Abstract
Administration of oxytocin has been proposed as a treatment for the core symptoms of autism spectrum disorder (ASD), including social-communicative deficit. Previous clinical trials have investigated the efficacy and safety of oxytocin intranasal single-dose and long-term administration for individuals with ASD. All studies suggest that single-dose and long-term administration are well tolerated, and no severe adverse events have been reported. However, the efficacy of long-term oxytocin administration is controversial. Some studies have reported significant improvement of the core symptoms of ASD by long-term oxytocin administration, while other studies showed no such improvement. To elucidate the factors influencing the efficacy of oxytocin administration, it is necessary to examine the effects of administration schedules (e.g., dosage amount, frequency per day) and participant characteristics (e.g., age, sex, intellectual ability). In addition to doubts about the efficacy of particular methods of administration, questions remain about the mechanism of action of intranasal oxytocin on the central nervous system. Examination of changes in the neural underpinnings of social behavior and simultaneous oxytocin levels in blood or cerebrospinal fluid could prove important in elucidating the pharmacokinetics of intranasal oxytocin administration, which could be essential for establishing optimal oxytocin treatments for individuals with ASD.
Keywords: Autism spectrum disorders, long-term administration, open-label trial, oxytocin, randomized controlled trials, single-dose, treatment
1. Introduction
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), autism spectrum disorder (ASD) are neurodevelopmental conditions characterized by “core symptoms” such as (1) persistent deficits in social communication and social interaction, and (2) restricted, repetitive patterns of behavior, interests, or activities [1]. Difficulty of social interaction includes both verbal and non-verbal communication. For instance, individuals with ASD tend to be incapable of sustaining a back-and-forth conversation, and frequently show abnormal eye contact and body language. Furthermore, they poorly integrate verbal and nonverbal communication. Based on these deficits, individuals with ASD have difficulties developing, maintaining, and understanding relationships, and exhibit an absence of interest in peers [1]. In addition to these social-communicative deficits, they frequently show stereotypic or repetitive motor movements, use of objects, or speech; they also exhibit hyper- or hyporeactivity to sensory input or otherwise show unusual interest in sensory aspects of the environment [1].
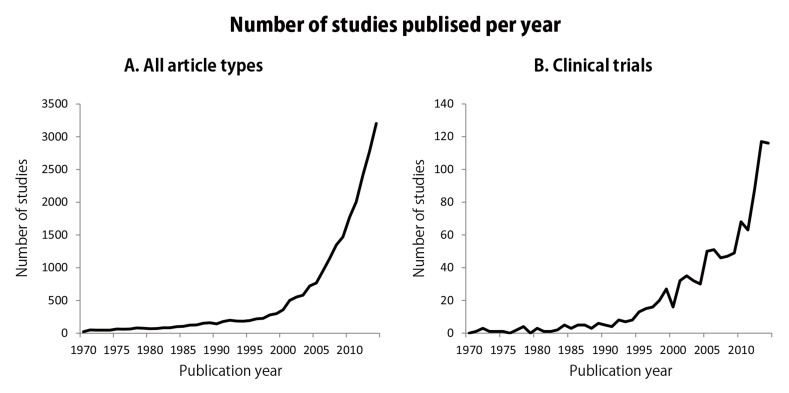
It has been suggested that the frequency of the ASD has been increasing at a high rate [2]. The prevalence ratio of ASD was thought to be 1 in 5,000 in 1975; however, it had risen to 1 in 110 in 2012 (around 50-fold in 40 years) [2]. Based on such a steep rise, the importance of elucidating the etiology, pathophysiology, and treatment of ASD has also increased. Fig. (1) shows the number of papers dealing with this topic published per year; it is obvious that studies of ASD have rapidly increased in recent years (Fig. 1A), as have clinical trials (Fig. 1B).
Fig. (1).
Illustration representing the number of studies published on autism spectrum disorder. The number of papers published per year according to a PubMed search for articles with the terms “autism”[All Fields] OR “autism spectrum disorder”[All Fields] OR “pervasive developmental disorder”[All Fields] OR “Asperger syndrome”[All Fields]; in all article types (A) and in clinical trials (B) between 1970 and 2014. The search was performed on October 6, 2015.
Despite a large number of clinical trials, intervention for ASD mainly relies on behavioral approaches such as applied behavioral analysis or treatment and education of autistic and related communication-handicapped children (TEACCH) [3]. In terms of medical treatment, several drugs have shown significant efficacy of some aspect for ASD patients [4]. For instance, risperidone can improve the irritability that can hamper social interaction. Although other medicines such as fenfluramine, naltrexone, and secretin have also been proposed, these medicines do not appear to be efficacious for core symptoms [4]. Therefore, there is still no medical treatment aimed at directly improving social-communicative deficits in individuals with ASD [3, 4].
However, recent animal and human studies found that oxytocin, a neuropeptide, plays an important role in modulating social-communicative behaviors [5, 6]. Furthermore, some studies have reported altered oxytocin levels in individuals with ASD [7, 8]. These studies indicate that disturbances in oxytocin levels lead to social and communicative dysfunction in individuals with ASD, and therefore suggest that exogenous oxytocin administration may be effective in reversing these symptoms, potentially constituting a medical treatment for individuals with ASD. To date, several studies have attempted to determine whether oxytocin administration in individuals with ASD improves core symptoms, and comorbid anxiety or depression. Recently, two systematic reviews concluded that the efficacy of long-term administration of oxytocin for improving core symptoms is controversial [9, 10], although validation is still in progress. In this review, we initially summarize the typical role of oxytocin in developing individuals, followed by the alteration of oxytocin levels in individuals with ASD. We then introduce and discuss studies examining the efficacy and safety of oxytocin administration for individuals with ASD, dividing core symptoms by their function (i.e., social-communicative function, restricted and repetitive patterns, and other comorbidities such as anxiety). We also advise caution in the use of oxytocin administration and describe the issues that remain to be addressed.
2. Role of oxytocin on social behaviors for typically developed individuals
Oxytocin is a neuropeptide synthesized in magnocellular neurons in the paraventricular and supraoptic nuclei of the hypothalamus, and is processed during its transport along the axonal projections to the posterior lobe of the pituitary. It has been well known that oxytocin has peripheral actions in parturition and childcare such as prompting galactopoiesis and contraction of the uterus. However, recent studies suggest that oxytocin also has central actions associated with social behavior [5, 6, 11-16]. Lee et al. proposed that oxytocin plays an important role in social behaviors over the course of one’s life. For instance, oxytocin promotes attachment between children and their mother by facilitating maternal behaviors during childhood. During adolescence, playing with friends might be mediated by oxytocin, and sexual behavior during adulthood is also associated with this hormone [6]. Heinrichs et al. explained the mechanisms of oxytocin function in social behavior, and showed that positive social interactions such as physical contact are associated with oxytocin release; reciprocally, oxytocin also promotes social approach behavior. Several studies have shown the association of oxytocin levels in the body and social interaction behaviors [17, 18]. For instance, Kim et al. examined the association between changes of plasma oxytocin levels of mothers during their interaction with their babies. They measured the maternal oxytocin response by calculating difference of oxytocin level in plasma between baseline period and their interaction time with their babies. They showed that maternal oxytocin response was also positively associated with the duration of gaze toward their babies. Furthermore, mothers with low or average oxytocin responses showed decreased gazing upon their babies during periods of infant distress; however, such decreased were not observed in mothers with high oxytocin response [17]. Collectively, these studies suggest that oxytocin secretion is modulated by social situations such as interpersonal interaction. In turn, oxytocin secretion prompts socially integrative behaviors.
