Abstract
Effects of nanoparticles (NPs) on skin corrosion and irritation using three-dimensional human skin models were investigated based on the test guidelines of Organization for Economic Co-operation and Development (OECD TG431 and TG439). EpiDermTM skin was incubated with NPs including those harboring iron (FeNPs), aluminum oxide (AlNPs), titanium oxide (TNPs), and silver (AgNPs) for a defined time according to the test guidelines. Cell viabilities of EpiDermTM skins were measured by the 3-(4, 5-dimethylthi-azol-2-yl)-2.5-diphenyltetrazolium bromide based method. FeNPs, AlNPs, TNPs, and AgNPs were non-corrosive because the viability was more than 50% after 3 min exposure and more than 15% after 60 min exposure, which are the non-corrosive criteria. All NPs were also non-irritants, based on viability exceeding 50% after 60 min exposure and 42 hr post-incubation. Release of interleukin 1-alpha and histopathological analysis supported the cell viability results. These findings suggest that FeNPs, AlNPs, TNPs, and AgNPs are ‘non-corrosive’ and ‘non-irritant’ to human skin by a globally harmonized classification system.
Keywords: Nanoparticles, Skin model, Alternative methods, Skin corrosion, Skin irritation
INTRODUCTION
Tests of skin corrosion and irritation have long been performed using animals including rabbits based on the test guideline (TG) 404 published by the Organization for Economic Co-operation and Development (1,2). However, the growing ethical recognition of animal welfare has prompted the replacement of in vitro testing of skin corrosion and irritation using animals by alternative methods (3). Regulatory classifications of chemicals with skin corrosion and/or irritation are transitioning from using in vivo data to in vitro data obtained from alternative methods using human skin models (4).
Recently, various three-dimensional (3D)-human skin models have become commercially available for the alternative test methods; EpiSkinTM (EpiSkin Research Institute, Lyon, France), EpiDermTM (MatTech Co., Ashland, MA, USA), and SkinEthicTM (EpiSkin Research Institute) are the most widely used for regulatory purposes. They make use of reconstructed human epidermis obtained from human derived non-transformed epidermal keratinocytes, which closely mimic the histological, morphological, biochemical, and physiological properties of the epidermal layer of human skin (5,6).
Nanoparticles (NPs), defined as small-scale materials less than 100 nm in at least one dimension, are used in a variety of areas including electronics, cosmetics, pharmaceuticals, and catalysts (7,8). The rapid development of nanotechnology and use of NPs has led to the concern of deleterious human and environmental NP exposure. Hazards of NPs include genotoxicity, reproductive toxicity, and inflammatory responses (7). However, further study is needed to identify human skin related toxicity that includes irritation, sensitization, and corrosion, since human skin can be the first target of NP exposure (9).
Acute dermal irritation and corrosion testing of silver NPs (AgNPs) using rabbits did not show any skin damage including erythema eschar or edema formation for 72 hr after exposure (10,11). However, AgNPs decreased viability and increased the inflammatory cytokines levels of interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α) in cultured human epidermal keratinocytes (12). Several recent studies of skin corrosion/irritation by NPs utilized in vitro testing with cultured skin-derived cell lines. In vitro data using 3D-human skin models are needed, given the dissimilarity in the data between cell systems and 3D-human skin models. Titanium NPs (TNPs) and quantum dot NPs decreased the viability of cultured HaCaT human keratinocytes cell line but did not decrease the viability of a human skin equivalent model (13,14). Nanosilica NPs also caused cell death in cultured human keratinocytes but were not cytotoxic in a human skin equivalent model (15).
In this study, iron NPs (FeNPs), aluminum oxide (Al2O3) NPs (AlNPs), titanium oxide (TiO2) NPs (TNPs), and silver NPs (AgNPs) were investigated using the EpiDermTM in vitro 3D-human skin model as an alternative test of skin corrosion and irritation.
