0 | 42 (7.3) | 1 (0.2) |
| Cough | 9 (28.1) | 0 | 121 (20.7) | 3 (0.5) | 7(21.2) | 0 | 116 (20.3) | 5 (0.9) |
| Nausea | 9 (28.1) | 0 | 159 (27.2) | 7 (1.2) | 4 (12.1) | 0 | 164 (28.7) | 9 (1.6) |
| Oropharyngeal pain | 8 (25.0) | 0 | 38 (6.5) | 0 | 5 (15.2) | 0 | 26 (4.5) | 0 |
| Peripheral sensory neuropathya) | 8 (25.0) | 0 | 63 (10.8) | 13 (2.2) | 8 (24.2) | 0 | 50 (8.7) | 4 (0.7) |
| Hematologic adverse events | ||||||||
| Neutropeniaa) | 27 (84.4) | 26 (81.3) | 312 (53.4) | 274 (46.9) | 24 (72.7) | 24 (72.7) | 255 (44.6) | 217 (37.9) |
| Febrile neutropenia | 14 (43.8) | 14 (43.8) | 86 (14.7) | 86 (14.7) | 4 (12.1) | 4 (12.1) | 57 (10.0) | 57 (10.0) |
| Anemia | 10 (31.3) | 3 (9.4) | 118 (20.2) | 15 (2.6) | 9 (27.3) | 2 (6.1) | 160 (20.8) | 32 (5.6) |
| Thrombocytopeniaa) | 3 (9.4) | 2 (6.3) | 79 (13.5) | 15 (2.6) | 0 | 0 | 32 (5.6) | 4 (0.7) |
| AESIs (categories) | ||||||||
| Bleeding/Hemorrhage | 11 (34.4) | 0 | 167 (28.6) | 15 (2.6) | 4 (12.1) | 0 | 88 (15.4) | 14 (2.4) |
| Pulmonary/Hemorrhage | 5 (15.6) | 0 | 42 (7.2) | 8 (1.4) | 3 (9.1) | 0 | 42 (7.3) | 8 (1.4) |
| GI hemorrhage | 2 (6.3) | 0 | 15 (2.6) | 4 (0.7) | 0 | 0 | 10 (1.7) | 2 (0.3) |
| GI perforation | 2 (6.3) | 2 (6.3) | 4 (0.7) | 3 (0.5) | 0 | 0 | 2 (0.3) | 2 (0.3) |
| Infusion-related reaction | 1 (3.1) | 0 | 22 (3.8) | 5 (0.9) | 3 (9.1) | 0 | 22 (3.8) | 3 (0.5) |
| Proteinuria | 1 (3.1) | 0 | 18 (3.1) | 1 (0.2) | 0 | 0 | 5 (0.9) | 0 |
Values are presented as number (%). AESIs, adverse events of special interest; GI, gastrointestinal.
Consolidated adverse event category comprising synonymous MedDRA ver. 16.1 preferred terms.
Table 4.
Summary of treatment-emergent adverse events at reduced (60 mg/m2) dose of docetaxel in ≥ 20% East Asian patients in the docetaxel+ramucirumab combination treatment arm
| Variable | Docetaxel+ramucirumab combination (60 mg/m2) (n=11) |
Docetaxel (docetaxel+placebo) (60 mg/m2) (n=13) |
||
|---|---|---|---|---|
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| Adverse event | ||||
| Decreased appetite | 3 (27.3) | 0 | 6 (46.2) | 0 |
| Stomatitis | 6 (54.5) | 0 | 2 (15.4) | 0 |
| Fatiguea) | 4 (36.4) | 0 | 5 (38.5) | 0 |
| Alopecia | 5 (45.5) | 0 | 3 (23.1) | 0 |
| Anemiaa) | 3 (27.3) | 0 | 2 (15.4) | 0 |
| Cough | 3 (27.3) | 0 | 5 (38.5) | 0 |
| Pneumonia | 3 (27.3) | 3 (27.3) | 1 (7.7) | 1 (7.7) |
| Hypertension | 3 (27.3) | 1 (9.1) | 0 | 0 |
| Hematologic adverse events | ||||
| Neutropeniaa) | 6 (54.5) | 6 (54.5) | 5 (38.5) | 5 (38.5) |
| Febrile neutropenia | 0 | 0 | 1 (7.7) | 1 (7.7) |
| Anemia | 3 (27.3) | 0 | 2 (15.4) | 0 |
| Thrombocytopenia | 2 (18.2) | 1 (9.1) | 0 | 0 |
| AESIs (categories)b) | ||||
| Bleeding/Hemorrhage | 3 (27.3) | 0 | 2 (15.4) | 0 |
| Pulmonary/Hemorrhage | 2 (18.2) | 0 | 1 (7.7) | 0 |
| Proteinuria | 2 (18.2) | 0 | 0 | 0 |
| Hypertension | 3 (27.3) | 1 (9.1) | 0 | 0 |
Values are presented number (%). AESIs, adverse events of special interest.
