Abstract
Due to undesirable hazardous interactions with biological systems, this investigation was undertaken to evaluate the effect of chronic exposure to silver on certain biochemical and some oxidative stress parameters with histopathological examination of brain, as well as the possible protective role of selenium and/or vitamin E as nutritional supplements. Thirty six male rats were divided into six groups of six each: the first group used as a control group. Group II given both vitamin E (400 mg/kg) of diet and selenium (Se) (1 mg/L) in their drinking water. Group III given silver as silver nitrate (AgNO3) (20 mg/L). Group IV given vitamin E and AgNO3. Group V given both AgNO3 and selenium. Group VI given AgNO3, vitamin E and Se. The animals were in the same exposure conditions for 3 months. According to the results which have been obtained; there was an increase in serum lactate dehydrogenase (LDH), lipase activities and cholesterol level, a decrease in serum total protein, calcium and alkaline phosphatase (ALP) activity in Ag-intoxicated rats. Moreover, the findings showed that Ag+ ions affected antioxidant defense system by decreasing superoxide dismutase (SOD) activity and increasing vitamin E concentration with a high level of malondialdehyde (MDA) in brain tissue. The histological examination also exhibited some nervous tissue alterations including hemorrhage and cytoplasm vacuolization. However, the co-administration of selenium and/or vitamin E ameliorated the biochemical parameters and restored the histological alterations. In conclusion, this study indicated that silver could cause harmful effects in animal body and these effects can be more toxic in high concentrations or prolonged time exposure to this metal. However, selenium and vitamin E act as powerful antioxidants which may exercise adverse effect against the toxicity of this metal.
Keywords: Silver, Rats, Oxidative stress, Brain tissue, Vitamin E, Selenium
INTRODUCTION
Each year, thousands of new chemicals are released into the environment, a substantial proportion of which are likely to elicit developmental neurotoxicity. Recently, there has been an explosive increase in the use of silver (Ag) in medical and consumer products, largely in the form of silver nanoparticles, leading to a corresponding increase in human and ecosystem exposures (1,2). Silver is considered toxic for humans, aquatic and terrestrial animals (3,4). The cellular pathology and the molecular mechanisms involved in their toxicity in mammalian cells are largely unknown. The recommendations of the World Health Organization (WHO) permit maximal concentrations of 0.1 mg/L of silver ions in drinking water disinfection (5) and it is known that chronic exposure leads to argyria, a clinical entity characterized by gray-blue pigmentation of the skin and other body viscera (6). In addition some studies have examined the neurotoxicity effects of silver in experimental animal studies and human clinical reports. Ohbo et al. (7) have concluded that the seizures observed in an argyric patient were likely to be due to the presence of silver in the patient’s central nervous system (CNS). Landas et al. (8) have observed the deposition of silver nitrate (AgNO3) in circumventricular organs (CVO) and in paraventricular and supraoptic nuclei of hypothalamus, thus, Rungby and Danscher (9) have suggested that the hypoactivity of mice treated by Ag is due to an influence of silver upon the functional status of the CNS. Rungby et al. (10) have found that a direct administration of silver salts to neonatal rats results in damage to particularly vulnerable areas of brain, such as the hippocampus. The role of antioxidants like vitamin E and selenium (Se) is known to alleviate harmful effects associated with heavy metals (11). Moreover, Se is a component of several seleno-proteins and selenoenzymes with essential biological functions (12). It has been widely recognized as an essential dietary component with numerous beneficial effects on health, namely by its pharmacotherapeutic efficacy against brain diseases (13). This trace element is known to antagonize the toxicity of silver ions (14). Vitamin E is a lipid-soluble chain-breaking antioxidant which protects, especially, biological membranes from lipid peroxidation (15). Deficiency of dietary vitamin E and selenium has been showed to induce a number of lesions in rats depending upon the degree of depletion (16). The functional interrelation ship between vitamin E and selenium has long been recognized (17). In view of the above considerations, the present study was carried out to estimate the toxicity of silver on animal body and to evaluate the efficacy of vitamin E and selenium to alleviate this toxicity by measuring some biological serum markers and oxidative stress markers including brain lipid peroxidation (LPO) and antioxidants parameters with brain histopathology examination.
MATERIALS AND METHODS
Chemicals
Silver nitrate (AgNO3), sodium selenite (Na2SeO3), vitamin E (α-tocopherol), CDNB (1-chloro-2.4 dinitrobenzene), DTNB (5,5′ dithio-bis-(2-nitrobenzoic acid)), GSH (reduced glutathione), GSSG (oxidized glutathione) and epinephrine bathophenanthroline and NADPH (nicotinamide adenine dinucleotidephosphate reduced form) were obtained from sigma Chemical Co. (St Louis, Saint-Quentin Fallavier, France) and all other chemicals used in the experiment were of analytical grade.
Experiment animals
All experiments were performed with ‘Wistar’ male rats weighing about 260~280 g, which were purchased from Pasteur Institute, Algiers, Algeria. The animals were kept under good ventilation and were maintained on standard diet and water throughout the experimental period. They were kept at 22 ± 2°C with the 12 hr light/dark cycle and 40% humidity. All animal experiments were carried out according to the National Institute of Health Guideline for animal care and approved by the Ethics Committee of our institution. After two weeks of adaptation, thirty six rats were randomly divided into six groups of six each. Group I fed standard diet and used as control group. Group II received vitamin E at a dose of 400 mg/Kg diet and selenium (1 mg/L) in their drinking water. Group III received AgNO3 in their drinking water (20 mg/L). Group IV given both AgNO3 (20 mg/L) and standard diet enriched with vitamin E (400 mg/kg). Group V received AgNO3 (20 mg/L) and selenium (1 mg/L). Group VI given AgNO3, vitamin E and Selenium. During the course of treatment, body weight gain, food intake and water consumption were recorded regularly. The doses of AgNO3 and the period of treatment were basically selected on previous study of Environmental Protection Agency (EPA) (18). Vitamin E and selenium doses were also chosen on the clinical application and on results from previous investigations of Kim et al. (19) and Qingzhi et al. (20) respectively. The treatments of rats continued for a period of three months. At the end of the experiment, animals were sacrificed by cervical decapitation without anesthesia to avoid animals stress, blood was transferred into non-heparinised tubes. Serum was obtained by centrifugation of the blood at 3000 rpm and then quickly frozen at −20°C for biochemical analysis. Brain samples were rapidly excised, rinsed in ice cold saline [0.9% (w/v) NaCl]. Then, one lobe of brain was homogenized in a twice volume of ice cold TBS (50 mM TRIS, 150 mM NaCl, pH 7.4), the homogenates were centrifuged at 10.000 g for 15 min at 4°C, and the resultant supernatant was frozen at −20°C for oxidative parameters analysis. The other lobe of brain was fixed in formol solution and used for histological examination.