MATERIALS AND METHODS
Materials
FeNPs were purchased from Sigma-Aldrich (St. Louis, MO, USA). As supplied, the NPs were a black powder. Analysis by transmission electron microcopy (TEM) revealed particle size ranging from 35~45 nm. Purity was 99.5% based on the trace metals analysis and oxygen content was less than 3 wt%. AlNPs were also purchased from Sigma-Aldrich. Following the product specification supplied by the manufacturer, they were prepared as 20 wt% suspension in water. TEM analysis showed a particle size range of 30~60 nm. AgNPs (Sigma-Aldrich) were prepared as a dark grey powder and contained polyvinyl pyrrolidone as dispersant. Purity was 99.5% based on the trace metals analysis and TEM-determined particle size was less than 100 nm. TNPs (P-25, 21 nm diameter size; Aeroxide®) were kindly provided by Evonik Industry (Ham, France) as part of an OECD program for nanomaterial assessments. P-25 was supplied as a fine white powder with a hydrophilic character due to surface hydroxyl groups (ca. five OH groups per nm2). It consists of aggregated primary particles. The aggregates were several hundred nm in size. Mean diameter of the primary particles was approximately 20 nm.
The EpiDermTM consists of normal human-derived keratinocytes, multiple viable cell layers, and functional stratum corneum. It is an OECD-validated skin model. EpiDermTM samples were provided on specially prepared cell culture inserts prepared by the manufacturer. Immunoassay kit for human IL-1α was purchased from R&D Systems (Minneapolis, MN, USA).
EpiDermTM and NP exposure
For the in vitro skin corrosion test, the EpiDermTM inserts were transferred to wells of 6-well plates containing 0.9 mL Dulbecco’s Modified Eagle’s Medium (DMEM) and pre-incubated in a 5% CO2 incubator overnight according to the supplier’s protocol. After pre-incubation, the EpiDermTM inserts were exposed to FeNPs, AlNPs, AgNPs, and TNPs for 3 min, and separately for 60 min based on OECD test guideline (TG) 431. As specified by the TG, the epidermis surface was moistened with deionized water if the 25 mg of test material was a powder (FeNPs, AgNPs, and TNPs) to improve contact. AlNPs were supplied as a liquid; 50 μL was directly applied to the epithelial insert. KOH (8 N) was used as positive chemical and phosphate buffred saline (PBS) was the normal control. Three replicates of EpiDermTM were used for each experiment. After exposure, the test materials were removed from the exposed skin models by repeated rinsing with PBS, and they were transferred to the new plates for viability testing as described below.
For the OECD TG439 in vitro irritation test, skin models were exposed to NPs for 60 min. Sodium dodecyl sulfate (5% solution) was used as the positive chemical. Following exposure, test materials were removed and repeatedly rinsed with PBS. Post-incubation of the exposed skin models was done in fresh medium for 42 hrs prior to the viability assay.
Viability assay
For viability testing, the insert was transferred to a well of a 24-well plate. 3-(4,5-Dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 300 μL of a 0.3 mg/mL solution) was added to each well. Samples were incubated in a 5% CO2 incubator for 3 hrs and insoluble formazan products of MTT were extracted by 2 mL isopropanol that was added to each well. A 200 μL portion of the isopropanol extract was transferred to a well of a 96-well plate and the optical density (OD) was measured at 570 nm.
IL-1α assay and histopathology
During the 42 hr post-incubation period of the skin irritation test, the medium was replaced with fresh medium at 24 hr. The replaced medium was kept for the IL-1α assay. After coating wells of a 96-well plate with capture antibody, 100 μL aliquots of the medium obtained at 24 hr were added to wells and incubated for 2 hr at room temperature. After washing the wells, detection antibody was added and the plate was incubated at room temperature for 2 hr. Following the washing steps, streptavidin-horse radish peroxidase-conjugated secondary antibody was added to the wells and the plate was incubated for 20 min. After washing, substrate solution was added and incubated for 20 min. After incubation, the reaction was stopped by adding 2 N sulfuric acid. The OD was measured at 450 nm using a microplate reader. The concentration was calculated using a standard curve.