In addition to these endogenous oxytocin-mediated actions during social behaviors, recent studies have shown that exogenous oxytocin administration also changes social behaviors for typically developed (TD) individuals. For instance, intranasal single-dose oxytocin administration studies in individuals with TD have shown that oxytocin changes social behaviors such as gazing on particular areas of faces [19], inferring mental states of other persons [12], and trust of other persons [13]. Guastella et al. examined changes of gazing behaviors on three facial parts, the eye region, nose and mouth region, and forehead and cheek region, by oxytocin administration. Fifty-two TD male adults participated in the study, and were administered 24 international units (IU) oxytocin or placebo. Participants who received oxytocin gazed longer and more frequently at the eye region than participants who received a placebo. Gazing at the other facial regions was not influenced by oxytocin [19]. As eye-contact is critical for interpersonal communication, the results suggest that oxytocin might facilitate interpersonal communication by improving eye contact. Domes et al. examined how oxytocin administration affects the ability to infer the mental state of others using the Reading the Mind in the Eyes test (RMET). The RMET was developed by Baron-Cohen and colleagues, where participants are shown photographs of eyes and choose appropriate term describing mental states [20]. Thirty TD male adults participated in the study and received either 24 IU oxytocin or placebo. Compared to placebo, oxytocin-treated subjects showed improved RMET scores. Furthermore, the improvement was more pronounced in the more challenging selections, indicating that oxytocin improves inference skills based on gazing at eyes for TD individuals [12]. Kosfeld et al. examined behavioral changes in a trust game that involved actual monetary stakes after single-dose oxytocin administration of 24 IU or placebo in TD male adults. In this game, two participants (investor and trustee) receive equivalent amounts of monetary units (MU). First, the investor sends an arbitrary MU amount to the trustee, which the experimenter triples. Then, the trustee chooses an amount to transfer back to the investor from his/her own endowment plus the tripled value of the investor’s amount. The experimenter does not triple the back transfer to the investor. More trusting investors will presumably send higher amounts of money to the trustee. In this experiment, the investor’s average transfer was significantly higher in the oxytocin-administered group than in the placebo group [13]. Because trust is one of the important components of prosocial behaviors, these results suggest that oxytocin influences prosocial behaviors for TD individuals. Hence, exogenous oxytocin administration may change a wide-range of social behaviors, from simple traits such as gaze to complex behaviors such as decision-making based on trust.
Oxytocin administration changes not only social behaviors but also neural responses [11, 21, 22]. For instance, Kirsch et al. examined changes in fear-related neural responses due to oxytocin administration using functional magnetic resonance imaging (fMRI). Thirteen TD adults participated in the study and received 27 IU oxytocin or placebo before scanning. The participants viewed fear-inducing visual stimuli (i.e. fearful faces and scenes) inside the MR scanner. Compared to the placebo group, oxytocin-administered subjects showed reduced activation in the amygdala when viewing fear-inducing visual stimuli [22]. Furthermore, Domes et al. found that oxytocin suppressed activation in the amygdala when viewing faces irrespective of valence. They conducted an fMRI study where 13 TD male adult participants observed happy, fearful, and angry faces. The participants received 24 IU oxytocin or placebo before MR scanning. Activation in the right amygdala upon exposure to all facial expressions was significantly reduced in the oxytocin-administered group compared to the placebo group [11]. Baumgartner et al. examined neural underpinnings of improvement of trust by oxytocin administration for TD individuals. In their study, 49 male TD participants played a trust game with fMRI evaluation while they were administered 24 IU oxytocin or placebo. They found oxytocin-induced effects in the amygdala, the midbrain regions, and the dorsal striatum [21].
Collectively, recent studies on TD individuals suggest that endogenous oxytocin plays an important role in facilitating socially appropriate behaviors. Furthermore, exogenous oxytocin administration (i.e. intranasal oxytocin spray) also improved social behaviors through modulating neural responses in TD individuals, indicating that oxytocin administration may be a treatment for individuals with ASD.
3. Altered oxytocin system in individuals with ASD
Because a social-communicative deficit is one of the core symptoms of ASD [1], alterations in the oxytocin system may be one of the pathophysiologies of ASD; i.e., alteration of the oxytocin system might lead to difficulty in social communication for individuals with ASD. Based on this hypothesis, several studies examined the oxytocin system in individuals with ASD, including both production of, and sensitivity to, oxytocin.
Modahl et al. initially reported abnormal plasma oxytocin levels in children with ASD [8]. The authors measured plasma oxytocin levels in 29 boys with ASD and 30 TD boys aged 6–11 years. The plasma oxytocin level was significantly lower in ASD boys than TD boys. Furthermore, the oxytocin level was differently associated with social and developmental measures. Specifically, greater interaction or daily living skill was associated with elevated oxytocin level in TD boys, while lower functionality was associated with elevated oxytocin level in ASD boys. Green et al. confirmed lower plasma oxytocin levels in children with ASD compared to TD children. Furthermore, they also found elevated extended forms of oxytocin in children with ASD, indicating that such children have a dysfunctional endocrine oxytocin system [23]. Following these studies, several others confirmed lower plasma oxytocin levels in individuals with ASD than in TD individuals [24-26]. In contrast, other studies found equivalent [27-29] or elevated plasma oxytocin levels in males with ASD, but not in females with ASD [30]. These inconsistent findings suggest that abnormal oxytocin production in individuals with ASD cannot simplify be due to reduced secretion. Furthermore, the inconsistent abnormality of oxytocin levels raises the possibility that modulation of oxytocin secretion in response to social situations might differ between individuals with ASD and TD individuals. To understand the oxytocin dynamics in natural situations for individuals with ASD, Feldman et al. measured changes in saliva oxytocin levels in children with ASD while interacting with their mothers or fathers. Forty preschool children with ASD and an equivalent number of age-matched TD preschool children participated in the study. The saliva oxytocin level of these participants was measured during pre-interaction, interaction, and post-interaction with the mother or father. They found that saliva oxytocin levels of children with ASD were lower pre- and post- interaction. During interaction, however, the oxytocin levels of these children was elevated, and attained similar levels as those of TD children [31]. The results suggest abnormal modulation of oxytocin levels occurs in children with ASD.
Although these findings suggest that an abnormality in oxytocin secretion or metabolism might exist in individuals with ASD, there are several fundamental problems to resolve. For instance, recent studies have questioned the methodology of measuring oxytocin levels [32, 33]. Specifically, they claimed that oxytocin levels are overestimated when measured using enzyme immunoassay techniques without prior extraction [32, 33]. As some of these studies utilized these immunoassay techniques, the findings should be re-examined using more accurate procedures such as radioimmunoassays or oxytocin extraction prior to employing immunoassays. Additionally, it is questionable if peripheral oxytocin levels (i.e. plasma, saliva, and urine) accurately reflect central oxytocin levels [32]. In fact, several studies have shown no correlation between oxytocin levels in the cerebrospinal fluid and that in plasma [34, 35]. Similarly, McCullough et al. questioned if saliva oxytocin levels reflect plasma or cerebrospinal fluid levels [32]. Thus, measuring oxytocin levels in the cerebrospinal fluid should provide stronger evidence of the relationship between altered oxytocin secretion and social and communicative dysfunction in individuals with ASD. In order to better understand the oxytocin system in individuals with ASD, further studies that overcome these issues are necessary.