Biochemical analysis
Serum biochemical markers: glucose, transaminases (glutamic pyruvic transaminase: GPT, glutamic oxaloacetic transaminase: GOT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), direct bilirubin, total proteins, albumin, cholesterol, triglycerides, total lipids, creatinine, urea, uric acid, lipase, α-Amylase, calcium, phosphor were assessed using Spinreact Laboratory Spain diagnostic kits using spectrophotometer (Jenway 6505, Jenway LTD, Essex, UK). The references were as follow: glucose-41011, GOT-1001161, GPT-1001171, ALP-1001131, LDH-1001260, direct bilirubin-1001044, total proteins-100129, albumin-1001020, cholesterol-1001091, triglycerides-100131, total lipids-1001270, creatinine-1001110, urea-1001331, uric acid-1001010, lipase-1001275, α-Amylase-41201, calcium-1001061, phosphor-1001150.
Determination of oxidative parameters
Lipid peroxidation level
Lipid peroxidation as evidenced by formation of thiobarbituric acid reacting substances (TBARS), were measured by the method of Esterbauer and Cheeseman (21). 250 microliters of tissue homogenate were added to 1.5 mL of 1% phosphoric acid (pH 2.0) and 1 mL of 0.6% of TBA in air light tubes and the samples was cooled to room temperature and MDA (malondialdehyde)-TBA was extracted with 2.5 mL of butanol. Organic phase was separated by centrifugation for 5 min at 2000 g and measured at 532 nm. A 99% TBARS are MDA, so TBARS concentration of the samples were calculated using the extinction coefficient of MDA is 1.56 × 105 M−1cm−1. Lipid peroxidation is expressed as nmol TBARS/mg prot.
Estimation of enzymatic antioxidants
The specific activity of brain superoxide dismutase (SOD) was determined according to the method described by Misra and Fridonich (22). 10 μL of tissue homogenate were added to 970 μL of ethylene diamine tetraacetic acid (EDTA) - Sodium carbonate buffer (0.05 M) at pH10.2. The reaction was started by adding 20 μL of epinephrine (30 mM) and the activity was measured at 480 nm for 4 min. A unit of SOD is defined as the amount of enzyme that inhibits by 50% the speed of oxidation of epinephrine and the results were expressed as UI/mg protein.
Glutathione peroxidase (GSH-Px) catalyzes the reduction of hydroperoxides by utilizing GSH as a reductant. Determination of tissue GSH-Px activity was carried out according to the method of Flohe and Gunzler (23). The reaction mixture contained 0.2 mL of TBS (Tris 50 mM, NaCl 150 mM, pH 7.4); 0.4 mL of GSH (0.1 mM), 0.2 mL of homogenate was added and allowed to equilibrate for 5 min at 25°C. The reaction was initiated by adding 0.2 mL of H2O2 (1.3 mM); reaction was terminated by addition of 1 mL of 1% Trichloroacetic acid (TCA). Tubes were centrifuged at 1500 g for 5 min and the supernatant was collected. To 0.48 mL of resultant supernatant, 2.2 mL of TBS (pH 7.4) and 0.32 mL of DTNB (1.0 mM) were added. After mixing, absorbance was recorded at 412 nm and the specific activity of this enzyme is expressed as μmol GSH/mg protein.
Glutathione-S-transferase (GST) activity of tissues was measured spectrophotometrically by the method of Habig et al. (24) using CDNB as electrophilic substrate that binds to GSH with the participation of the enzyme and forms a colored GSH-substrate complex, detected at 340 nm. The activity of GST was expressed in terms of μmol CDNB-GSH conjugate formed/min/mg protein.
Glutathione reductase (GR) activity was based on the method of Goldberg and Spooner (25). The enzymatic activity was assayed photometrically by measuring, NADPH consumption. In the presence of oxidized glutathione (GSSG) and NADPH, GR reduces GSSG and oxidizes NADPH, resulting in a decrease of absorbance at 340 nm. Quantification was based on the molar extinction coefficient of 6.22 mM−1cm−1 of NADPH, 1 unit of GR was defined as the amount of enzyme that reduced 1 μmol GSSG (corresponding to the consumption of 1 μmol of NADPH) per minute at 25°C. The GR activities were expressed as 1 unit per milligram protein.
Catalase activity (CAT) measured using the method of Sinha (26). It is based on the fact that dichromate in acetic acid is reduced to chromic acetate when heated in the presence of H2O2, with the formation of perchromic acid as an unstable intermediate. The chromic acetate thus produced is measured calorimetrically at 570~610 nm. Since dichromate has no absorbance in this region, the presence of the compound in the assay mixture does not interfere at all with the colorimetric determination of chromic acetate. The catalase preparation is allowed to split H2O2 for different periods of time. The reaction is stopped at a particular time by the addition of dichromate/acetic acid mixture and the remaining H2O2 is determined by measuring chromic acetate calorimetrically after heating the reaction mixture.
Non-enzymatic antioxidants measurement
GSH concentration was performed with the method described by Ellman (27) based on the development of a yellow color when DTNB is added to compounds containing sulfhydryl groups. In brief, 0.8 mL of tissue homogenate was added to 0.2 mL of 0.25% sulphosalylic acid and tubes were centrifuged at 2500 g for 15 min. Supernatant (0.5 mL) was mixed with 0.025 mL of 0.01 M DTNB and 1 mL TBS (pH 7.4). Finally, absorbance at 412 nm was recorded. Total GSH content was expressed as nmol GSH/mg prot.
Vitamin E estimated by the method of Desai (28), to 1 mL of tissue homogenate, 1 mL of ethanol and 3 mL of petroleum ether were added, shaken rapidly and centrifuged at 4000 rpm for 10 min, 2 mL of supernatant was evaporated to dryness at 80°C, to that added 0.2 mL of bathophenanthroline, 0.2 mL of ferric chloride and 0.2 mL of phosphoric acid kept in dark for 5 min and then complete with 3 mL ethanol. The color developed was read at 530 nm, vitamin E levels were expressed as mg vitamin E/mg protein.
Protein determination
The protein content of tissue samples were measured by the method of Bradford (29) using bovine serum albumin as a standard.
