Abstract
Background
The Anesthesia Patient Safety Foundation has advocated the use of continuous electronic monitoring of oxygenation and ventilation to preemptively identify opioid-induced respiratory depression. In adults, capnography is the gold standard in respiratory monitoring. An alternative technique used in sleep laboratories is respiratory inductance plethysmography (RIP). However, it is not known if either monitor is well tolerated by pediatric patients for prolonged periods of time.
Aim
The goal of this study was to determine whether capnography or RIP is better tolerated in non-intubated, spontaneously breathing pediatric patients being treated with intravenous patient-controlled analgesia (IVPCA).
Methods
Nasal cannula capnography with oral sampling and thoracic and abdominal inductance plethysmography bands were placed along with routine monitors on pediatric patients being treated for acute pain with IVPCA. Study monitors were left in place for as long as they were tolerated by the patient, for a maximum of 24 consecutive hours. If the patient did not wear a particular study monitor for any reason, but tolerated the remaining monitor, participation in the study continued. If the patient would not wear either monitor, participation was terminated.
Results
Twenty-six patients (18 female, 8 male, average age 10.1 ± 5.5 years) consented to participate, but only 14 patients attempted to wear one or both devices. Among those who wore either device, median time to device removal was 8.33 hours (range 0.3–23.6 hours) for capnography and 23.5 hours (range 0.7–24 hours) for RIP bands.
Conclusion
Children did not tolerate wearing capnography cannulae for prolonged periods of time, limiting the usefulness of this device as a continuous monitor of ventilation in children. RIP bands were better tolerated; however, they require further assessment of their utility. Until more effective, child-friendly monitors are developed and their utility validated, guidelines recommended for adult patients cannot be extended to children.
Keywords: capnography, plethysmography, opioid, analgesia, patient controlled, patient monitoring
Introduction
In patients of all ages, opioids are the analgesics most commonly used to treat moderate to severe pain. Although rare, respiratory depression is a real and potentially fatal consequence of opioid therapy, regardless of the opioid chosen or its method of administration.1, 2 Indeed, even intravenous patient-controlled analgesia (IVPCA), which is often considered the safest mode of opioid delivery because patients must be conscious to initiate a demand dose, is associated with occasional respiratory insufficiency and/or arrest.1–5 To minimize these risks, the Anesthesia Patient Safety Foundation and others have advocated that continuous electronic monitoring of oxygenation and ventilation be used to preemptively identify and potentially prevent opioid-induced respiratory.6
Ideally, respiratory monitoring should continuously and accurately measure respiratory rate, airflow, carbon dioxide (CO2) tension, and oxygenation, and it should have a low incidence of false (positive or negative) alarms. The most common monitoring methods in current practice are continuous pulse oximetry, which measures heart rate and oxygen saturation, and transthoracic impedance plethysmography, which estimates respiratory rate by measuring changes in electrical impedance associated with chest movement during respiration.5, 7, 8 Both monitors produce a high incidence of false alarms and have significant limitations. For example, supplemental oxygen circumvents the value of pulse oximetry, and transthoracic impedance plethysmography cannot detect airway obstruction.7–9 In contrast, capnography measures end-tidal CO2, provides a direct measure of airflow, and offers a more accurate assessment of respiratory rate than impedance plesthysmography.10 Consequently, it is used ubiquitously in operating rooms, endoscopy suites, and critical care units as a respiratory monitor for anesthetized and sedated.11, 12 Overdyk and colleagues9 extended the use of capnography with nasal and oral sampling outside of these environs to non-intubated, adult, postoperative patients receiving IVPCA. They found a 58.4% incidence of hypopnea (respiratory rate < 10 breaths per minute) even though they observed only a 21.4% incidence of desaturation, supporting capnography’s potential use as an early warning monitor of impending respiratory insufficiency.