In addition to oxytocin production, several genetic studies have suggested that ASD is associated with alteration of the genetic background of the oxytocin receptor [7]. Oxytocin receptor genes are known to have a number of single nucleotide polymorphisms (SNPs). Based on studies examining the SNPs of the oxytocin receptor for individuals with ASD, LaParo et al. conducted a meta-analysis to examine whether specific SNPs of the oxytocin receptor are associated with ASD. Of 16 candidate SNPs of the oxytocin receptor, they found four (rs7632287, rs237887, rs2268491, and rs2254298) that were significantly associated with ASD [7]. These four SNPs are linked to social behaviors in both individuals with and without ASD. For instance, rs2268491 and rs2254298 are considered to be associated with empathy in TD individuals [36]. Wu et al. tested if SNPs of the oxytocin receptor are associated with cognitive and emotional empathy measured by an interpersonal reactivity index for TD individuals, and found that rs2268491 and rs2254298 were associated with cognitive empathy [36]. Thus, alteration of these oxytocin receptor SNPs are potentially associated with social dysfunction in individuals with ASD.
Although there are still several issues to resolve, these studies have collectively suggested that individuals with ASD have abnormalities in the oxytocin system involving both secretion and responsiveness of oxytocin. The disruption of the oxytocin system may be one of causes of the social-communicative dysfunction of individuals with ASD, as shown in an animal study [37]. Takayanagi et al. examined the role of the oxytocin system on social behavior using oxytocin knockout mice as well as oxytocin receptor knockout mice. They showed that deficits of social discrimination, aggressive behavior, and maternal behavior were altered by knocking out oxytocin and/or the oxytocin receptor. Because an altered oxytocin system may constitute one of the pathophysiologies of ASD, recent studies have explored whether administration of oxytocin improves abnormal social behaviors of individuals with ASD. In the next section, we introduce studies examining the efficacy and safety of oxytocin administration for individuals with ASD, including both single-dose and long-term administration.
4. Oxytocin administration studies in individuals with ASD
4.1. Single-Dose Studies
Several single-dose studies have reported positive effects for oxytocin administration intranasally [38-46] and by drip infusion [47, 48] in individuals with ASD (Table 1). As shown Tables 1 and 2, the acute effect of oxytocin administration was not only examined in behavioral studies [38, 41, 45, 47, 48] but also in neuroimaging studies [39, 40, 42-44, 46].
Table 1. Behavioral studies of single-dose oxytocin administration for individuals with ASD.
| Study | ASD Participants | Dosage | Measures | |||||
|---|---|---|---|---|---|---|---|---|
| N | Age | Gender | IQ | Amount | Procedure | |||
| Social Communicative Function | ||||||||
| Hollander et al., 2007 | [47] | 15 | 32.9 (19-56) | 14M 1F | 112 | 10U/ml (10ml/h ~ 700ml/h) | Drip infusion | Emotion comprehension of affective speech |
| Guastella et al., 2010 | [45] | 16 | 14.9 (12-19) | 16M | 18 or 24 IU | Intranasal spray | RMET | |
| Andari et al., 2010 | [38] | 13 | 26 (17-39) | 11M 2F | 92 | 24IU | Intranasal spray | Social ball tossing game Gaze during obsrerving face |
| Auyeung et al., 2015 | [41] | 32 | 36.4 (18-56) | 32M | 116 | 24IU | Intranasal spray | Gaze to human face parts during talk with experimenter |
| Restricted and Repetitive Patterns | ||||||||
| Hollander et al., 2003 | [48] | 15 | 32.9 (19-55) | 14M 1F | 90 | 10U/ml (10ml/h ~ 700ml/h) | Drip infusion | Repetitive behavior scale (need to know, repeating, ordering, need to tell/ask, self-injury, touching) |
| Comorbidity | ||||||||
| No study | ||||||||
Amount, dosage amount per single administration; N, number of participants; ASD, autism spectrum disorder; M, males; F, Females; RMET, Reading the Mind in the Eyes Test.
Table 2. Neuroimaging studies of single-dose oxytocin administration for individuals with ASD.
| Study | ASD Participants | Dosage | Task | |||||
|---|---|---|---|---|---|---|---|---|
| N | Age | Gender | IQ | Amount | Procedure | |||
| fMRI | ||||||||
| Social Communicative Function | ||||||||
| Domes et al., 2013a | [42] | 14 | 24 | 14M | 112 | 24IU | Intranasal spray | Face- and house- matching task |
| Domes et al., 2013b | [43] | 14 | 24 | 14M | 112 | 24IU | Intranasal spray | Facial emotion recognition task of eye or mouth |
| Gordon et al., 2013 | [44] | 21 | 13.2 (8-16) |
18boys 3girls | 18 or 24 IU | Intranasal spray | Emotion judgement (RMET) Category of automobile judgement |
|
| Watanabe et al., 2014 | [46] | 33 | 28.5 | 33M | >80 | 24IU | Intranasal spray | Judgement of friend or foe based on speeach and facial expression |
| Aoki et al., 2014a | [40] | 20 | 30.8 (22-41) | 20M | 109 | 24IU | Intranasal spray | Emotion and belief understanding by catoorn |
| Restricted and Repetitive Patterns | ||||||||
| No study | ||||||||
| Comorbidity | ||||||||
| No study | ||||||||
| MRS | ||||||||
| Social Communicative Function | ||||||||
| Aoki et al., 2014b | [39] | 40 | 30 | 40M | >80 | 24IU | Intranasal spray | |
| Restricted and Repetitive Patterns | ||||||||
| No study | ||||||||
| Comorbidity | ||||||||
| No study | ||||||||
Amount, dosage amount per single administration; gender, participants’ gender; age, range of participant age; N, number of participants; M, males; F, Females; RMET, Reading the Mind in the Eyes Test.
4.1.1. Behavioral Studies
To date, one behavioral study examined the efficacy of oxytocin in restricted and repetitive behaviors in individuals with ASD [48], while others examined the efficacy of oxytocin administration for social-communicative deficit [38, 41, 45, 47] (Table 1).