Histological studies
For histological examination, brain was dissected and immediately fixed in formalin solution for 24 hr, processed by using a graded ethanol series, and then embedded in paraffin. The paraffin sections were cut into 5 μm thick slices and stained with hematoxylin and eosin for light microscopic examination (30). The sections were then viewed and photographed.
Statistical analysis
All the results were expressed as mean values ± SEM. Comparisons between the groups were performed by one-way ANOVA followed by student’s t-test. Differences were considered significant at p < 0.05.
RESULTS
Effect of treatments on body weight, food intake, water consumption and relative brain weight
AgNO3 dose (20 mg/L) did not affect clinical appearance, all animals survived until the termination of the study. It was observed that AgNO3 had no effect on body weight gain and relative brain weight, food intake and water consumption (Table 1), except AgNO3 + vit E + Se treated group showed significant decrease (p < 0.05) in water consumption as compared to AgNO3 exposed animals.
Table 1.
Body weight gain, relative brain weight, food intake and water consumption of control and experimental rats
| Treatments | Control (n = 6) | vit E + Se (n = 6) | AgNO3 (n = 6) | AgNO3 + vit E (n = 6) | AgNO3 + Se (n = 6) | AgNO3 + vit E + Se (n = 6) |
|---|---|---|---|---|---|---|
| Body weight gain1 | 88.57 ± 32.63 | 80.71 ± 20.34 | 97.14 ± 57.21 | 75.72 ± 51.12 | 97.14 ± 18.42 | 66.71 ± 47.97# |
| Relative brain weight2 | 0.33 ± 0.05 | 0.31 ± 0.06 | 0.33 ± 0.07 | 0.35 ± 0.04 | 0.33 ± 0.01 | 0.38 ± 0.06# |
| Food intake3 | 21.22 ± 2.02 | 23.45 ± 4.07 | 23.5 ± 4.21 | 24.58 ± 3.12 | 25.47 ± 1.69 | 22.5 ± 3.55# |
| Water consumption4 | 32.46 ± 4.02 | 30.06 ± 5.10 | 32.92 ± 6.46 | 32.38 ± 4.05++ | 31.66 ± 5.45 | 30.05 ± 4.62# |
Values are means ± SEM, n: number of animals in each group.
p < 0.05: significantly different from AgNO3,
p < 0.01: statistical difference between AgNO3+vit E and AgNO3 + vit E + Se,
Units:
g;
%;
g/rat/day;
mL/rat/day.
Effect of treatments on biochemical parameters
As seen from Table 2, AgNO3 induced a significant increase (p < 0.05) in LDH and lipase activities, a significant decrease (p < 0.05) in ALP activity and calcium content, a highly significant increase (p < 0.01) in cholesterol and a highly significant decrease (p < 0.01) in total proteins compared to the corresponding control values. Supplementation of vitamin E and/or Se to AgNO3-treated group restored the levels of ALP, total protein, LDH and cholesterol, the two later parameters presented a remarkable decreases (LDH: p < 0.01, AgNO3 + vit E + Se and cholesterol: p < 0.001, AgNO3 + vit E, AgNO3 + vit E + Se, p < 0.01, AgNO3 + Se) compared to metal exposed animals, while an amelioration in ALP activity (p < 0.01, AgNO3 + Se), and total protein concentration (p < 0.05, AgNO3 + vit E, p < 0.01, AgNO3 + Se) compared to AgNO3 group. In addition, total serum lipids were decreased in AgNO3 + vit E + Se group compared to AgNO3 (p < 0.001), AgNO3 + vit E (p < 0.01) and AgNO3 + Se (p < 0.05).
Table 2.
Variation biochemical parameters in serum of control group and experimental rats
| Treatments | Control (n = 6) | vit E + Se (n = 6) | AgNO3 (n = 6) | AgNO3 + vit E (n = 6) | AgNO3 + Se (n = 6) | AgNO3 + vit E + Se (n = 6) |
|---|---|---|---|---|---|---|
| Glucose1 | 0.13 ± 0.02 | 0.14 ± 0.013 | 0.13 ± 0.01 | 0.14 ± 0.03 | 0.15 ± 0.022 | 0.14 ± 0.01 |
| GOT2 | 56.99 ± 12.94 | 76.05 ± 12.05 | 72.01 ± 16.86 | 66.20 ± 21.50 | 64.17 ± 18.34 | 72.92 ± 10.24 |
| GPT2 | 34.22 ± 16.57 | 27.27 ± 10.39 | 36.70 ± 16.74 | 43.07 ± 14.59 | 36.55 ± 9.12 | 45.28 ± 11.15 |
| LDH2 | 481.2 ± 114.3 | 411.23 ± 113.8 | 654.9 ±141.9* | 436.9 ± 156.1 | 443.8 ± 160.7 | 370.2 ± 141## |
| APL2 | 93.06 ± 28.65 | 116.78 ± 29.65 | 58.85 ±17.73* | 77 ± 5.16 | 101.7 ± 34.85## | 83.6 ± 28.14 |
| Direct bilirubin1 | 2.08 ± 0.68 | 2.07 ± 0.6 | 2.08 ± 0.87 | 1.22 ± 0.25++ | 2.12 ± 0.53 | 2.32 ± 0.59 |
| Total proteins1 | 7.28 ± 1.68 | 6.46 ± 0.87 | 4.78 ± 1.06** | 5.86 ± 0.66# | 7.80 ± 1.01##++ | 5.2 ± 0.41 |
| Albumin1 | 3.27 ± 0.84 | 2.94 ± 0.97 | 3.49 ± 0.65 | 3.14 ± 0.33 | 3.75 ± 0.52 | 3.14 ± 0.9 |
| Cholesterol3 | 1.86 ± 0.62 | 1.66 ± 0.76 | 3.12 ± 0.62** | 1.26 ± 0.46### | 1.64 ± 0.65## | 1.32 ± 0.31### |
| Triglycerides3 | 1.11 ± 0.35 | 1.20 ± 0.48 | 1.18 ± 0.65 | 1.23 ± 0.13 | 1.52 ± 0.4 | 1.26 ± 0.4 |
| Total lipids4 | 262.59 ± 71.2 | 326.26 ± 131.9 | 341.67 ± 55.4 | 323.07 ± 68.5++ | 323.77 ± 29.4+ | 219.6 ± 61.9### |
| Creatinine4 | 0.69 ± 0.29 | 1.03 ± 0.14 | 1.015 ± 0.24 | 1.052 ± 0.52 | 0.86 ± 0.23 | 0.95 ± 0.37 |
| Urea4 | 35.2 ± 11.53 | 32.41 ± 8.69 | 33.33 ± 5.42 | 36.61 ± 5.35 | 37.21 ± 6.42 | 36.08 ± 5.00 |
| Uric acid4 | 4.05 ± 0.88 | 4.73 ± 0.89 | 4.02 ± 1.52 | 4.45 ± 1.26 | 4.86 ± 0.82 | 4.22 ± 1.44 |
| Lipase2 | 179.43 ± 46.4 | 123.41 ± 35.47 | 247.7 ± 39.5* | 224.1 ± 63.3 | 221.43 ± 64.8 | 170.16 ± 73.5 |
| α-Amylase2 | 531.6 ± 93.5 | 622.62 ± 95.93 | 548.7 ± 90.5 | 537.1 ± 49.7+ | 605.4 ± 152.5 | 635.8 ± 95.5 |
| Calcium4 | 11.95 ± 1.24 | 11.48 ± 2.84 | 9.73 ± 2.46* | 10.20 ± 2.91 | 11.56 ± 2.94 | 11.69 ± 2.06 |
| Phosphor4 | 7.73 ± 1.81 | 6.22 ± 0.73 | 6.25 ± 0.80 | 6.15 ± 1.11 | 6.28 ± 1.19 | 6.48 ± 0.7 |
Values are means ± SEM, n: number of animals in each group.