Consolidated adverse event category comprising synonymous MedDRA ver. 16.1 preferred terms,
Not subjected to the ≥ 20% criterion.
At the 75 mg/m2 dose of docetaxel, the number of East Asian patients with at least 1 grade ≥ 3 treatment-emergent adverse event (TEAE) was 31 (96.9%) for the docetaxel+ ramucirumab combination treatment arm versus 26 (78.8%) for the docetaxel treatment arm (Table 3). The number of non–East Asian patients with at least 1 grade ≥ 3 TEAE, regardless of causality, was 458 for the docetaxel+ramucirumab combination treatment arm (78.4%) versus 411 for the docetaxel treatment arm (71.9%).
At the reduced starting dose of docetaxel (60 mg/m2), the number of East Asian patients with at least 1 grade ≥ 3 TEAE was six in the docetaxel+ramucirumab combination treatment arm (54.5%) versus seven in the docetaxel treatment arm (53.8%) (Table 4).
At the 75 mg/m2 dose of docetaxel, the number of East Asian patients with grade ≥ 3 neutropenia was 26 (81.3%) in the docetaxel+ramucirumab combination treatment arm versus 24 (72.7%) in the docetaxel treatment arm. The number of non–East Asian patients with grade ≥ 3 neutropenia was 274 (46.9%) in the docetaxel+ramucirumab combination treatment arm versus 217 (37.9%) in the docetaxel treatment arm (Table 3).
At the reduced starting dose of 60 mg/m2 of docetaxel, the number of East Asian patients with grade ≥ 3 neutropenia was six in the docetaxel+ramucirumab combination treatment arm (54.5%) and five in the docetaxel treatment arm (38.5%) (Table 4).
At the 75 mg/m2 dose, the number of East Asian patients with febrile neutropenia was 14 in the docetaxel+ramucirumab combination treatment arm (43.8%) versus four in the docetaxel treatment arm (12.1%). The number of non–East Asian patients with febrile neutropenia was 86 in the docetaxel+ramucirumab combination treatment arm (14.7%) versus 57 in the docetaxel treatment arm (10.0%).
At the reduced starting dose of 60 mg/m2 of docetaxel, the number of East Asian patients with febrile neutropenia was 0 in the docetaxel+ramucirumab combination treatment arm and one (7.7%) in the docetaxel+treatment arm.
In East Asian patients, at the 75 mg/m2 dose of docetaxel, the categories of adverse events of special-interest (AESIs), regardless of grade, and potentially associated with the VEGF pathway, that occurred more frequently in the docetaxel+ ramucirumab combination treatment arm versus the docetaxel treatment arm were bleeding/hemorrhage events (11 patients [34.4%] vs. 4 patients [12.1%]), pulmonary hemorrhage events (5 patients [15.6%] vs. 3 patients [9.1%]), gastrointestinal hemorrhage events (2 patients [6.3%] vs. 0 patient), gastrointestinal perforation (2 patients [6.3%] vs. 0 patient), and proteinuria (1 patient [3.1%] vs. 0 patient). In non–East Asian patients, at the 75 mg/m2 dose of docetaxel, any grade AESIs that occurred more frequently in the docetaxel+ramucirumab combination treatment arm versus the docetaxel treatment arm were bleeding/hemorrhage events (167 patients [28.6%] vs. 88 patients [15.4%]), gastrointestinal hemorrhage events (15 patients [2.6%] vs. 10 patients [1.7%]), gastrointestinal perforation (4 patients [0.7%] versus 2 patients [0.3%]), and proteinuria (18 patients [3.1%] vs. 5 patients [0.9%]) (Table 3).