Hollander et al. examined if oxytocin administration reduces restricted and repetitive behaviors for individuals with ASD. Fourteen men and one woman with ASD received continuous infusion of oxytocin or placebo over four hours. The amount of oxytocin infusion was gradually increased. In this experiment, a repetitive behavior scale involving six components (need to know, repeating, ordering, need to tell/ask, self-injury, and touching) was measured five times every hour during oxytocin or placebo infusion. In contrast to placebo subjects, oxytocin-administered subjects had a significantly reduced total score on the repetitive behavior scale [48]. Thus, oxytocin administration was effective in reducing repetitive behaviors in individuals with ASD. The efficacy of oxytocin administration is not only observed in those with restricted and repetitive behaviors. Hollander et al. also examined oxytocin-induced changes in understanding others’ emotions through speech based on prosody. Fourteen men and one woman with ASD received continuous infusion of oxytocin or placebo over four hours, as in the previous study [48]. The participants listened to four sentences, and judged the mood of the speaker as being one of four emotions (happy, indifferent, angry and sad). They found that the accuracy in emotion discernment via speech was increased with oxytocin administration. Oxytocin also improved the understanding of human expressions of facial or vocal emotion [47]. Guastella et al. measured the effect of single-dose oxytocin administration on the behavioral performance of 16 male adolescents with ASD using RMET. Participants received 18 or 24 IU of nasal oxytocin spray or placebo spray. Similar to TD participants, oxytocin administration improved behavioral performance on the RMET compared to the placebo condition [19]. However, in contrast to TD participants, improvement of emotional understanding was restricted to easily-discernible situations for individuals with ASD, while accuracy for more challenging questions was constantly low regardless of oxytocin administration. Based on this, the investigators concluded that oxytocin improves emotional recognition skills only for situations of moderate difficulty for each person [45]. Other than recognition of emotion in faces or voices, improvement in gazing behavior was also observed. Andari et al. conducted a single-dose study of the effects of administration of 24 IU oxytocin on 11 men and two women with ASD. They examined fixation time on particular facial areas such as the eye when shown photographs of faces, and found that such fixation time was increased by oxytocin administration relative to placebo [38]. Auyeung et al. confirmed that improvement of gazing behaviors was also observed during natural social interactions. Thirty-two men with ASD received 24 IU of oxytocin or placebo, and their gazing behavior while conversing with the experimenter was measured using an eye-tracking system. Oxytocin administration enhanced gazing at the eyes, and the effect was more pronounced in participants with severe gazing difficulty at baseline [41]. Furthermore, Andari et al. showed that trust in, and preference for, another person was changed in a social ball-tossing game, as well as gazing behavior. Participants engaged in a multi-round ball-toss game with three other computer-simulated players. The authors created different cooperative behavior profiles (good player, bad player, and neutral player) by manipulating the amount of reciprocation exhibited by the three fictitious players (i.e. percentages of passes to the study subjects). TD individuals without oxytocin administration sent the ball more often to the good player than to the bad or neutral player. Placebo-treated individuals with ASD passed the ball to all three players equally, while oxytocin-treated ASD patients passed the ball more often to the good player than to the bad player. Thus, oxytocin administration increases sensitivity to players’ behavior and might enhance trust in them [38].
Taken together, these behavioral studies suggest that oxytocin improves a wide-range of social behaviors including facial or vocal recognition of emotion, gazing at the eyes, and trust in or preference for another person; this facilitates socially appropriate behaviors while restricting repetitive behaviors.
4.1.2. Neuroimaging Studies
What neural underpinnings underlie the behavioral improvements in individuals with ASD upon oxytocin administration? Recent neuroimaging studies attempted to elucidate the mechanisms of the beneficial effects of oxytocin administration on individuals with ASD, specifically in social communication [39, 40, 42-44, 46] (Table 1). Although all
studies found significant changes of brain activation by oxytocin administration, few showed a direct relationship between behavioral improvement and changes in brain activity.
For example, Gordon et al. and Domes et al. showed improvement of neural response by oxytocin administration, but they failed to find significant improvement of behavioral results. Gordon et al. (2013) conducted an fMRI study where participants judged mental states by viewing photographs of a human eye using RMET (social task) and judging the category of automobiles by viewing photographs of vehicles (nonsocial task). Twenty-one children and adolescents with ASD received 12, 18 or 24 IU oxytocin or placebo. Regardless of task, oxytocin enhanced activation in the various regions, including the middle frontal gyrus, striatum, superior frontal gyrus, orbitofrontal cortex, medial prefrontal cortex, and superior temporal sulcus. A greater oxytocin effect during social tasks relative to non-social tasks was also found in the precentral gyrus, striatum, nucleus accumbens, cerebellum, pons, posterior cingulate cortex, precuneus, parahippocampal gyrus, inferior parietal lobule, and superior temporal sulcus. However, significant behavioral improvement was not observed in this study; thus, it is unknown if changes of neural response in these regions contribute to improvement of social behaviors [44]. In another fMRI study of 14 men with ASD, Domes et al. examined the effect of oxytocin on neural response in face- and house-matching tasks. The subjects received 24 IU oxytocin or placebo before MR scanning. They found that amygdala activation in the ASD group was significantly increased by oxytocin compared to placebo. Interestingly, the TD group showed opposite effects in neural response in the amygdala; i.e., oxytocin administration reduced activation in the amygdala. Based on these results, the authors concluded that oxytocin reduced the responsiveness of the amygdala when suppressing fear and stress in TD individuals, while increasing its responsiveness for saliency of face/place recognition in individuals with ASD. Although oxytocin appears to exert adaptive effects on social cognition, it did not improve the accuracy of the subjects’ ability to match faces or houses [42]. Aoki et al. found improvement of both fMRI and behavioral results, but did not observe a significant relationship between these improvements. In their study, 20 men with ASD received 24 IU of oxytocin or placebo, and were asked to judge another’s emotion or belief using a cartoon. When participants judged the emotion compared to the belief of another person, oxytocin treatment enhanced activation in the anterior insula, as well as the cuneus, anterior middle temporal gyrus, and inferior frontal gyrus relative to placebo subjects. In contrast, no brain region showed enhancement by oxytocin administration when participants judged the belief of another person. They found that the rate of accurate emotion judgment was higher in oxytocin-treated subjects than in placebo subjects. However, the behavioral improvement was not associated with increased activation in the anterior insula [40].
In contrast to these studies, two other fMRI studies found a relationship between behavioral changes and neural responses [43, 46]. Domes et al. conducted fMRI studies in 14 men with ASD where participants judged emotion based on a photograph of a human’s eye or mouth. These participants received 24 IU of oxytocin and placebo. Oxytocin-treated participants with ASD showed a greater activation in their amygdala when judging emotion using both the eyes and mouth. In addition to the amygdala, various brain regions including the temporal pole, superior temporal cortex, inferior frontal gyrus, supplementary motor area, cerebellum, and superior parietal lobule were strongly activated with oxytocin treatment. Oxytocin administration also improved accuracy of emotion recognition in individuals with ASD. Furthermore, the difference of amygdala activation between oxytocin and placebo conditions was positively correlated with the difference of behavioral performance vis-à-vis emotion recognition [43]. In a study by Watanabe et al., 40 men with ASD received 24 IU of oxytocin or placebo. The participants then watched movies with an actor uttering positive or negative words using gentle or scowling faces, and were asked to judge the actor as a friend or foe. When words and facial expressions were inconsistent, judgment depended more on the actor’s facial expression in oxytocin-treated subjects. In fMRI results, oxytocin increased activation in the anterior cingulate cortex, dorsal medial prefrontal cortex, inferior frontal gyrus, anterior insula, superior frontal gyrus, and superior temporal sulcus. In these regions, activity in the anterior cingulate cortex and dorsal medial prefrontal cortex was positively correlated with behavioral results in oxytocin-treated subjects, but not in placebo subjects. Thus, participants with greater activation of the anterior cingulate cortex and dorsal medial prefrontal cortex showed increased judgment based on facial expression. In their study, they also found decreased activation of the amygdala [46], in contrast to the study of Domes et al. Furthermore, Aoki et al. demonstrated that levels of N-acetylaspartate, a marker of neuronal energy demand, was associated with MR signal changes in the ventromedial prefrontal cortex/anterior cingulate gyrus after oxytocin administration in the previous fMRI study [39].