p<0.05,
p < 0.01: significantly different from control group,
p<0.05,
p<0.01,
p < 0.001: significantly different from AgNO3,
p<0.05,
p < 0.01: statistical difference between AgNO3+vit E, AgNO3 +Se and AgNO3 +vit E+Se.
Units:
g/dL;
U/L;
mmol/L;
mg/dL.
Effect of treatments on oxidative stress parameters
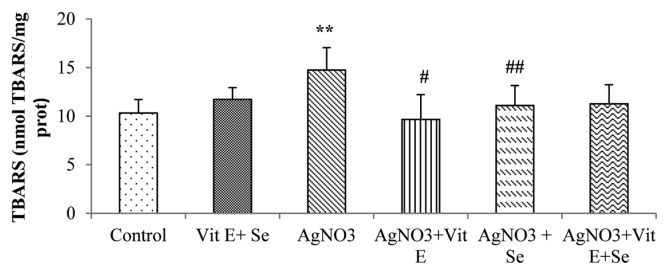
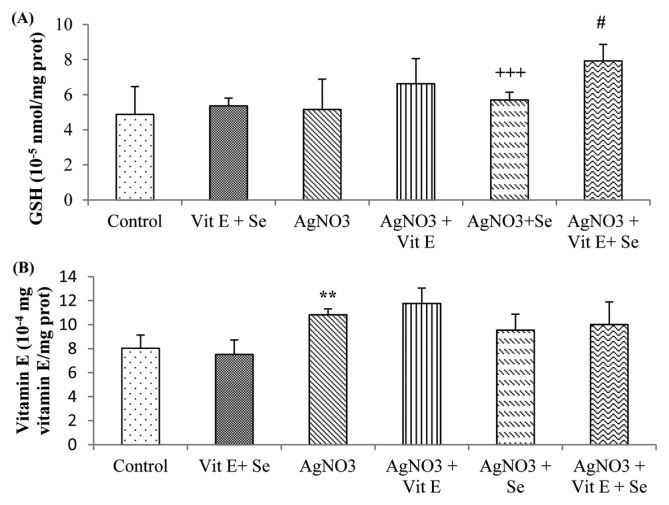
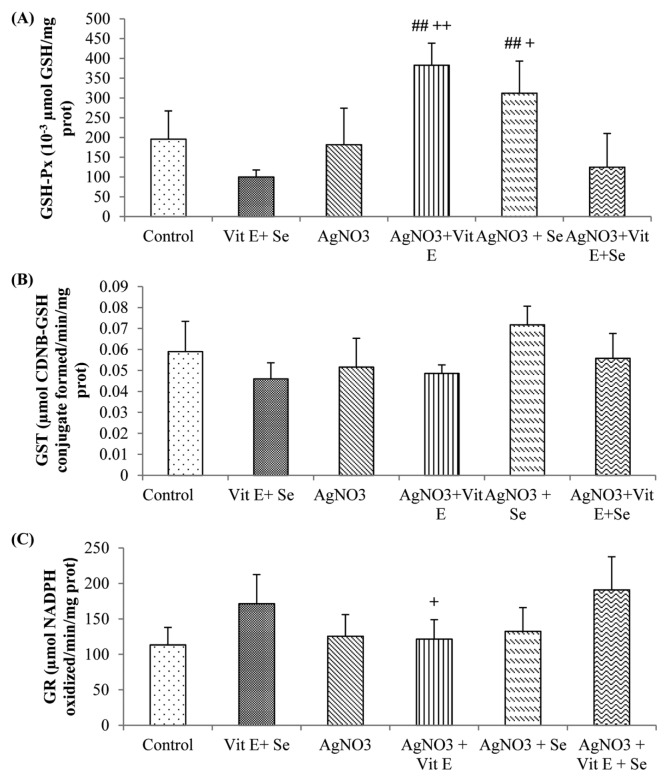
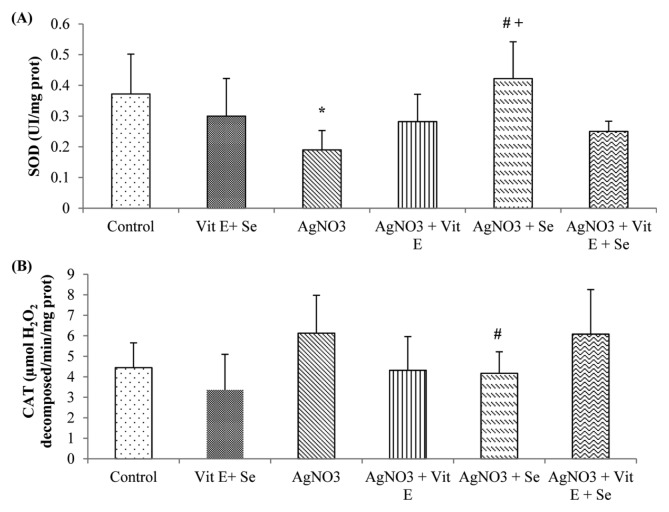
As shown in Fig. 1, exposure to AgNO3 produced a highly significant rise (p < 0.01) in TBARS levels in brain compared to control group. However, this parameter was reduced by vitamin E (p < 0.05) or selenium (p < 0.01) supplementation compared to Ag-intoxicated group. Brain GSH content was not affected in Ag-exposure, while a very highly significant decrease (p < 0.001) in Se supplemented group was observed in comparison to AgNO3 + vit E + Se treated animals. Meanwhile, the latter group presented a significant increase (p < 0.05) in GSH concentration compared to metal exposed rats (Fig. 2A). Furthermore, AgNO3 was found to increase brain vitamin E quantity (p < 0.01) (Fig. 2B). Data on GSH-Px, GST, GR, CAT and SOD activities are presented in Fig. 3 and 4. Brain GSH-Px, GR, GST and CAT activities were not altered under AgNO3. However, it was noted that silver caused a significant decline (p < 0.05) in SOD activity. Moreover, vitamin E treatment revealed a highly significant elevation (p < 0.01: AgNO3, p < 0.01: AgNO3 + vit E + Se) in GSH-Px activity. Similarly this activity was increased by selenium treatment (p < 0.01: AgNO3, p < 0.05: AgNO3 + vit E + Se). It was also observed that Se increased significantly (p < 0.05: AgNO3, AgNO3 + vit E + Se) SOD activity and decreased significantly (p < 0.05: AgNO3) CAT activity.