For the histopathological examination, EpiDermTM was taken and prefixed in 10% (v/v) neutral buffered formaldehyde. The fixed tissues were trimmed, dehydrated, embedded in paraffin, sectioned, mounted on glass slides, stained with hematoxylin and eosin, and examined by light microscopy.
Interpretation of in vitro test results
According to OECD TG431, the test material is considered to be corrosive to the skin if the viability is < 50% after 3 min exposure. Although the viability after 3 min exposure is ≥ 50%, it is corrosive if the viability is < 15% after 60 min exposure. The material is non-corrosive if the viability is ≥ 50% after 3 min exposure and ≥ 15% after 60 min exposure. Regarding the irritation test, the test material is considered to be irritant to skin if the tissue viability after exposure/post-incubation is ≤ 50%. It is a non-irritant if the viability is > 50% in accordance with OECD TG439.
RESULTS
In vitro skin corrosion test
In the corrosion test, cell viability of EpiDermTM exposed to the positive chemical 8 N KOH for 3 min was decreased to 9.8%. However, the mean cell viability of EpiDermTM treated with NPs for 3 min was 87.5~96.3%. When the exposure time was increased to 60 min, viability decreased to 49.8% in FeNP treated samples, 58.7% in AgNP treated samples, 97.7% in AlNP treated samples, and 85.3% in TNP treated samples (Table 1). The decreased viability by FeNPs and AgNPs was not indicative of skin corrosive behavior. Based on OECD TG431, all the tested NPs were non-corrosive because the viability exceeded 50% and 15% after 3 and 60 min exposure, respectively.
Table 1.
Cell viabilities of 3D human skin model EpiDermTM in skin corrosion test
| Cell viability (%) | FeNPs | AlNPs | TNPs | AgNPs | KOH |
|---|---|---|---|---|---|
| 3 min | 87.5 ± 4.3*** | 90.9 ± 4.6** | 96.3 ± 2.4 | 89.0 ± 1.9*** | 9.8 ± 1.6*** |
| 60 min | 49.8 ± 28.6*** | 97.9 ± 5.8 | 85.3 ± 3.9 | 58.7 ± 43.1** | 8.3 ± 0.7*** |
EpiDerm™ were treated with nanoparticles for 3 min and 60 min, respectively and MTT assay was performed. Data were expressed as the mean ± S.D. (N = 3). Significant differences,
p < 0.01,
p < 0.001.
In vitro skin irritation test
In the irritation test, EpiDermTM cells were exposed to NPs and 5% sodium dodecyl sulfate (SDS) as the positive chemical for 60 min. The treated materials were washed out completely and the skins of EpiDermTM were post-incubated for 42 hr. EpiDermTM cell viability was 8.4 ± 0.2% in the 5% SDS-treated group after 42 hr. However, the viabilities of the NP-treated groups were not as comparably low: 80.9 ± 17.2% with FeNP treatment, 71.6 ± 31.0% with AlNP treatment, 94.4 ± 2.5% with TNP treatment, and 81.3 ± 1.8% with AgNP treatment (Table 2). Based on OECD TG439, all the NP preparations were non-irritants because the viability exceeded 50% after 60 min exposure and 42 hr incubation.
Table 2.
Cell viability of 3D human skin model in skin irritation test
| FeNPs | AlNPs | TNPs | AgNPs | SDS | |
|---|---|---|---|---|---|
| Cell viability (%) | 80.9 ± 17.2 | 71.6 ± 31.0** | 94.4 ± 2.5 | 81.3 ± 1.8 | 8.4 ± 0.2*** |
EpiDerm™ were treated with nanoparticles for 60 min and post-incubated for 42 hr and MTT assay was performed. Data were expressed as the mean ± S.D. (N = 3). Significant differences,
p < 0.01,
p < 0.001.