Collectively, previous fMRI studies found significant changes in activation of various brain regions by oxytocin administration, including the cortical and subcortical regions. However, only activation in the amygdala, the dorsal medial prefrontal cortex, and the anterior cingulate cortex showed a direct relationship with behavioral results, especially in understanding facial expressions and using it to judge others. No other tasks were found to be associated with brain activity or behavioral improvements.
4.1.3. Summary of Single Dose Studies of Individuals with ASD
In single dose studies, oxytocin was found to improve a wide-range of social behaviors and was also found to restrict repetitive behaviors. Furthermore, neuroimaging studies elucidated underlying brain mechanisms of oxytocin administration that were associated with behavioral improvements, i.e., an association between emotional facial expressions and amygdala activation, and between using non-verbal information for judging others and activation of the anterior cingulate cortex and dorsal medial prefrontal cortex. However, several issues ought to be addressed in future studies. For instance, although behavioral studies suggested that oxytocin improves core symptoms of ASD, including social-communicative function and restricted and repetitive behaviors, Preti et al. have proposed that there are several biases in these studies. They have conducted systematic reviews of randomized controlled trials for oxytocin administration in individuals with ASD including those of Hollander et al. (2003), Hollander et al. (2007), Guastella et al., and Andari et al. They proposed that these four studies have a higher risk of selection bias, and also did not reject the possibility of an existing publication bias [9]. Of note, 40% of recent fMRI studies (two out of five studies) mentioned in this review failed to observe a significant improvement in behavioral performance, in contrast to behavioral studies showing greater improvement with oxytocin administration. This fact also raises a possibility of the existence of a publication bias; i.e., there may be behavioral studies that failed to find significant improvements of oxytocin administration. Additionally, there were no studies examining neural mechanisms underlying various behavioral changes in individuals with ASD, including social-communicative function (comprehension of affective speech or trust) and restricted/repetitive behaviors. Thus, further fMRI studies using a broader range of testing tasks are necessary.
4.2. Long-Term Administration Studies
Although single-dose studies can elucidate the acute effects of oxytocin administration, the sustained dosage period of a single dose of oxytocin is relatively short. To constitute an acceptable treatment for ASD, oxytocin must have prolonged efficacy. Thus, whether behavioral changes were observed under long-term administration of oxytocin ought to be examined. In the following sections, we introduce open-label studies and randomized, controlled trials (RCTs) of oxytocin administration to individuals with ASD.
4.2.1. Open-Label Studies
Similar to single-dose studies, open-label studies, including case reports, have shown the efficacy of oxytocin administration in individuals with ASD [49-52]. Munesue et al. reported the efficacy of long-term intranasal oxytocin administration in a 23-year-old man with ASD and intellectual disability. After oxytocin administration twice daily, he showed improvement in social behaviors, including more frequent eye contact combined with smiling, as well as the ability to answer yes/no questions concerning his daily life. No adverse effects were observed [52]. This study was the first to show the efficacy and safety of long-term oxytocin administration. Kosaka et al. examined the efficacy of intranasal long-term oxytocin administration to a 16-year-old girl with high-functioning ASD. She received 8 IU of oxytocin per day for six months. Beginning one month after the start of oxytocin treatment, her social behavior gradually improved. For instance, she began to greet other people and make small talk with them, and to show empathy for others’ sickness and worries. Interestingly, her score on the Aberrant Behavior Checklist (ABC), scored by her mother, decreased from 69 to 15 two months after commencing oxytocin treatment. Her Clinical Global Impression (CGI)-Severity score was also decreased from 6 to 3. There were no obvious adverse effects [50]. Tachibana et al. examined the efficacy of oxytocin administration in eight boys with ASD for six months. Six out of eight participants showed improvement in the communication and social-interaction domains of the Autism Diagnostic Observation Schedule-Generic (ADOS-G), while no significant changes were observed in Child Behavior Checklist or ABC scores. Five out of eight caregivers of the boys reported positive effects of oxytocin therapy. As in other studies, no severe adverse effects of oxytocin were found [51]. Anagnostou et al. conducted an open-label study of 15 youths with ASD, including both boys and girls, for 12 weeks. They examined changes in various aspects of behavior including social cognition, social functioning, repetitive behavior, and anxiety. They showed that long-term oxytocin administration improves several social functions, including social cognition, repetitive behaviors, and anxiety measures, with no severe adverse events [49]. These studies suggest that long-term oxytocin administration ameliorates the core symptoms of ASD, regardless of sex, age, or intellectual ability.
4.2.2. Randomized, Controlled Trials
Although these open-label studies provide detailed information on how the behavior of participants with ASD is changed by long-term oxytocin administration, evaluation of participants’ behavior in an open-label study is considered biased. To objectively evaluate the efficacy of long-term oxytocin administration, an RCT is required. Table 3 lists clinical RCTs of long-term oxytocin administration registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR), ClinicalTrials.gov, and the Australian New Zealand Clinical Trials Registry. As shown in Table 3, the duration, dosage, and participant characteristics (i.e., sex, age, and number) differed among trials. More specifically, there was great variability in the duration of the clinical trials; the University of North Carolina planned to perform a remarkably long trial lasting over one year. Dosage amounts ranged from 3 IU to 40 IU, but most trials used 24 IU twice per day. A majority of the trials were restricted to male participants, while the remainder included both males and females with ASD. Over half of the trials planned to conduct RCTs on children with ASD, while the other trials examined the effects of oxytocin administration on adults with ASD. Furthermore, recent trials have included a large number of participants (N > 100). Five of these trials (NCT00490802, ACTRN12609000513213, ACTRN12609000784213, ACTRN12611000061932, and UMIN000007122) reported the efficacy and safety of long-term oxytocin administration [53-57]. In this section, we cover the results of these studies (Table 4).
Table 3. Clinical randomized controlled trials of long-term oxytocin administration registered with the University Hospital Medical Information Network Clinical Trial Registry, Clinicaltrials.gov, and the Australian New Zealand Clinical Trials Registry.