Children receiving opioids via IVPCA face similar, or perhaps even greater, respiratory risks.3 It is unknown, however, if Overdyk’s conclusions supporting the use of capnography can be extrapolated to children. Although capnography is used for multiple indications in pediatrics and can accurately monitor ventilation in sedated and anesthetized children, whether it can be used in conscious children for prolonged periods has not been ascertained.11 Appropriate clinical use of capnography monitoring requires detection of low tidal volumes exhaled from both the mouth and nose, as well as patient cooperation in wearing a specially designed capnography cannula for periods up to or in excess of 24 hours.11 If the cannula is uncomfortable or if it interferes with normal activities, such as eating or talking, children may either refuse to wear it or remove it, generating a high incidence of false positive alarms and limiting the monitor’s accuracy and value.
An alternative respiratory monitoring method used in sleep laboratories is respiratory inductance plethysmography (RIP).13 For RIP, two soft bands are placed around the chest and abdomen to measure respiratory rate, airflow, and chest and abdominal wall asynchrony (airway obstruction). Although it is not designed as a monitor (it has no alarm components), RIP may provide a useful measure of respiration in conscious patients being treated with opioids. However, it is not known if conscious pediatric patients will tolerate wearing the bands for prolonged periods of time. If this were to prove to be the case, it could provide an impetus for the development of an appropriate alarm system. Thus, in this study, we compared the tolerability of capnography and RIP in children to determine which method might be the most useful for prolonged respiratory monitoring in pediatric patients being treated with IVPCA opioids.
Methods
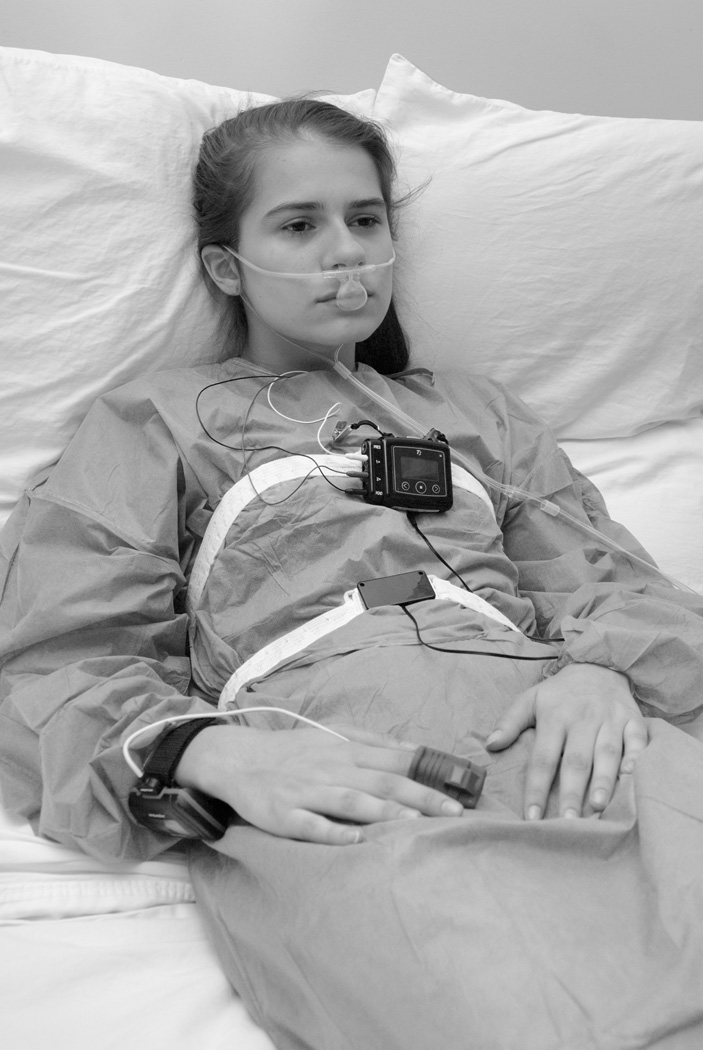
This pilot study was approved by the Johns Hopkins Institutional Review Board. During the study period, 50 patients who were to be treated for acute pain with IVPCA or parent/nurse-controlled analgesia (PNCA) therapy were approached for written informed parental consent and, when appropriate, patient assent. The Pediatric Acute Pain Service made all therapeutic decisions concerning pain therapy, including the analgesics prescribed and method of analgesic delivery. As per routine hospital policy, all patients were monitored for the first 24 hours of IVPCA therapy (CADD-Solis, Smiths Medical, Dublin, OH) with continuous electrocardiography and pulse oximetry. In addition to standard monitors, patients were asked to wear two study monitors: the Smart Capnoline Plus O2 nasal cannula and the Nox T3 monitoring system (Figure 1). The Smart Capnoline Plus O2 nasal cannula (Covidien, Mansfield, MA) is a noninvasive device that features side-stream capnography. It is designed to monitor spontaneously breathing patients with natural (i.e., non-intubated) airways, and has previously been used successfully to monitor ventilation in adult patients receiving PCA.9 Exhaled gas is aspirated through a specially designed cannula that samples expired carbon dioxide from both the mouth and nose. Oxygen, if needed, can be administered through the cannula without affecting carbon dioxide measurement. Effective gas sampling requires the cannula to be properly positioned over the patient’s mouth and nares. The Nox T3 monitoring system monitors both respiratory rate and airway patency with a chest wall audio microphone (Nox Medical, Reykjavík, Iceland), a pulse oximeter probe (Nonin Medical Inc., Plymouth, MN), and dual thoracic and abdominal bands (Nox Medical).