IL-1α assay and histopathology
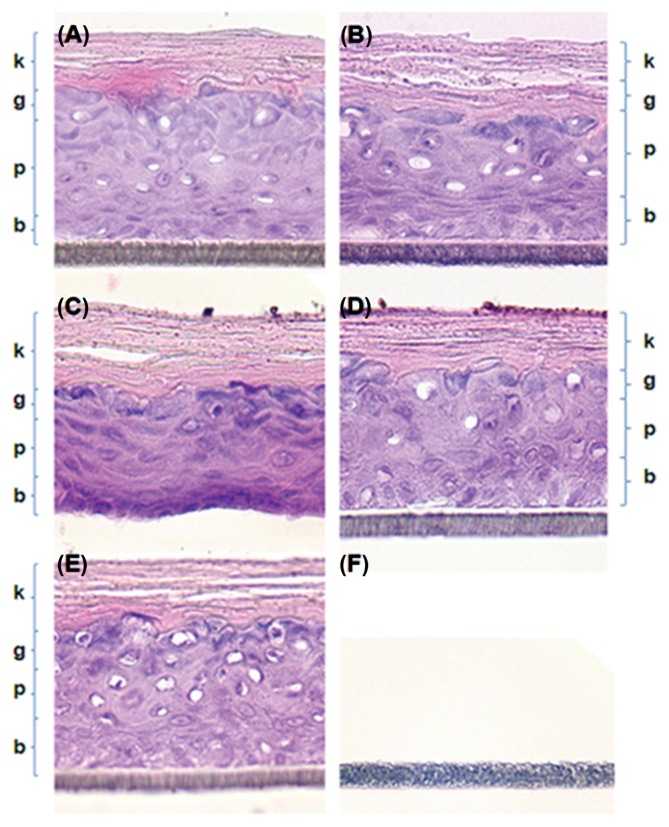
IL-1α is a marker of pro-inflammation. IL-1α in irritated skin was increased to 769.2 ± 7.6 pg/mL in the culture medium obtained from the 5% SDS treated group (Table 3), while the level of IL-1α in the PBS control was 189.8 ± 9.3 pg/mL. However, no significant increase was observed for any of the NP preparations, supporting their non-irritant nature. No histopathological changes were evident in EpiDerm TM skin treated with NPs (Fig. 1). NP treatment did not damage the keratin, granular, prickle cell, and basal layers (Fig. 1A–1D). PBS treatment was also not damaging (Fig. 1E). The keratin layer was characteristically dense and sufficient granules were evident in the granular layer. However, serious corrosion and destruction of skin structure was observed upon treatment with 8 N KOH, with corrosion and removal of cell layers evident (Fig. 1F).
Table 3.
IL-1α concentration in the media of cultured 3D human skin model EpiDermTM in skin irritation test
| FeNPs | AlNPs | TNPs | AgNPs | SDS | PBS | |
|---|---|---|---|---|---|---|
| IL-1α (pg/mL) | 85.4 ± 4.6 | 97.4 ± 6.3 | 67.7 ± 0.7 | 131.1 ± 4.9 | 769.2 ± 7.6 | 189.8 ± 9.3 |
EpiDerm™ were treated with nanoparticles for 60 min and post-incubated for 42 hr. During the 42 hr post-incubation period of skin irritation test, the media were replaced by fresh media at 24 hr and IL-1α was measured in the replaced media. Data were expressed as the mean ± S.D. (N = 3).
Fig. 1.