In East Asian patients, at the reduced starting dose of 60 mg/m2 of docetaxel, any grade AESIs potentially associated with the VEGF pathway and that occurred more frequently in the docetaxel+ramucirumab combination treatment arm vs. the docetaxel treatment arm were bleeding/hemorrhage events (3 patients [27.3%] vs. 2 patients [15.4%]), pulmonary hemorrhage events (2 patients [18.2%] vs. 1 patient [7.7%]), proteinuria (2 patients [18.2%] vs. 0 patient), and hypertension (3 patients [27.3%] vs. 0 patient) (Table 4).
Discussion
Although this subgroup analysis of the East Asian population vs. the non–East Asian population is not powered to demonstrate significant improvement, in the East Asian subgroup that received docetaxel+ramucirumab combination treatment, compared with the East Asian subgroup treated with docetaxel+placebo, the trend of prolonged OS (15.4 months vs. 10.2 months, respectively) (Fig. 1A), PFS (4.9 months vs. 2.8 months, respectively) (Fig. 2A), and ORR (25.6% vs. 8.7%, respectively) (Table 2) is consistent with the treatment effect observed in the overall ITT population in the REVEL trial.
Pharmacological studies of docetaxel have indicated a slower plasma clearance of docetaxel in Asian patients compared with Caucasian patients [21]. In addition, according to several studies, adverse events (AEs), predominantly hematological AEs, are higher in Asians than in Caucasians receiving docetaxel [21,22], with neutropenia being the predominant toxicity [22]. Studies in East Asian patients with NSCLC have indicated the need to decrease the dose of docetaxel or use the prophylactic support of growth factors in this ethnic group [22]. In a Japanese study, a reduction in the starting dose of docetaxel from 75 to 60 mg/m2 was associated with an improved safety profile and a reduction in the incidence of neutropenia and febrile neutropenia in Japanese patients [23].
In the current study, a decrease in the dosage of docetaxel resulted in decreased incidence of neutropenia in East Asian patients to a rate similar to that observed in non–East Asian patients (54.5% in East Asian patients in the 60 mg/m2 docetaxel group vs. 53.4% in non–East Asian patients). In addition, the incidence of febrile neutropenia in East Asian patients showed a similar decrease with the lowering of the starting docetaxel dose (0 in East Asian patients in the 60 mg/m2 docetaxel group vs. 14.7% in non–East Asian patients).
This analysis did not show an increased risk of sepsis, and there was no significant increase in thromboembolic events in the docetaxel+ramucirumab combination treatment group in both the East Asia and non–East Asia subgroups. Hypertension was mild and bleeding events in patients who received docetaxel+ramucirumab combination treatment were mainly due to grade 1-2 epistaxis in both the East Asia and non–East Asia subgroups.
Ramucirumab binds specifically to the extracellular domain of VEGFR-2, rather than the VEGF ligand; therefore, the effects of ramucirumab may be localized to abnormal vasculature [24]. Several studies of small-molecule VEGF inhibitors have not resulted in improved OS; however, some have been associated with benefits in PFS, particularly in the setting of NSCLC [25]. In the current study, ramucirumab showed an advantage in OS in previously treated NSCLC patients, while bevacizumab has been shown to prolong survival as first-line therapy for non-squamous NSCLC [12].
The current analysis of the REVEL trial had some limitations. First, the REVEL trial was not powered to show a significant improvement in East Asian patients. Second, as mentioned earlier, due to the higher incidence of EGFR mutations in East Asian NSCLC patients [13,14], a post hoc analysis would have been beneficial in factoring in the number of patients with EGFR mutations; however, EGFR mutation testing was not required for study entry, and the number of patients with known EGFR-positive mutation status in the overall REVEL population was very small (33 patients) and even smaller in the East Asia subgroup (n=3) (Table 1) [20].
Conclusion
In the REVEL trial, docetaxel (75 mg/m2)+ramucirumab combination treatment was well tolerated in the non-East Asia subgroup and at the reduced starting dose of 60 mg/m2 in the East Asia subgroup. The reduced dose of docetaxel in the East Asia subgroup had a positive effect on safety with a decrease in the incidence of neutropenia and febrile neutropenia and a decrease in the overall frequency of grade ≥ 3 TEAEs. REVEL demonstrated a favorable benefit-risk profile in East Asian patients with stage IV NSCLC who showed disease progression after platinum-based chemotherapy. Consistent with the ITT population results, this subgroup analysis showed a trend in the prolongation of median OS and PFS and improved ORR in the East Asia patient population treated with docetaxel+ramucirumab combination treatment, in conjunction with favorable tolerability based on manageable AEs.