Figure 1.
The Smart Capnoline Plus O2 nasal cannula and Nox T3 monitoring system worn by an adolescent. Effective gas sampling requires the Smart Capnoline Plus O2 nasal cannula to be positioned over both the mouth and nares. The Nox T3 monitoring system includes dual circumferential thoracic and abdominal respiratory inductance plethysmography bands to monitor both respiratory rate and airway patency. Photo courtesy of J. Craig Shearman.
Study monitors were left in place for as long as they were tolerated by the patient, up to a maximum of 24 consecutive hours. If the patient did not wear one study monitor for any reason, but tolerated the remaining monitor, participation in the study continued. If the patient would not wear either monitor, the patient’s participation in the study was terminated. Data are summarized as median with range, and the nonparametric Wilcoxon signed-rank test was used to compare results.
Results
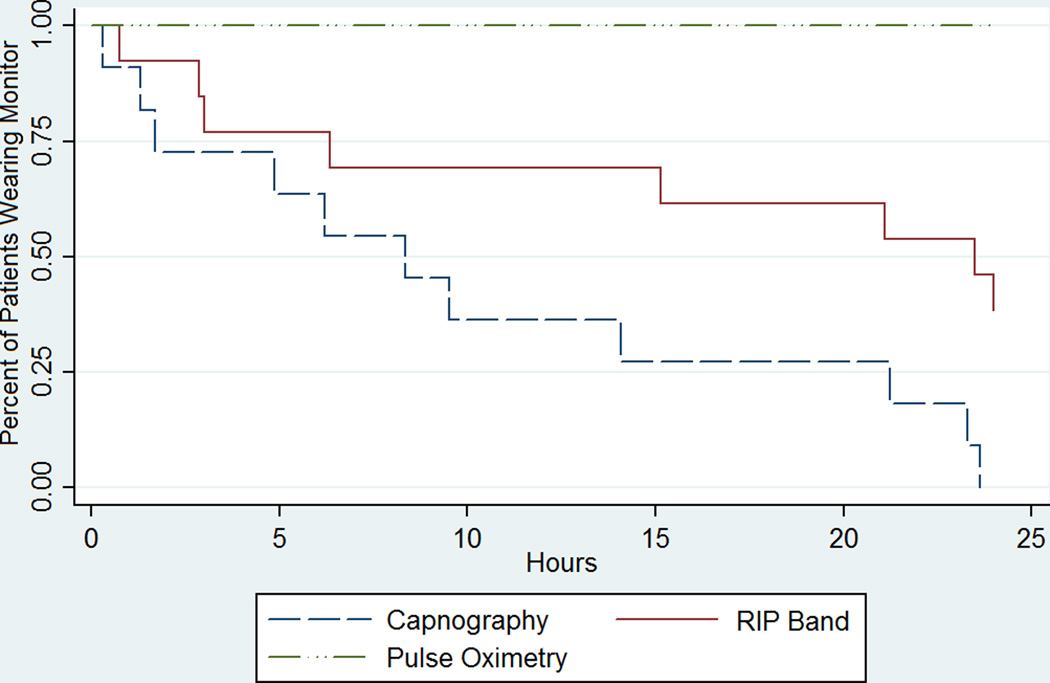
Of 50 patients approached for enrollment, 24 (48%) refused to participate in the study once they had seen or tried on the Smart Capnoline Plus O2 nasal cannula. Reasons for refusal included concern that wearing additional monitoring equipment postoperatively would be uncomfortable and/or would exacerbate discomfort. Of the 26 patients who consented to participate (18 female, 8 male, average age 10.1 ± 5.5 years), 14 attempted to wear one or both devices. Demographics for these patients are shown in Table 1. Of these 14 patients, 3 refused to wear the nasal cannulae, and one refused to wear the RIP bands. Median time to device removal was 8.33 hours (range 0.3–23.6 hrs, 95% CI [1.6, 21.8]) for nasal capnography and 23.5 hours (range 0.7–24 hrs, 95% CI [5.8, 24]) for RIP bands (p < 0.06), suggesting marginally greater tolerability of RIP over nasal capnography (Figure 2). No patient tolerated capnography monitoring for the full 24-hour study period. The cannula was removed during sleep, because it interfered with eating, or, most commonly, because the patient found wearing it physically uncomfortable. Three patients (27% of patients who tried the cannula, 11.5% of patients willing to be enrolled) wore the nasal cannula for >20 hours, all of whom also wore the RIP bands for a comparable period of time. However, only 3 of 7 subjects who wore the RIP bands for >20 hours tolerated capnography for a comparable time.