Histopathological examination of the EpiDermTM 3D-human skin model by hematoxylin and eosin (H & E) staining (×400). Histopathologically significant changes were not observed in the NP treated groups (A)~(D) and normal control groups (E). Keratin layer (k), granular layer (g), prickle cell layer (p), and basal layer (b) were obvious in NP treated groups (A)~(D) as well as in the normal control group (E). However, skin layers seem to be completely corroded out by treatment with 8N KOH (F). (A) FeNPs treated group, (B) AlNPs treated group, (C) TNPs treated group, (D) AgNPs treated group, (E) Normal control group, (F) 8 N KOH treated group.
DISCUSSION
Industrial and consumer uses of engineered NPs have increased to include applications as catalysts, and in the oil, textile, and cosmetic industries, and in medicine (16,17). As the uses of NPs have grown, the risks of environmental and human toxicity have also increased. Several studies that investigated NP-related hazards reported inflammatory responses, immune defects, genotoxicity, reproductive toxicity, developmental toxicity, and other related adverse effects, which may be caused by NP-mediated generation of reactive oxygen species (ROS) (18,19).
TNPs have a variety of applications. Most commonly they are used in pigments, paints, and cosmetics. The generation of ROS and cytotoxicity has been described when TNPs were added to cultured cells. However, information concerning TNP-related skin irritation is still limited. Ultraviolet and Visible-A (UV-A) irradiation of TNPs can induce significant cell damage of human skin keratinocytes. The damage is mediated by lipid and protein peroxidation. This phototoxicity is reportedly mediated by ROS generated during UV-A irradiation (20). However, another study reported that TNPs did not induce phototoxicity, acute cutaneous irritation, or skin sensitization in a human skin model (13). The penetration of nanomaterials in sunscreen formulations was observed when the mixtures of TNPs and zinc NPs were applied to UV-B damaged skin, but no transdermal absorption was detected (21). NPs made from iron or iron oxide, have also reportedly mediated ROS-linked redox reactions. A recent study showed that alveolar macrophages exposed to FeNPs increased their production of ROS, nitric oxide, and cytokines (22). Exposure to FeNPs can alter cell morphology, cytoskeleton of cells, and cell motility (23). Genotoxic effects of FeNPs are mainly influenced by direct interaction with leached iron ions from FeNPs or various indirect factors, such as excessive production of ROS (24). However, information concerning FeNP-related skin toxicity is scant, as is information on the effects of AlNPs and AgNPs on skin corrosion and skin irritation. In the present study, we sought to find direct evidence of an effect of NPs on skin corrosion and skin irritation using a 3D-human skin model, and explored whether the skin model would be good for regulatory purposes.
As specified in the OECD TG431 and TG439, test materials were directly applied to the skin model submerged into culture medium. Slight aggregation occurred when NPs were applied to the skin surface. The EpiDermTM was completely destroyed by 8 N KOH, with most cells killed after only a 3-min exposure (Table 1). Cell viability was not further decreased when the exposure time was increased to 60 min. So, 8 N KOH was toxic enough to cause corrosion in a very short time. EpiDermTM viability decreased with FeNP, TNP, and AgNP treatment as the exposure time increased from 3 min to 60 min, while AlNPs did not decrease the viability of EpiDermTM. A marked difference in EpiDerm TM viability was evident upon FeNP treatments; viability decreased from 87.5 ± 4.3% at 3 min exposure to 49.8 ± 28.6% at 60 min exposure. According to OECD TG431, EpiDermTM viability is judged to be non-corrosive when viability is ≥ 50% after 3 min exposure and > 15% at 60 min exposure. Thus, all NPs tested in this study were non-corrosive.
Concerning skin irritation, viability upon treatment with 5% SDS as the positive chemical was decreased to 8.4 ± 0.2%, while the viabilities upon treatment with all NP preparations exceeded the 50% limit value for skin irritation. The difference between the skin corrosion and irritation tests was the 42-hr post-incubation after the 60 min exposure. During the post-incubation period, reversibly damaged cells can be restored to be viable. Viabilities of the NP preparations, except for the AlNP-treated group, were higher in the skin irritation test compared to the skin corrosion test. Although the viability of AlNPs in the skin irritation test was lower than in the skin corrosion test, it was in the range of standard deviation. As shown in Table 1 and Table 2, the test nanoparticles were not corrosive and irritant based on the OECD TG criteria. However, they showed week cytotoxicity to EpiDermTM. It means that NPs may cause adverse effects in skin cells when they are exposed to NPs. Further studies on the toxic mechanism of NPs in skin cell including cell death mechanism will be necessary.