|
Starting
Date |
Registered Database | Institute | Country | Duration | Dosage | Gender | Age | N | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Amount | Frequency | |||||||||
| 2006.06. | NCT00490802 | [53] | Mount Sinai School of Medicine |
USA | 6 weeks | 24 IU | Twice per day | Both | 18-60 y.o. | 19 |
| 2009.06. | ACTRN12609000513213 | [55] | University of Sydney | Australia | 8 weeks | 18 or 24 IU | Twice per day | Male | 12-18 y.o. | 50 |
| 2009.09. | ACTRN12609000784213 | [54] | The University of New South Wales |
Australia | 4 days | 12 or 24 IU | Once per day | Male | 8-16 y.o. | 60 |
| 2009.10. | NCT01337687 | Albert Einstein College of Medicine |
USA | 8 weeks | 24 IU | Twice per day | Both | 18-55 y.o. | ||
| 2010.12. | ACTRN12611000061932 | [57] | University of Sydney | Australia | 5 weeks | Upward titration |
Twice per day | Both | 3-8 y.o. | 40 |
| 2011.03. | NCT01308749 | University of North Carolina |
USA | 8 weeks | 24 or 32 IU | Twice per day | Both | 3-17 y.o. | 25 | |
| 2011.03. | UMIN000005211 | University of Fukui | Japan | 12 weeks | 8 or 16 IU | Twice per day | Both | 15- y.o. | 60 | |
| 2011.09. | NCT01417026 | Children's Hospital of Philadelphia | USA | 5 days | 24 IU | Once per day | Male | 12-17 y.o. | 68 | |
| 2012.03. | UMIN000007122 | [56] | The University of Tokyo | Japan | 6 weeks | 24 IU | Twice per day | Male | 18-55 y.o. | 20 |
| 2012.03. | UMIN000007250 | Kanazawa University | Japan | 8 weeks | 8 IU | Twice per day | Male | 15-39 y.o. | 30 | |
| 2012.06. | NCT01624194 | Stanford University | USA | 4 weeks | 24 IU | Twice per day | Both | 6-12 y.o. | 50 | |
| 2012.11. | UMIN000009075 | Osaka University | Japan | 4 weeks | 24 IU | Twice per day | Male | 6-9 y.o. | 40 | |
| 2013.09. | NCT01908205 | Holland Bloorview Kids Rehabilitation Hospital/ University of Minnesota |
Canada/ USA |
24 weeks | 0.4 IU/kg (max 24IU) |
Twice per day | Both | 10-17 y.o. | 60 | |
| 2013.11. | NCT02007447 | University of Sao Paulo General Hospital | Brazil | 8 weeks | 24 IU | Twice per day | Male | 12-18 y.o. | 30 | |
| 2014.03. | NCT02090829 | University of Minnesota Department of Psychiatry | USA | 5 days | 24 IU | Once per day | Male | 12-17 y.o. | 52 | |
| 2014.04. | NCT01914939 | Massachusetts General Hospital | USA | 12 weeks | 24 IU | Once per day | Male | 18-30 y.o. | 150 | |
| 2014.06. | NCT01788072 | Holland Bloorview Kids Rehabilitation Hospital/ McMaster University |
Canada | 12 weeks | 24 IU | Twice per day | Both | 18-45 y.o. | 146 | |
| 2014.08. | NCT01944046 | University of North Carolina |
USA | 18 months | Upward titration |
Twice per day | Both | 3-17 y.o. | 300 | |
| 2014.10. | UMIN000015264 | The University of Tokyo/ Kanazawa University/ University of Fukui/ Nagoya |
Japan | 6 weeks | 24 IU | Twice per day | Male | 18-55 y.o. | 114 | |
| 2015.06. | UMIN000017876 | Osaka University | Japan | 4 weeks | 24 IU | Twice per day | Male | 6-10 y.o. | 10 | |
Duration, duration of clinical trial; amount, dosage amount per single administration; frequency, dosage frequency per day; N, number of participants. y.o.: years old.
Among these five RCTs, two did not report significant improvement in several aspects of ASD under oxytocin administration [54, 55], while the other three showed improvements of several outcomes including social-communicative functioning [53, 56, 57]. Dadds et al. conducted an RCT with 38 boys with ASD aged 7 to 16 years [54]. Dosage was 12 or 24 IU, depending on participant weight, once per day for four days. Although the authors measured various behaviors through video-coding of participants and their families, they did not find any improvement in outcomes such as social-communicative functions, restricted and repetitive behaviors, or diagnostic and severity changes [54]. Guastella et al. examined long-term oxytocin administration in 50 adolescent boys with ASD aged 12 to 18 years [55]. Participants were administered 18 or 24 IU twice per day over eight weeks. In this trial, the primary endpoints were the Social Responsiveness Scale (SRS) and the CGI; secondary endpoints were the Developmental Behavior Checklist (DBC), the Repetitive Behavior Scale-revised (RBS), the RMET, the Diagnostic Analysis of Non-Verbal Accuracy test (DANVA), and behavioral results of biological motion tasks (i.e., the accuracy of identification of an action, emotion, subjective state, or object). Thus, they measured changes in social-communicative aspects, restricted and repetitive behaviors, and general symptoms. Consistent with the result of Dadds et al., participants did not show improvement in either primary or secondary endpoints [55]. Anagnostou et al. performed an RCT involving 19 individuals with high-functioning ASD aged 18 to 60 years. Participants were administered 24 IU of oxytocin intranasally twice per day over six weeks [53]. DANVA, CGI, and RBS were measured as the primary endpoints; RMET, the World Health Organization Quality of Life (WHO-QOL) scale, the Yale Brown Obsessive Compulsive Scale (Y-BOCS), and the SRS were measured as secondary endpoints. Although they did not observe significant improvement in primary outcomes, the authors found improved scores on the RMET and WHO-QOL in the oxytocin group relative to the placebo group [53]. The results suggest that some social-cognitive functions and quality of life was improved by oxytocin administration. Recently, Watanabe et al. also found significant improvement in social functioning in adults with ASD [56]. Twenty male adults with ASD participated in this trial, and dosage was 24 IU twice per day. ADOS and the Childhood Autism Rating Scale were the primary endpoints. Secondary endpoints included various behavioral measures such as the autism-spectrum quotient, SRS, RBS, State and Trait Anxiety Inventory, the Centre for Epidemiologic Studies Depression Scale, the WHO-QOL, CGI, Global Assessment of Functioning, and neural responses as measured by fMRI. In this trial, the authors found significant improvement in the ADOS reciprocity score, task-dependent neural responses, and task-independent resting-state functional connectivity [56]. Yatawara et al. examined the efficacy of long-term oxytocin administration in 31 children with ASD who received 12 IU oxytocin twice per day [57]. SRS and RBS scores were the primary endpoints, while DBC, CGI, ADOS,
and caregiver strain questionnaire outcomes were secondary endpoints. They found significant improvements in parent-rated SRS and CGI-I following oxytocin administration [57]. Of note, none of these trials reported severe adverse effects [53-57].
4.2.3. Summary of Long-term Administration Study of Individuals with ASD
The efficacy of long-term oxytocin administration is controversial. One of the possible reasons for the equivocal results in the trials described above is participant age (Table 4). Two out of three RCTs conducted in children with ASD failed to reveal significant improvements [54, 55, 57], even though significant behavioral changes are observed in all RCTs involving adults with ASD [53, 56]. Based on this, we speculate that long-term oxytocin administration may be less effective for children with ASD than for adults. Alternatively, it is possible that, as children with ASD show greater changes in social and cognitive functioning through natural development, improvement due to oxytocin administration may be obscured. This explanation is applicable to single dose studies. Almost all single dose studies have been conducted on adolescents or adults with ASD [38-43, 45-48], while only one study was conducted in children [44]. In the fMRI study of Gordon et al., children with ASD did not show any behavioral improvement, although they did show
Table 4. Clinical randomized controlled trials of long-term oxytocin administration.