Table 1.
Demographic Data for the 26 Patients Composing the Study Population
| Parameter | Patients who consented number (%) |
Patients who wore cannula and/or RIP bands number (%) |
|---|---|---|
| Age | ||
| 12–18 years | 13 (50%) | 10 (38.5%) |
| 6–11 years | 5 (19.2%) | 3 (11.5%) |
| 3–5 years | 5 (19.2%) | 1 (3.8%) |
| 7 months–2 years | 3 (11.5%) | 0 (0%) |
| Sex | ||
| Male | 8 (30.8%) | 7 (26.9%) |
| Female | 18 (69.2%) | 7 (26.9%) |
| Race/Ethnicity | ||
| White | 16 (61.5%) | 8 (30.8%) |
| Black | 9 (34.6%) | 5 (19.2%) |
| Other | 1 (3.8%) | 1 (3.8%) |
| Reason for PCA/PNCA Administration | ||
| Spine surgerya | 19 (73.1%) | 12 (46.2%) |
| Peripheral orthopedic surgeryb | 4 (15.4%) | 0 (0%) |
| Bladder exstrophy repair | 1 (3.8%) | 0 (0%) |
| Pectus excavatum repair | 1 (3.8%) | 1 (3.8%) |
| T-cell lymphoma | 1 (3.8%) | 1 (3.8%) |
PCA = patient-controlled analgesia; PNCA = parent/nurse-controlled analgesia; RIP = respiratory inductance plethysmography.
Spinal surgery included posterior spinal fusion, laminectomy, suboccipital craniectomy, growing rod revision, spinal cord untethering.
Peripheral orthopedic surgery included tenotomy, osteotomy, club foot repair.
Figure 2.
Time to Failure of Monitoring Devices. Kaplan Meier plot showing time to patient removal of capnography cannula and RIP bands (n=14). Each patient wore a continuous pulse oximetry probe for the entire 24-hour study duration as part of routine monitoring. RIP = respiratory inductance plethysmography
Discussion
In this pilot study, we found that pediatric patients did not tolerate wearing the Smart Capnoline Plus O2 capnography cannulae for prolonged periods of time. Consequently, this monitor, as currently designed, is unlikely to be effective as a continuous monitor of ventilation in spontaneously breathing children being treated with IVPCA opioids. RIP bands were better tolerated, but they also had significant problems. These findings underscore the need for child-friendly respiratory monitors and a need to reconsider the applicability of adult monitoring guidelines in children until such devices are available.