The results of the REVEL trial indicate a significant new second-line therapeutic option for East Asian patients with advanced NSCLC after platinum-based chemotherapy.
Acknowledgments
We thank the patients, their families, the study sites, and the study personnel who participated in this clinical trial.
Medical writing support was provided by Marissa Philpott and Andrew Sakko, and editorial support was provided by Jeanne Claypoole of inVentiv Health Clinical and funded by Eli Lilly and Company.
Footnotes
Jin-Hyoung Kang and Jin-Yuan Shih received honoraria from Eli Lilly and Company. Annamaria Hayden Zimmermann, Pablo Lee, Ekaterine Alexandris, Tarun Puri, and Mauro Orlando are employees of Eli Lilly & Company. Tarun Puri and Mauro Orlando hold stock with Eli Lilly and Company. All remaining authors have declared no conflicts of interest.
This study was sponsored by Eli Lilly and Company.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet] Lyon: International Agency for Research on Cancer; 2013. [cited 2014 Jan 27]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Schnabel PA, Smit E, Carpeno JC, Lesniewski-Kmak K, Aerts J, Kraaij K, et al. Influence of histology and biomarkers on first-line treatment of advanced non-small cell lung cancer in routine care setting: baseline results of an observational study (FRAME) Lung Cancer. 2012;78:263–9. doi: 10.1016/j.lungcan.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol. 2012;19(Suppl 1):S52–8. doi: 10.3747/co.19.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber DE, Schiller JH. Maintenance chemotherapy for advanced non-small-cell lung cancer: new life for an old idea. J Clin Oncol. 2013;31:1009–20. doi: 10.1200/JCO.2012.43.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 7.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 8.Jantus-Lewintre E, Sanmartin E, Sirera R, Blasco A, Sanchez JJ, Taron M, et al. Combined VEGF-A and VEGFR-2 concentrations in plasma: diagnostic and prognostic implications in patients with advanced NSCLC. Lung Cancer. 2011;74:326–31. doi: 10.1016/j.lungcan.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Tugues S, Koch S, Gualandi L, Li X, Claesson-Welsh L. Vascular endothelial growth factors and receptors: anti-angiogenic therapy in the treatment of cancer. Mol Aspects Med. 2011;32:88–111. doi: 10.1016/j.mam.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997;3:1222–7. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21:1804–9. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–7. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 16.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 17.Wu YL, Zhong WZ, Li LY, Zhang XT, Zhang L, Zhou CC, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007;2:430–9. doi: 10.1097/01.JTO.0000268677.87496.4c. [DOI] [PubMed] [Google Scholar]
- 18.Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol. 2009;4:1083–93. doi: 10.1097/JTO.0b013e3181b27b15. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa Y, Kawaguchi T, Kubo A, Ando M, Shiraishi J, Isa S, et al. Ethnic difference in hematological toxicity in patients with non-small cell lung cancer treated with chemotherapy: a pooled analysis on Asian versus non-Asian in phase II and III clinical trials. J Thorac Oncol. 2011;6:1881–8. doi: 10.1097/JTO.0b013e31822722b6. [DOI] [PubMed] [Google Scholar]
- 20.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–73. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 21.Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol. 2002;20:3683–90. doi: 10.1200/JCO.2002.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Goh BC, Lehnert M, Lim HL, Ng AW, Chan CC, Kong HL, et al. Phase II trial of docetaxel in Asian patients with inoperable stage III non-small cell lung cancer. Acta Oncol. 2000;39:225–9. doi: 10.1080/028418600430824. [DOI] [PubMed] [Google Scholar]
- 23.Kenmotsu H, Tanigawara Y. Pharmacokinetics, dynamics and toxicity of docetaxel: why the Japanese dose differs from the Western dose. Cancer Sci. 2015;106:497–504. doi: 10.1111/cas.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, Ashton SE, et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res. 2010;16:3548–61. doi: 10.1158/1078-0432.CCR-09-2797. [DOI] [PubMed] [Google Scholar]
- 25.Crino L, Metro G. Therapeutic options targeting angiogenesis in nonsmall cell lung cancer. Eur Respir Rev. 2014;23:79–91. doi: 10.1183/09059180.00008913. [DOI] [PMC free article] [PubMed] [Google Scholar]