Fig. 1.
Lipid peroxidation levels in brain tissue of control and experimental rats. **p < 0.01: significantly different from control group, #p<0.05, ##p < 0.01: significantly different from AgNO3.
Fig. 2.
Brain non-enzymatic antioxidant levels of control and experimental rats. (A) Reduced glutathione (GSH); (B) vitamin E. **p < 0.01: significantly different from control group, #p < 0.05: significantly different from AgNO3, +++p < 0.001: statistical difference between AgNO3+vit E, AgNO3+Se and AgNO3+vit E + Se.
Fig. 3.
Brain enzymatic antioxidant levels of control and experimental rats. (A) GSH-Px; (B) GST, C: GR. ##p < 0.01: statistical difference from AgNO3, +p<0.05, ++p < 0.01: statistical difference between AgNO3 +vit E, AgNO3 +Se and AgNO3 +vit E + Se.
Fig. 4.
Brain enzymatic antioxidant levels of control and experimental rats. (A) SOD, (B) CAT. *p < 0.05: significantly different from control group, #p < 0.05: statistical difference from AgNO3, +p < 0.05: statistical difference between AgNO3 +vit E, AgNO3 +Se and AgNO3 +vit E + Se.
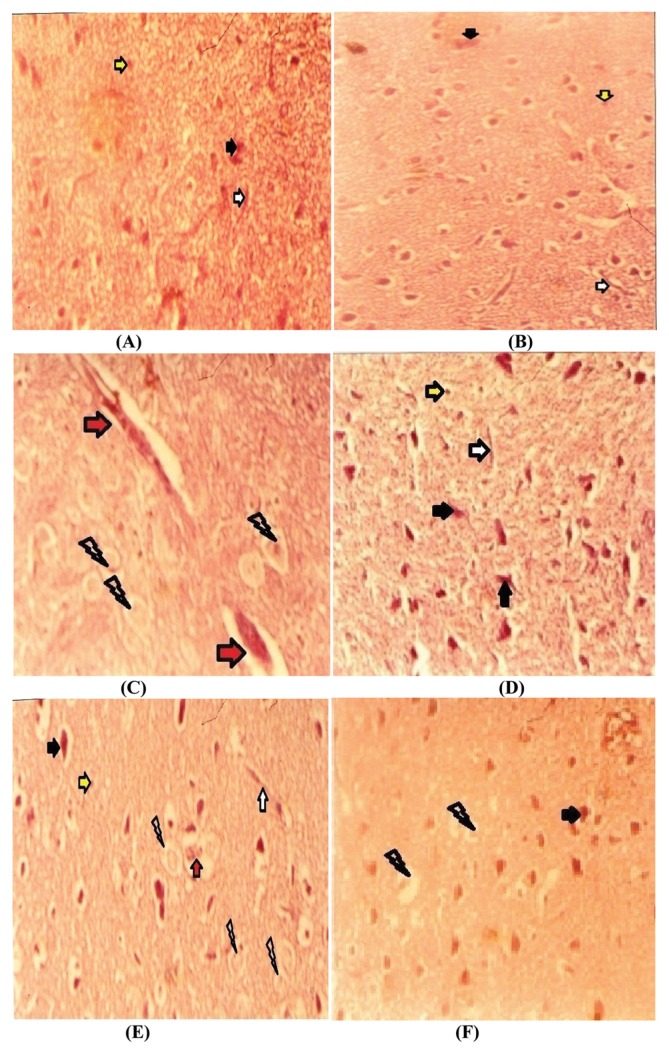
Histological results
Upon histological examination of control rats and vitamin E + Se treated rats, tissue presented normal histoarchitecture (Fig. 5A, 5B). In the Ag-treated rats, histological sections showed abnormalities (Fig. 5C) when compared to controls. Histopathological changes were alleviated in the administration of vitamin E (Fig. 5D) or Se (Fig. 5E), or both Se and vitamin E (Fig. 5F).
Fig. 5.
Photographs showing histological changes in cerebral tissue in different groups; Control group (A); (Vit E + Se)-treated group (B), histological picture showed normal brain tissue; Ag-treated group (C) showed some abnormalities; nervous tissue exhibited hemorrhage, and vacuolated spaces in the affected area; (Ag + Vit E)-treated group (D), (Ag + Se)-treated group (E) and (Ag + Vit E + Se)-treated group (F) ameliorated brain tissue histological pictures with less vacuolated cytoplasm especially in vitamin E supplementation, histological damages significantly decreased. Optic microscopy section were stained using the haematoxylin-eosin method, 40×. neurons; glial cells; a net work of finely branching small blood vessels; hemorrhage; vacuolated cytoplasm.