IL-1α production was not elevated in the medium of each NP-treated group compared to the positive SDS control (Table 3). Although the levels of IL-1α in the tested groups are not same level but lower than the PBS control group, it does not distract the conclusion for non-irritation/non-corrosive. None of the NP preparations induced cell death or release of IL-1α in levels indicative of skin irritation. The non-corrosive nature of the NPs was supported by the histopathology observations that indicated lack of damage (Fig. 1). KOH, which is corrosive, completely damaged the skin structure.
In summary, FeNPs, AlNPs, TNPs, and AgNPs were tested for skin corrosion and irritation in vitro based on the OECD TG431 and TG439. The NPs were non-corrosive and non-irritant.
ACKNOWLEDGMENTS
This work was supported by National Institute of Environmental Research, Korea.
REFERENCES
- 1.OECD. Test No. 404: Acute dermal irritation/corrosion, OECD guidelines for the testing of chemicals, section 4. 2002 Available from: http://dx.doi.org/10.1787/9789264070622-en.
- 2.OECD. Test No. 404: Acute dermal irritation/corrosion, OECD guidelines for the testing of chemicals, section 4. 2015 Available from: http://dx.doi.org/10.1787/9789264242678-en.
- 3.Murthy PB, Kishore AS, Surekha P. Assessment of in vitro skin irritation potential of nanoparticles: RHE model. Methods Mol Biol. 2012;926:219–234. doi: 10.1007/978-1-62703-002-1_16. [DOI] [PubMed] [Google Scholar]
- 4.Basketter D, Jírova D, Kandárová H. Review of skin irritation/corrosion hazards on the basis of human data: A regulatory perspective. Interdiscip Toxicol. 2012;5:98–104. doi: 10.2478/v10102-012-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OECD. Test No. 431: In vitro skin corrosion: reconstructed human epidermis (RHE) test method, OECD guidelines for the testing of chemicals, section 4. 2015 Available from: http://dx.doi.org/10.1787/9789264242753-en.
- 6.OECD. Test No. 439: In vitro skin irritation: reconstructed human epidermis test method, OECD guidelines for the testing of chemicals, section 4. 2015 Available from: http://dx.doi.org/10.1787/9789264242845-en.
- 7.Hofmann-Amtenbrink M, Grainger DW, Hofmann H. Nanoparticles in medicine: current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomedicine. 2015;11:1689–1694. doi: 10.1016/j.nano.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Hussain I, Singh NB, Singh A, Singh H, Singh SC. Green synthesis of nanoparticles and its potential application. Biotechnol Lett. 2016;38:545–560. doi: 10.1007/s10529-015-2026-7. [DOI] [PubMed] [Google Scholar]
- 9.Franken A, Eloff FC, Du Plessis J, Du Plessis JL. In vitro permeation of metals through human skin: a review and recommendations. Chem Res Toxicol. 2015;28:2237–2249. doi: 10.1021/acs.chemrestox.5b00421. [DOI] [PubMed] [Google Scholar]
- 10.Kim JS, Song KS, Sung JH, Ryu HR, Choi BG, Cho HS, Lee JK, Yu IJ. Genotoxicity, acute oral and dermal toxicity, eye and dermalirritation and corrosion and skin sensitisation evaluation of silvernanoparticles. Nanotoxicology. 2013;7:953–960. doi: 10.3109/17435390.2012.676099. [DOI] [PubMed] [Google Scholar]
- 11.Paladini F, Sannino A, Pollini M. In vivo testing of silver treated fibers for the evaluation of skin irritation effect and hypoallergenicity. J Biomed Mater Res Part B Appl Biomater. 2014;102:1031–1037. doi: 10.1002/jbm.b.33085. [DOI] [PubMed] [Google Scholar]