The most common methods of monitoring respiratory depression in current practice are transthoracic impedance plethysmography, which estimates respiratory rate by measuring changes in electrical impedance associated with chest movement during respiration, and continuous pulse oximetry, which measures heart rate and oxygen saturation. Transthoracic impedance plethysmography readings can be inaccurate for a variety of reasons, including inaccurate ECG electrode placement, motion artifact, and physiological events unrelated to respiration that cause chest wall movement, such as coughing and crying. Furthermore, transthoracic impedance plethysmography has a high false alarm rate and is insensitive to obstructive apnea, that is, the presence of chest wall movement without any actual gas exchange.7, 8 These limitations make the technique of limited value as an early warning device for respiratory depression.
Pulse oximetry measures heart rate and oxygen saturation. However, oxygenation and ventilation are separate, albeit related, physiologic processes, and desaturation and bradycardia are only surrogate markers of gas exchange. As such, they are late rather than early warning signs of respiratory insufficiency, particularly if patients are receiving supplemental oxygen.7–9 Although the sensitivity of pulse oximetry as a respiratory monitor can be enhanced if supplemental oxygen is not given, postoperative patients treated with opioids often receive supplemental oxygen either preemptively by well-intentioned staff or in response to low or falling oximetry readings. Limiting “prophylactic” supplemental oxygen may be possible, but experience suggests that supplemental oxygen is often required after many major surgeries (e.g., posterior spinal fusion, Nuss procedure, thoracotomy) in the early post-operative period. This need circumvents a potential safety mechanism and puts patients at increased risk of unrecognized hypoventilation by increasing the time between apnea and desaturation.
Because capnography provides a direct measure of airflow and a more accurate assessment of respiratory rate than impedance plesthysmography, it has been studied as an early warning monitor of impending respiratory insufficiency in adults.6, 9, 10 We believe that this is the first study to investigate the tolerability of capnography and RIP for long-term respiratory monitoring in pediatric patients being treated for pain in noncritical care settings. Our results differ from those reported in a similar study that used the same capnography cannulae in adult patients9 but they are consistent with a respiratory monitoring study by Patino, et. al.,14 in which only 62.5% of pediatric patients tolerated capnography for an unspecified period of time. Although our sample size was small, our patients were generally old enough that we could expect them to be cooperative. Hence, the degree to which capnography failed as a monitor in our study makes it unlikely that increasing our sample size would significantly alter our results. Rather, we suggest that the inability of pediatric patients with a natural airway to tolerate prolonged use of nasal capnography is not unexpected. We can only speculate as to whether an alternate design, such as one that sampled exhaled gas via a simple nasal cannula, might have been better tolerated by our subjects. Though this might have been the case, we specifically chose to study the Oridion cannula in large part because it has already been validated in adult6, 9 and short-term pediatric studies14 and because we had concerns that a simpler design would not accurately monitor ventilation in mouth-breathing patients, potentially leading to inaccurate data and frequent false (positive and negative) alarms. Finally, this cannula is advertised as the most comfortable on the market because the plastic that sits on the face is soft rather than stiff, and this property was particularly appealing to us in the research design.
RIP bands were generally tolerated for longer periods of time than the capnography cannulae were, despite the fact that almost all of our study patients had undergone chest or spine surgery, a situation wherein placement of these bands might have caused additional discomfort. This finding further supports our belief that monitor design, rather than an unwillingness to be monitored, was responsible for the failure of long-term capnography monitoring in our patients. RIP uses circumferential soft bands with impregnated electrical coils that monitor both respiratory rate and airway patency. Respiratory movements induce changes in the cross sectional area and electrical self-inductance of the coils contained in the dual thoracic and abdominal bands; then respiratory rate is calculated from measured changes in body surface area. In addition, by identifying asynchrony or increasing lag between movement of the thorax and abdomen, the dual-band RIP can detect upper airway obstruction. Although the utility of this technique has been demonstrated in the sleep lab, where, by definition, subjects are asleep and relatively motionless, additional study is required to determine the usefulness of RIP in patients who are awake, ambulating, eating, talking, and occasionally sleeping. In our setting, we found that using RIP bands in patients who were awake and mobile was frequently tolerated, but movements produced a substantial amount of artifact in the recordings, in part owing to constant shifting around the chest and abdomen (data not shown). These findings lead us to acknowledge RIP’s tolerability but question its potential utility as a long-term ventilatory monitor in awake subjects in the absence of a change in design.