DISCUSSION
During three months of experiment, no clinical appearance or body weight loss have been observed after silver exposure. Also, relative brain weight, food intake and water consumption were not affected following oral administration of metal, these results were accentuated by some other reports (31). Simultaneously, the treatment with selenium and/or vitamin E did not resulted any variations in body weight gain, relative brain weight and food intake except for a lower consumption of water in AgNO3 + vit E + Se treated-group which could be a spurious finding and not an indication of toxicity effects. This study explored the influences of silver on some biochemical serum markers and oxidative stress status in brain. So that the findings showed an increases in LDH and lipase activities, cholesterol concentration and a decrease in ALP activity, total proteins and calcium in serum of AgNO3 rats. Increased levels of LDH could be attributed to liver disease, myocardial infarction, muscular dystrophy or augmentation of anaerobic respiration (32), the ALP is an enzyme which is presented in rat with high proportion in intestine, a decreased serum ALP may be due to hypothyroidism, hypoparathyroidism, malnutrition and/or gastrointestinal disease (33). Similarly to these results, some reports found an alteration in blood serum enzymes activities following oral administration of ionic silver or silver nanoparticles (34). Hypercholesterolemia might be resulted from hypothyroidism, hyperlipoproteinemia and/or liver toxicity (35). According to the later parameter, the rise in lipase activity may be due to adyslipidemia. However, the decrease in serum total protein of Ag-treated rats might be due to changes in proteins synthesis and/or metabolism (36) and for the hypocalcaemia may be due to mal absorption or hypoparathyroidism (37). Corresponding to these findings, we could not discuss about hepatic damage without alterations in transaminase activity and direct bilirubin level, some studies have proven the toxic effects of silver on different organs including intestine, bone, thyroid and heart (38,39). Meanwhile, animals treated with vitamin E and/or selenium presented lower blood LDH and cholesterol and higher ALP and total protein values than those of metal exposed animals, Calcium level and lipase activity are too normalized in these treatments, a findings which have also been observed by other reports (40). In the other side, data showed an increase in brain TBARS levels as biomarkers of lipid peroxidation, vitamin E content and a decrease in SOD activity. These results are correlated with our previous study which indicated a decline in SOD activity and an augmentation in vitamin E concentration in liver of AgNO3 intoxicated animals (41). Powers et al. (1) were also found that the longer exposure of neuronotypic PC12 cells to AgNO3 led to oxidative stress, loss of viability and reduced numbers of cells. Similarly, Hadrup et al. (42) was suggested that Ag+ affected brain noradrenalin and dopamine in vivo and caused apoptotic death mechanisms in vitro. Moreover, under normal conditions, the brain is susceptible to oxidative damage due to its high oxygen consumption rate (in humans, the brain uses ~20% of the circulating glucose, 20% of blood’s oxygen and occupies 2% of the body weight), to high levels of polyunsaturated fatty acids (PUFA), and to a progressive accumulation of iron levels with aging (13,43,44) and paradoxically its deficient oxidant defense mechanisms and its diminished cellular turn over (45). The antioxidant system (AOS) spans over a wide range of mechanisms such as enzymatic antioxidant system including SOD, GSH-Px and CAT to small molecular weight compounds such as vitamin E and GSH though in relatively low concentrations (44). The function of antioxidant systems is to modify the highly reactive oxygen species (ROS) to form less reactive intermediate which no longer pose a threat to the cell. These species can attack and damage all types of macromolecules (46); they are the main culprit for development of oxidative stress contributing to the development of cytotoxicity (47,48). However, there must be a balance between oxidation and antioxidant’s level in the system for healthy biological integrity to be maintained. Oxidants such as superoxide anions (O2∘−), hydroxyl radical (OH∘), hydrogen peroxide (H2O2), and singlet oxygen (1O2) may contribute to neuronal loss in cerebral ischemia and hemorrhage, and may be involved in the degeneration of neurons during normal aging and also in various diseases (49–51). In addition, the decrease in brain SOD activity could be explained by the investigation of Park et al. (51) who found that the major form of ROS generated by silver ions was the O2∘−, H2O2 was not induced. Cortese - Krott et al. (52) showed that AgNO3 increased the production of O2∘− within mitochondria. Thus, it might be due to the reaction with SH groups of enzymes belonging to the respiratory chain (51–53). Whereas, the augmentation of brain vitamin E levels is corresponding to some transition metal as cadmium (Cd) which increases the concentration of vitamin E in rat tissue (54). Van Der Zande et al. (55) have indicated that the silver content in blood was 2~3 times lower than the silver content in brain; the authors have noticed the persistent of metal in this organ after two months of treatment. Evidence of silver transfer across the blood brain-barrier is alarming, but more researches are necessary.
Treatment with vitamin E and/or Se has appeared to reduce harmful effects in nervous tissue as demonstrated by our previous study (41) and by other investigators (13–15, 44). Therefore, vitamin E has appeared to diminish free radicals in brain tissue. In contrast, it has ameliorated GSH-Px activity, the supplementation have also increased the cerebral content of this vitamin. Vitamin E may attenuate the toxic effects of ROS (13). It may also be a regulatory agent in intermediary metabolism, act synergistically as an antioxidant with GSH and ascorbate, and promote humoral antibody formation, because, it is not synthesized in the body and its concentration is dependent on the uptake (43). Its deficiency results mainly in neurological symptoms including impaired balance and coordination, injury to the sensory nerves, muscle weakness and damage to the retina of eye (13–43). In fact, selenium has diminished lipid peroxidation levels in brain, decreased CAT activity and increased SOD and GSH-Px activities, this micronutrient accessed the central nervous system via cerebral capillary seleno-protein receptors and could be incorporated in newly synthesized selenoproteins. Se supplementation increased the activities of selenoproteins, by the high incorporation of selenocysteine in selenoproteins which may decrease free radical-mediated LPO and regenerate glutathione. This trace element reduces oxidative stress in cerebral ischemia, Alzheimer’s and Parkinson’s diseases (13–49). Under microscopic examination, distortions in cellular architecture were observed in brain tissue of Ag-treated rats. Neurodegenerative changes can be due to ROS (56). Vitamin E and selenium are able to restore or repair brain damages against the harmful effects of Ag, but this restoration is clearer in vitamin E treated rats which is also supported by Yin et al. (57).
In conclusion, this study revealed that the contamination by silver is responsible for certain cellular disorders disturbing some blood and brain parameters, like xenobiotics, toxicity is depending on the dose and time of exposure. For that, an equilibrate diet rich in antioxidants is always requested.
Footnotes
CONFLICT OF INTEREST
Authors declare that no conflicts of interest.