| Trial | Participants | Oxytocin Group | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
Dosage
Frequency Duration |
General Symptom | Autism Core Symptoms | Social Communicative |
Restricted and Repetitive
Behavior |
Comorbidity | Other | |||
| Anagnostou et al., 2012 | [53] | Gender: Both N: 10 (9M1F) / 9 (7M2F) Age: 33.8 (12.7) / 32.9 (14.4) |
24 IU Twice per day 6 weeks |
Primary Outcomes | |||||
| CGI-social DANVA-face DANVA-paralanguage |
RBS higher order RBS lower order |
||||||||
| Secondary Outcomes | |||||||||
| *RMET SRS |
Y-BOCS | *WHO-QOL | |||||||
| Dadds et al., 2014 |
[54] | Gender: Male N: 19 / 19 Age: 11.8 (2.8) / 10.7 (2.4) |
12 or 24 IU Once per day 4 days |
Diagnosis changes (OSU, CARS, DISCAP-ASD) |
SSRS Video coding: child eye contact Video coding: parent eye contact Video coding: positive non-verbal behavior Video coding: verbal content Video coding: global rating UNSW facial emotion task |
Video coding Social reciprocity scale |
|||
| Guastella et al., 2015 |
[55] | Gender: Male N: 26 / 24 Age: 13.8 (1.5) / 14.0 (2.0) |
18 or 24 IU Twice per day 8 weeks |
Primary Outcomes | |||||
| CGI | SRS | ||||||||
| Secondary Outcomes | |||||||||
| RMET DANVA biological motion |
RBS | DBC | |||||||
| Watanabe et al., 2015 | [56] | Gender: Male N: 9 / 9 Age: 35.1 (7.6) / 29.3 (5.9) (cross over: oxytocin-initially group / placebo-initially group) |
24 IU Twice per day 6 weeks |
Primary Outcomes | |||||
| CARS | *ADOS: reciprocity ADOS: communication |
ADOS: repetitive | |||||||
| Secondary Outcomes | |||||||||
| CGI-EI CGI-SI GAF |
AQ | SRS *NVJ number NVJ response time *Fixation time for eye area |
RBS | STAI state CES-D |
WHO-QOL *fMRI: NVJ-specific brain activity in the ACC *fMRI: NVJ-specific brain activity in the dmPFC *fMRI: Functional brain connectivity during task *rsfMRI: Functional brain connectivity of ACC-dmPFC (systolic blood pressure) (diastolic blood pressure) (pulse rate) |
||||
| Yatawara et al., in press |
[57] | Gender: Both N: 15 (14M1F) / 16 (13M3F) Age: 5.7 (1.5) / 6.7 (1.8) (cross over: oxytocin-initially group / placebo-initially group) |
12 IU Twice per day 5 weeks |
Primary Outcomes | |||||
| *SRS-P | RBS-R-P | ||||||||
| Secondary Outcomes | |||||||||
| *CGI-I | ADOS: reciprocity ADOS: communication |
DBC-P CSQ |
|||||||
Participants properties were shown in oxytocin/placebo and from oxytocin to placebo/from placebo to oxytocin. Asterisk indicates outcomes showing significantly greater improvement in the oxytocin group than the placebo group.
M, males; F, Females; SSRS: Social Skills Rating Scale, OSU: Ohio State University autism rating scale-DSM IV, DISCAP-ASD: Diagnostic Interview Schedule for Children, Adolescents, and Parents-ASD diagnostic clinical interview, CARS: Childhood Autism Rating Scale, CGI-I: Clinical Global Impression-Improvements subscale, SRS: Social Responsiveness Scale, DBC: Developmental Behavior Checklist, RBS: Repetitive Behavior Scale-Revised, RMET: Reading the Mind in the Eyes Test, DANVA: Diagnostic Analysis of Nonverbal Accuracy, YBOCS: Yale Brown Obsessive Compulsive Scale, WHO-QOL: World Health Organization Quality of Life Questionnaire, AQ: Autism spectrum quotient, ADOS: Autism Diagnostic Observation Scale, GAF: Global Assessment of Functioning, NVJ: non-verbal information-based judgment, STAI: State and Trait Anxiety Inventory, CESD: Centre for Epidemiologic Studies Depression Scale, ACC: Anterior cingulate cortex, dmPFC: Dorsal medial prefrontal cortex, rsfMRI: Resting state functional magnetic resonance imaging, SRS-P: caregiver-rated social responsiveness, RBS-R-P: caregiver-rated severity of repetitive behavior on the Repetitive Behavior Scale-Revised, DBC-P: caregiver-rated social and emotional difficulties on the Developmental Behavior Checklist, CSQ: caregiver stress on the Caregiver Strain Questionnaire.
significant changes in brain activity by oxytocin administration [44]. As for parameters improved by oxytocin administration in adults with ASD, Table 4 shows that the rate of significant improvement in social cognitive endpoints following oxytocin administration was relatively high at 36% (4 out of 11). By contrast, none of the endpoints related to general symptoms (0 out of 3), comorbidity (0 out of 2), or autism core symptoms including social-communication plus restricted and repetitive behavior (0 out of 2) and restricted and repetitive behavior alone (0 out of 5) showed significant improvements. These results therefore suggest that long-term oxytocin administration in adults with ASD might improve social-communicative functions but not general symptoms such as restricted and repetitive behavior and comorbidity. However, the variability in factors, such as participants’ characteristics (e.g. age, intellectual ability, and sex), administration schedules (e.g. dosage amount, frequency per day, and duration of trial), and indices measured as endpoints, may have caused inconsistencies in the results. Additionally, as shown in Table 3, there are a number of clinical trials examining the efficacy and safety of long-term oxytocin administration for individuals with ASD that have not yet reported their results; some of these trials are ongoing. Thus, determining whether oxytocin is effective for improving social-communicative function in individuals with ASD is partly contingent on the upcoming results of these clinical studies.
5. Outstanding issues with oxytocin administration, and further studies
Although, a number of studies have shown beneficial effects of oxytocin administration in individuals with ASD, there are still some obstacles impeding the acceptance of oxytocin treatment for ASD. We discuss some of these, including safety, administration schedule, and mechanisms of long-term oxytocin administration, in this section.
5.1. Safety of Oxytocin Administration
Although, none of the previous studies reported severe adverse effects, the safety of oxytocin administration remains to be confirmed in future studies. We discuss three issues in this section. First, as previous long-term administration trials were conducted within half-year time spans, there are no data on the efficacy and safety of longer-term (>1 year) oxytocin administration. As shown in Table 3, the University of North Carolina plans to conduct an RCT of long-term oxytocin administration for 18 month (NCT01944046); thus, efficacy of longer-term oxytocin administration ought to be clarified in the near future. Second, long-term follow-up studies are necessary to confirm the safety of oxytocin administration. For instance, a recent rodent study cautioned against long-term oxytocin administration during early childhood [58]. In that study, the authors tested whether long-term oxytocin administration during early life affects lifelong social behavior. Soon after treatment with oxytocin during early life commenced, the social behavior of male voles increased. However, long-term developmental treatment with low doses of intranasal oxytocin caused deficits in various partner preference behaviors when male voles reached adulthood [58]. These findings suggest the possibility that long-term developmental treatment with oxytocin impairs later-developing social behaviors. Although the study was conducted on rodents, several RCTs and open-label studies have been performed in children with ASD [51, 54, 55, 57]. While none of these studies have shown severe adverse events, there is a possibility that negative effects occur when the participants reach adulthood. This possibility should be carefully examined in longer-term follow-up studies of these participants. Third, although there were some studies performed on female participants [50, 53], there is still no standardized clinical test to confirm safety in females. Because oxytocin is involved in parturition and childcare, including galactopoiesis and contraction of the uterus, observation of female-specific vital signs is critical. Two previous studies did not evaluate safety. In our RCT (UMIN000005211 in Table 3), we planned to measure sex hormones, menstrual cycle, or uterine peristalsis activity by cine MRI. Thus, standardized clinical tests for confirmation of safety in female participants is a priority for future studies.