In most instances during this study, capnography cannulae were removed volitionally while patients were awake. Although one might argue that these cannulae might have been tolerated for more of the study period if we had required that they be worn only while patients were sleeping, this monitoring approach is impractical. Patients being treated for pain with opioids frequently drift back and forth between sleep and wakefulness, making it essential that children tolerate the monitor while awake. Indeed, this situation is where pulse oximetry shines. Previous studies have shown that despite its known limitations in monitoring respiration, pulse oximetry is well tolerated for long periods in both awake and sleeping patients, and pulse oximetry probes were worn by all of our study patients for the entire 24-hour observation period. Finally, it may also be argued that adherence could have been improved if use of these devices had been mandated (as was pulse oximetry) rather than voluntary. In the context of a clinical study, however, this limitation remains an inherent bias as patient/parent consent and assent are mandatory for ethical trial design.15 Nevertheless, outside of the study setting, even simple mandated monitoring in adults has been shown to be incompletely used, suggesting that mandating the monitoring of end-tidal CO2 in children would not necessarily translate into long-term compliance.
In conclusion, to reduce the likelihood of unrecognized, clinically significant, opioid-induced respiratory depression, capnography and/or other continuous electronic monitors of ventilation and oxygenation are commonly mandated in adults whenever IVPCA is used. We believe that the need for respiratory monitoring may be even greater for children treated with opioids because young children may be more sensitive to the respiratory depressant effects of opioids.3, 16 In addition, they may receive continuous opioid infusions or surrogate opioid dosing either in the form of nurse administered as needed (prn) opioids or IVPCA demand doses initiated by a caregiver (PNCA or PCA by proxy).17, 18 Thus, we agree that continuous ventilatory monitoring is needed to detect and prevent opioid-induced respiratory depression. However, our pediatric patients simply did not tolerate wearing modified nasal cannulae designed for accurate capnographic gas sampling for prolonged periods of time. Therefore, it is essential to develop better tolerated or alternative respiratory monitoring devices to ensure patient safety. However, until such child-friendly monitors are available and their utility validated, guidelines and recommendations validated in adult patients cannot be extended to children.
Summary.
Continuous electronic monitoring of both oxygenation and ventilation has been endorsed as a means to minimize the risk of opioid-induced respiratory depression.
In this study of two ventilatory monitoring techniques in non-intubated, spontaneously breathing children, we found that capnography monitoring was not tolerated for prolonged periods; respiratory inductance plethysmography was better tolerated but may be of limited utility.
Respiratory monitoring techniques effective in adult patients may not be appropriate for children, highlighting a need to develop and validate effective respiratory monitors for pediatric patients.
Acknowledgments
Disclosures: This study was approved by the Johns Hopkins Medicine Office of Human Subjects Research Institutional Review Boards.
Funding: This work was funded by Smiths Medical, Inc. and the Richard J. Traystman endowed chair. Dr. Kudchadkar was supported by the Johns Hopkins CTSA Award Number 5KL2RR025006 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: No conflicts of interest declared.