REFERENCES
- 1.Powers CM, Wrench N, Ryde IT, Smith AM, Seidler FJ, Slotkin TA. Silver impairs neurodevelopment: studies in PC12 cells. Environ Health Perspect. 2010;118:73–79. doi: 10.1289/ehp.0901149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y, Choi J, Kim P, Choi K, Kim S, Shon W, Park K. A transfer of silver nanoparticles from pregnant rat to offspring. Toxicol Res. 2012;28:139–141. doi: 10.5487/TR.2012.28.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roh JY, Eom HJ, Choi J. Involvement of caenohabditis elegans MAPK signaling pathways in oxidative stress response induced by silver nanoparticles exposure. Toxicol Res. 2012;28:19–24. doi: 10.5487/TR.2012.28.1.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon JT, Minai-Tehrani A, Hwang SK, Kim JE, Shin JY, Yu KN, Chang SH, Kim DS, Kwon YT, Choi IJ, Cheong YH, Kim JS, Cho MH. Acute pulmonary toxicity and body distribution of inhaled metallic silver nanoparticles. Toxicol Res. 2012;28:25–31. doi: 10.5487/TR.2012.28.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Guidelines for drinking-water quality. 2nd edition. Vol. 2. World Health Organization; Geneva: 1996. Silver in drinking-water; pp. 338–343. [Google Scholar]
- 6.Holler JS, Fawler BA, Nordberg GR. Handbook on the toxicology of metals: Silver. 4th edition. Elsevier; 2015. pp. 1209–1216. [DOI] [Google Scholar]
- 7.Ohbo Y, Fukuzako H, Takeuchi K, Takigawa M. Argyria and convulsive seizures caused by ingestion of silver in a patient with schizophrenia. Psychiatry Clin Neurosci. 1996;50:89–90. doi: 10.1111/j.1440-1819.1996.tb01669.x. [DOI] [PubMed] [Google Scholar]
- 8.Landas S, Fischer J, Wilkin LD, Mitchell LD, Johnson AK, Turner JW, Theriac M, Moore KC. Demonstration of regional blood-brain barrier permeability in human brain. Neurosci Lett. 1985;57:251–256. doi: 10.1016/0304-3940(85)90500-2. [DOI] [PubMed] [Google Scholar]
- 9.Rungby J, Danscher G. Hypoactivity in silver exposed mice. Acta Pharmacol Toxicol (Copenh) 1984;55:398–401. doi: 10.1111/j.1600-0773.1984.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 10.Rungby J, Slomianka L, Danscher G, Andersen AH, West MJ. A quantitative evaluation of the neurotoxic effect of silver on the volumes of the components of the developing rat hippocampus. Toxicology. 1987;43:261–268. doi: 10.1016/0300-483X(87)90085-0. [DOI] [PubMed] [Google Scholar]
- 11.Serafín Muñoz AH, Wrobel K, Gutierrez Corona JF, Wrobel K. The protective effect of selenium inorganic forms against cadmium and silver toxicity in mycelia of Pleurotus ostreatus. Mycol Res. 2007;111:626–632. doi: 10.1016/j.mycres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Letavayová L, Vlasáková D, Spallholz JE, Brozmanová J, Chovanec M. Toxicity and mutagenicity of selenium compounds in Saccharomyces cerevisiae. Mutat Res. 2008;638:1–10. doi: 10.1016/j.mrfmmm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Ben Amara I, Soudani N, Hakim A, Troudi A, Zeghal KM, Boudawara T, Zeghal N. Selenium and vitamin E, natural antioxidants, protect rat cerebral cortex against dimethoate-induced neurotoxicity. Pestic Biochem Physiol. 2011;101:165–174. doi: 10.1016/j.pestbp.2011.08.012. [DOI] [Google Scholar]
- 14.Peterson RP, Jensen LS. Poult Sci. 1972;51:1849. [Google Scholar]
- 15.Kabay SC, Ozden H, Guven G, Ustuner MC, Degirmenci I, Olgun EG, Unal N. Protective effects of vitamin E on central nervous system in streptozotocin-induced diabetic rats. Clin Invest Med. 2009;32:E314–E321. doi: 10.25011/cim.v32i5.6918. [DOI] [PubMed] [Google Scholar]
- 16.Chow CK. Effects of dietary vitamin E and selenium on rats: pyruvate kinase, glutathione peroxidase and oxidative damage. Nutr Res. 1990;10:183–194. doi: 10.1016/S0271-5317(05)80606-2. [DOI] [Google Scholar]
- 17.Chow CK. Interrelation ship of antioxidant defense systems. In: Chow CK, editor. Cellular Antioxidant Defense Mechanisms. Vol. 2. CRC Press Inc; Boca Raton: 1988. pp. 217–237. [Google Scholar]
- 18.US Environmental Protection Agency. Ambient water quality criteria for silver. Washington: 1980. (EPA 440/5-80-071) [Google Scholar]
- 19.Kim KR, Kim JK, Rhee SJ. Effects of vitamin E on arachidonic acid cascade in platelets and aorta of acute cadmium-poisoned rats. Nutr Res. 2001;21:657–665. doi: 10.1016/S0271-5317(01)00265-2. [DOI] [Google Scholar]
- 20.Wu Q, Huang K, Xu H. Effects of long-term selenium deficiency on glutathione peroxidase and thioredoxin reductase activities and expressions in rat aorta. J Inorg Biochem. 2003;94:301–306. doi: 10.1016/S0162-0134(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 21.Esterbauer H, Cheeseman K. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Meth Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-H. [DOI] [PubMed] [Google Scholar]
- 22.Misra HP, Fridovich I. Superoxide dismutase: “positive” spectrophotometric assays. Anal Biochem. 1977;79:553–560. doi: 10.1016/0003-2697(77)90429-8. [DOI] [PubMed] [Google Scholar]
- 23.Flohe L, Gunzler WA. Analysis of glutathione peroxidase. Meth Enzymol. 1984;105:114–121. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 24.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 25.Goldberg DM, Spooner RJ. In: Methods of enzymatic analysis. Bergmeyen HV, editor. VerlogChemie; Deerfield Beach: 1983. pp. 258–265. [Google Scholar]
- 26.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 27.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Desai ID. Vitamin E analysis method for animal tissue. Methods Enzymol. 1984;105:138–147. doi: 10.1016/S0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantities of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Hould R. Techniques d’histopathologie et de cytopathologie. Ed Maloine. 1984;19–21:225–227. [Google Scholar]
- 31.Kim YS, Song MY, Park JD, Song KS, Ryu HR, Chung YH, Chang HK, Lee JH, Oh KH, Kelman BJ, Hwang IK, Yu IJ. Subchronic oral toxicity of silver nanoparticles. Part Fibre Toxicol. 2010;7:20. doi: 10.1186/1743-8977-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burtis CA, Ashwood ER. Tietz textbook of clinical chemistry. 3rd edition. Saunders; Philadelphia: 1999. [Google Scholar]