- 12.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ Health Perspect. 2010;118:407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park YH, Jeong SH, Yi SM, Choi BH, Kim YR, Kim IK, Kim MK, Son SW. Analysis for the potential of polystyrene and TiO2 nanoparticles to induce skin irritation, phototoxicity, and sensitization. Toxicol In Vitro. 2011;25:1863–1869. doi: 10.1016/j.tiv.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Jeong SH, Park YH, Choi BH, Kim JH, Sohn KH, Park KL, Kim MK, Son SW. Assessment of the skin irritation potential of quantum dot nanoparticles using a human skinequivalent model. J Dermatol Sci. 2010;59:147–148. doi: 10.1016/j.jdermsci.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Park YH, Kim JN, Jeong SH, Choi JE, Lee SH, Choi BH, Lee JP, Sohn KH, Park KL, Kim MK, Son SW. Assessment of dermal toxicity of nanosilica using cultured keratinocytes, a human skin equivalent model and an in vivo model. Toxicology. 2010;267:178–181. doi: 10.1016/j.tox.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Contado C. Nanomaterials in consumer products: a challenging analytical problem. Front Chem. 2015;3:48. doi: 10.3389/fchem.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark WJ, Stoessel PR, Wohlleben W, Hafner A. Industrial applications of nanoparticles. Chem Soc Rev. 2015;44:5793–5805. doi: 10.1039/C4CS00362D. [DOI] [PubMed] [Google Scholar]
- 18.Khalili Fard J, Jafari S, Eghbal MA. A review of molecular mechanisms involved in toxicity of nanoparticles. Adv Pharm Bull. 2015;5:447–454. doi: 10.15171/apb.2015.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Vijver MG, Peijnenburg WJ. Summary and analysis of the currently existing literature data on metal-based nanoparticles published for selected aquatic organisms: applicability for toxicity prediction by (Q)SARs. Altern Lab Anim. 2015;43:221–240. doi: 10.1177/026119291504300404. [DOI] [PubMed] [Google Scholar]
- 20.Yin JJ, Liu J, Ehrenshaft M, Roberts JE, Fu PP, Mason RP, Zhao B. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes--generation of reactive oxygen species and cell damage. Toxicol Appl Pharmacol. 2012;263:81–88. doi: 10.1016/j.taap.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro-Riviere NA, Wiench K, Landsiedel R, Schulte S, Inman AO, Riviere JE. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: an in vitro and in vivo study. Toxicol Sci. 2011;123:264–280. doi: 10.1093/toxsci/kfr148. [DOI] [PubMed] [Google Scholar]
- 22.Ahamed M, Alhadlaq HA, Alam J, Khan MA, Ali D, Alarafi S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr Pharm Des. 2013;19:6681–6690. doi: 10.2174/1381612811319370011. [DOI] [PubMed] [Google Scholar]
- 23.Astanina K, Simon Y, Cavelius C, Petry S, Kraegeloh A, Kiemer AK. Superparamagnetic iron oxide nanoparticles impair endothelial integrity and inhibit nitric oxide production. Acta Biomater. 2014;10:4896–4911. doi: 10.1016/j.actbio.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Mesárošová M, Kozics K, Bábelová A, Regendová E, Pastorek M, Vnuková D, Buliaková B, Rázga F, Gábelová A. The role of reactive oxygen species in the genotoxicity of surface-modified magnetite nanoparticles. Toxicol Lett. 2014;226:303–313. doi: 10.1016/j.toxlet.2014.02.025. [DOI] [PubMed] [Google Scholar]