References
- 1.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. British journal of anaesthesia. 2004;93(2):212–223. doi: 10.1093/bja/aeh180. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro A, Zohar E, Zaslansky R, Hoppenstein D, Shabat S, Fredman B. The frequency and timing of respiratory depression in 1524 postoperative patients treated with systemic or neuraxial morphine. J. Clin. Anesth. 2005;17(7):537–542. doi: 10.1016/j.jclinane.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Voepel-Lewis T, Marinkovic A, Kostrzewa A, Tait AR, Malviya S. The prevalence of and risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesth. Analg. 2008;107(1):70–75. doi: 10.1213/ane.0b013e318172fa9e. [DOI] [PubMed] [Google Scholar]
- 4.Macintyre PE. Safety and efficacy of patient-controlled analgesia. British journal of anaesthesia. 2001;87(1):36–46. doi: 10.1093/bja/87.1.36. [DOI] [PubMed] [Google Scholar]
- 5.Nelson KL, Yaster M, Kost-Byerly S, Monitto CL. Current trends in pediatric acute pain management: Results of a national survey of American pediatric anesthesiologists. Anesthesia and analgesia. 2009 doi: 10.1213/ANE.0b013e3181ca749c. [DOI] [PubMed] [Google Scholar]
- 6.Maddox RR, Oglesby H, Williams CK, Fields M, Danello S. Continuous Respiratory Monitoring and a "Smart" Infusion System Improve Safety of Patient-Controlled Analgesia in the Postoperative Period Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 4: Technology and Medication Safety) Rockville MD: 2008. [Google Scholar]
- 7.Nassi N, Piumelli R, Lombardi E, Landini L, Donzelli G, de MM. Comparison between pulse oximetry and transthoracic impedance alarm traces during home monitoring. Arch. Dis. Child. 2008;93(2):126–132. doi: 10.1136/adc.2007.118513. [DOI] [PubMed] [Google Scholar]
- 8.Wiklund L, Hok B, Stahl K, Jordeby-Jonsson A. Postanesthesia monitoring revisited: frequency of true and false alarms from different monitoring devices. J. Clin. Anesth. 1994;6(3):182–188. doi: 10.1016/0952-8180(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 9.Overdyk FJ, Carter R, Maddox RR, Callura J, Herrin AE, Henriquez C. Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth. Analg. 2007;105(2):412–418. doi: 10.1213/01.ane.0000269489.26048.63. [DOI] [PubMed] [Google Scholar]
- 10.Gaucher A, Frasca D, Mimoz O, Debaene B. Accuracy of respiratory rate monitoring by capnometry using the Capnomask(R) in extubated patients receiving supplemental oxygen after surgery. British journal of anaesthesia. 2012;108(2):316–320. doi: 10.1093/bja/aer383. [DOI] [PubMed] [Google Scholar]
- 11.Lightdale JR, Goldmann DA, Feldman HA, Newburg AR, DiNardo JA, Fox VL. Microstream capnography improves patient monitoring during moderate sedation: a randomized, controlled trial. Pediatrics. 2006;117(6):e1170–e1178. doi: 10.1542/peds.2005-1709. [DOI] [PubMed] [Google Scholar]
- 12.Eipe N, Doherty DR. A review of pediatric capnography. Journal of clinical monitoring and computing. 2010;24(4):261–268. doi: 10.1007/s10877-010-9243-3. [DOI] [PubMed] [Google Scholar]
- 13.Hammer J, Newth CJ. Assessment of thoraco-abdominal asynchrony. Paediatr. Respir. Rev. 2009;10(2):75–80. doi: 10.1016/j.prrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Patino M, Redford DT, Quigley TW, Mahmoud M, Kurth CD, Szmuk P. Accuracy of acoustic respiration rate monitoring in pediatric patients. Paediatric anaesthesia. 2013;23(12):1166–1173. doi: 10.1111/pan.12254. [DOI] [PubMed] [Google Scholar]
- 15.Yaster M, Galinkin J, Schreiner MS. An introduction to the ethical design, conduct, and analysis of pediatric clinical trials. In: Gregory GA, Andropoulos DB, editors. Gregory's Pediatric Anesthesia. 5th. Oxford, UK: Wiley-Blackwell; 2012. pp. 40–59. [Google Scholar]
- 16.Yaster M, Kost-Byerly S, Maxwell LG. Opioid Agonists and Antagonists. In: Schechter NL, Berde CB, Yaster M, editors. Pain in Infants, Children, and Adolescents. 2. Philadelphia: Lippincott Williams and Wilkins; 2003. pp. 181–224. [Google Scholar]
- 17.Anghelescu DL, Burgoyne LL, Oakes LL, Wallace DA. The safety of patient-controlled analgesia by proxy in pediatric oncology patients. Anesth. Analg. 2005;101(6):1623–1627. doi: 10.1213/01.ANE.0000184198.13285.33. [DOI] [PubMed] [Google Scholar]
- 18.Monitto CL, Greenberg RS, Kost-Byerly S, et al. The safety and efficacy of parent-/nurse-controlled analgesia in patients less than six years of age. Anesth. Analg. 2000;91(3):573–579. doi: 10.1097/00000539-200009000-00014. [DOI] [PubMed] [Google Scholar]