- 33.Evans GO, O’Brien PJ, Watterson CL. Animal clinical chemistry. Taylor & Francis Group; Boca Raton: 2009. [DOI] [Google Scholar]
- 34.Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, Kwon IH, Jeong J, Han BS, Yu IJ. Twenty-eight-day oral toxicity, genotoxicity, and gender related tissue distribution of silver nanoparticles in Sprague Dawley rats. Inhal Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- 35.Sung JH, Ji JH, Park JD, Yoon JU, Kim DS, Jeon KS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Chang HK, Lee JH, Cho MH, Kelman BJ, Yu IJ. Subchronic inhalation toxicity of silver nanoparticles. Toxicol Sci. 2009;108:452–61. doi: 10.1093/toxsci/kfn246. [DOI] [PubMed] [Google Scholar]
- 36.Das KK, Dasgupta S. Effect of nickel on testicular nucleic acid concentrations of rats on protein restriction. Biol Trace Elem Res. 2000;73:175–180. doi: 10.1385/BTER:73:2:175. [DOI] [PubMed] [Google Scholar]
- 37.Clarck WL, Balinski EL, Maie SS, Zak B. Spectrometric study of a direct determination of Serum Calcium. Microchem J. 1975;20:22–32. doi: 10.1016/0026-265X(75)90108-3. [DOI] [Google Scholar]
- 38.Olcott CT. Experimental argyrosis; hypertrophy of the left ventricle of the heart in rats ingesting silver salts. AMA Arch Pathol. 1950;49:138–149. [PubMed] [Google Scholar]
- 39.Fung MC, Bowen DL. Silver products for medical indications: risk-benefit assessment. J Toxicol Clin Toxicol. 1996;34:119–126. doi: 10.3109/15563659609020246. [DOI] [PubMed] [Google Scholar]
- 40.Chow CK. Vitamin E and blood. World Rev Nutr Diet. 1985;45:133–166. doi: 10.1159/000410266. [DOI] [PubMed] [Google Scholar]
- 41.Gueroui M, Kechrid Z. Effect of the joint supplementation of vitamin E and selenium on chronic silver induced liver injury in male (wistar) albino rats. Int J Pharm Sci Rev Res. 2015;34:176–182. [Google Scholar]
- 42.Hadrup N, Loeschner K, Mortensen A, Sharma AK, Qvortrup K, Larsen EH, Lam HR. The similar neurotoxic effects of nanoparticulate and ionic silver in vivo and in vitro. Neurotoxicology. 2012;33:416–423. doi: 10.1016/j.neuro.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJ. Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem. 1994;62:45–53. doi: 10.1046/j.1471-4159.1994.62010045.x. [DOI] [PubMed] [Google Scholar]
- 44.Devi SA, Kiran TR. Regional responses in antioxidant system to exercise training and dietary Vitamin E in aging rat brain. Neurobiol Aging. 2004;25:501–508. doi: 10.1016/S0197-4580(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 45.Ilhan A, Gurel A, Armutcu F, Kamisli S, Iraz M, Akyol O, Ozen S. Ginkgo biloba prevents mobile phone-induced oxidative stress in rat brain. Clin Chim Acta. 2004;340:153–162. doi: 10.1016/j.cccn.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Lee J. Use of antioxidants to prevent cyclosporine a toxicity. Toxicol Res. 2010;26:163–170. doi: 10.5487/TR.2010.26.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nirwane A, Sridhar V, Majumdar A. Neurobehavioural changes and brain oxidative stress induced by acute exposure to GSM900 mobile phone radiations in Zebrafish (Danio rerio) Toxicol Res. 2016;32:123–132. doi: 10.5487/TR.2016.32.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JG, Noh WJ, Kim H, Lee MY. Generation of reactive oxygen species contributes to the development of carbon black cytotoxicity to vascular cells. Toxicol Res. 2011;27:161–166. doi: 10.5487/TR.2011.27.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bourre JM, Dumonta O, Cleament M, Dinha L, Droy-Lefaix MT, Christen Y. Vitamin E deficiency has different effects on brain and liver phospholipid hydroperoxide glutathione peroxidase activities in the rat. Neurosci Lett. 2000;286:87–90. doi: 10.1016/S0304-3940(00)01095-8. [DOI] [PubMed] [Google Scholar]
- 50.Otitoju O, Onwurah INE, Otitoju GTO, Ugwu CE. Oxidative stress and superoxide dismutase activity in brain of rats fed with diet containing permethrin. Biokemistri. 2008;20:93–98. [Google Scholar]
- 51.Park HJ, Kim JY, Ki J, Lee JH, Hahn JS, Gu MB, Yoon J. Silver-ion-mediated reactive species generation affecting bactericidal activity. Water Res. 2009;43:1027–1032. doi: 10.1016/j.watres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Cortese-Krott MM, Münchow M, Pirev E, Hebner F, Bozkurt A, Uciechowski P, Pallua N, Kröncke KD, Suschek CV. Silver ions induce oxidative stress and intracellular zinc release in human skin fibroblasts. Free Radic Biol Med. 2009;47:1570–1577. doi: 10.1016/j.freeradbiomed.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 53.Almofti MR, Ichikawa T, Yamashita K, Terada H, Shinohara Y. Silver ion induces a cyclosporine a-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome C. J Biochem. 2003;134:43–49. doi: 10.1093/jb/mvg111. [DOI] [PubMed] [Google Scholar]
- 54.Ognjanovic BI, Pavlovic SZ, Maletic SD, Zikic RV, Stajn AS, Radojicic RM, Saicic ZS, Petrovic VM. Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol Res. 2003;52:563–570. [PubMed] [Google Scholar]
- 55.van der Zande M, Vanderbriel RJ, Van Doren E, Kramer E, Herrera Rivera Z, Serrano-Rojero CS, Gremmer ER, Mast J, Peters RJ, Hollman PC, Hendriksen PJ, Marvin HJ, Peijnenburg AA, Bouwmeester H. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano. 2012;6:7427–7442. doi: 10.1021/nn302649p. [DOI] [PubMed] [Google Scholar]
- 56.Liu F, Mahmood M, Xu Y, Watanabe F, Biris AS, Hansen DK, Inselman A, Casciano D, Patterson TA, Paule MG, Slikker W, Jr, Wang C. Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front Neurosci. 2015;9:115. doi: 10.3389/fnins.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin N, Yao X, Zhou Q, Faiola F, Jiang G. Vitamin E attenuates silver nanoparticle-induced effects on body weight and neurotoxicity in rats. Biochem Biophys Res Commun. 2015;458:405–410. doi: 10.1016/j.bbrc.2015.01.130. [DOI] [PubMed] [Google Scholar]