5.2. Efficiency of Administration Schedules
The most efficient oxytocin administration schedules, which would likely depend on individual patient characteristics, remain unclear. For example, it is unknown if dosage amount or frequency affect the efficacy of oxytocin administration in individuals with ASD. Dose-dependent efficacy was found in patients with schizophrenia [59]. In a single-dose oxytocin administration study, participants with schizophrenia were administered 20 IU or 10 IU oxytocin. While the higher oxytocin dose ameliorated deficits in emotion recognition, the lower dose aggravated these deficits [59]. Although such dose-dependency might also be applicable to ASD, there are no reports of the efficacy of dose-dependent oxytocin treatments for individuals with ASD. We are currently conducting RCTs to examine the dose dependent efficacy of oxytocin administration in adults with ASD (UMIN000005211 in Table 3). Therefore, data on dose-dependent efficacy should be available in due course. In term of frequency of oxytocin administration, none of clinical trials listed in Table 4 planned to manipulate frequency of dosages. Because the duration of the acute effect is relatively short, further investigation of the effect of frequency is required.
We believe that the most efficient schedule for oxytocin administration will vary according to participants’ characteristics. First, participants’ age may be linked to the efficacy of oxytocin administration. However, no study has directly compared the efficacy of oxytocin between children and adults with ASD. As administration schedules and endpoints may affect the efficacy of oxytocin administration, we should only compare studies of similar administration schedules and endpoints. Second, severity of symptoms and of alteration of the oxytocin system should affect the efficacy of oxytocin administration. For general symptoms, Scheele et al. measured the effect of single-dose oxytocin administration in TD males and showed that the efficacy of oxytocin administration was greater in subjects with lower autistic traits than in those with higher autistic traits [60]. Based on such findings, we propose a possibility that patients with severe autistic symptoms may require more intensive treatments (e.g., higher dose, higher frequency, or longer-term administration) than patients with mild autistic symptoms. In terms of the severity of alteration of the oxytocin system, it is possible that some ASD patients may exhibit heavy alteration of this system while alteration may be relatively mild in others, especially because ASD patients are a highly heterogeneous group (including in their etiologies). The severity of alteration of the oxytocin system might be linked to the efficacy of oxytocin administration. Hence, examining the association between the extent of alteration of the oxytocin system (by measuring oxytocin levels before treatment as well as determining oxytocin receptor SNPs) and the efficacy of oxytocin administration is necessary. Third, personal history might also influence efficacy. For instance, Bakermans-Kranenburg et al. conducted a meta-analysis of oxytocin administration in TD individuals and in several clinical groups. In TD individuals, they suggested that the oxytocin-induced effect was modulated by personal history; for instance, positive oxytocin effects on behavior and neurobiology is attenuated in individuals with negative childhood experiences [61]. Thus, investigations of early childhood and developmental history may be helpful in understanding variations in the effect of oxytocin.
5.3. Mechanisms Underlying Oxytocin Efficacy
The mechanisms underlying the efficacy of oxytocin are not fully understood, including how intranasally administered oxytocin functions or how acute and chronic administration of oxytocin change brain function. For a period of time, it was even unclear whether intranasally administered oxytocin reached the brain at all. However, recent studies on human and non-human primates provided evidence that cerebrospinal fluid (CSF) oxytocin levels were elevated by intranasally administered oxytocin [62-65]. Striepens et al. examined CSF oxytocin levels after intranasal oxytocin administration in humans and found that levels in CSF were elevated at 75 min after administration [65]. Elevated levels following intranasally injected oxytocin were also confirmed in non-human primates [62-64]. Although these studies provide evidence that intranasally administered oxytocin reaches to brain, several points remain unclear. First, the route through which intranasally administered oxytocin reaches the brain is unknown. Macdonald et al. proposed possible routes in their review [66]. The administered oxytocin may either cross the blood-brain barrier and enter the brain, or utilize extraneuronal/perineuronal routes along the trigeminal or olfactory nerve pathway. Additionally, they suggested that bulk flow, lymphatic channels, intraneuronal transport, and active or passive transport by the vasculature may contribute to the effect of intranasal oxytocin on the brain [66]. However, direct evidence for this is lacking. Second, it is debatable whether the incremental amount of CSF oxytocin increase due to intranasal oxytocin administration is sufficient to modulate brain function and behaviors [67]. Leng and Ludwig have suggested that only 0.005% of intranasally injected oxytocin enters the brain. Therefore, they concluded that the incremental increase in CSF oxytocin level compared to the originally administered amount is too small to modulate brain function; see also [68, 69]. However, although there have been no studies comparing CSF oxytocin levels between individuals with and without ASD, almost all studies that incidentally measured plasma oxytocin levels showed differences between TD individuals and individuals with ASD of below two fold [8, 23, 24, 27-30]. Because CSF oxytocin levels were approximately 1.5–2.0 fold higher in TD subjects than in individuals with ASD [62-65], we postulate that exogenous oxytocin administered to individuals with ASD is sufficient to compensate for the deficit in CSF oxytocin levels. Third, Leng and Ludwig suggested that peripheral oxytocin activity can modulate behaviors. In fact, previous studies have shown that intravenously administered oxytocin enhanced emotion recognition skills and reducing restricted and repetitive behaviors in individuals with ASD [47, 48]. In a study on non-human primates, intranasal oxytocin administration induced elevated oxytocin levels in the CSF, while no such elevated levels were observed upon intravenous oxytocin administration [64]. If intravenously administered oxytocin does not reach the brain in humans or macaques, improvement of core symptoms of ASD may be because of peripheral activity in addition to central action. Thus, further investigation examining the effect of oxytocin on core symptoms of individuals with ASD due to both central and peripheral activity is necessary. Fourth, differences in the mechanisms of acute vs. chronic effects of oxytocin administration are unclear. In studies with mice, chronic oxytocin administration reduced oxytocin receptor levels throughout the brains of healthy mice, which led to social dysfunction [70]. However, it is unknown if chronic administration reduces oxytocin receptors in mice, causing an oxytocin system deficit. Furthermore, there has been no study examining the difference in mechanisms between acute and chronic effects in primates, including humans, regardless of the dysfunction of the oxytocin system. Thus, to establish oxytocin as a treatment for individuals with ASD, there are still a number of issues to be resolved. Beyond the clinical trials described in this paper, basic studies will be necessary for elucidating the mechanisms of the positive and negative effects of oxytocin administration.
6. Conclusion
In this review, we covered studies examining oxytocin administration to individuals with ASD. In clinical trials involving such individuals, the results from RCTs investigating long-term oxytocin administration are inconsistent with those of RCTs involving single-dose administration. Specifically, almost all single-dose and open-label studies of oxytocin administration have reported positive effects; in contrast, RCTs of long-term oxytocin administration have produced equivocal findings. To alleviate this controversy, research to identify the factors affecting oxytocin efficacy will be necessary. We also emphasized that, although no study has reported a severe adverse event, longer-term follow-up is required to confirm the safety of this hormone. Basic studies are also necessary to elucidate the mechanisms of the positive and negative effects of oxytocin administration, in addition to the proposed clinical trials.
ACKNOWLEDGEMENTS
Part of this research was the result of the Center of Community from MEXT, and the project “Integrated research on neuropsychiatric disorders,” which was carried out under the Strategic Research Program for Brain Sciences from MEXT and AMED. This work was partly funded by the Grants-in-Aid for Scientific Research from the JSPS (15K08093).
List of abbreviations
- ABC
Aberrant Behavior Checklist
- ADOS-G
Autism Diagnostic Observation Schedule-Generic
- ASD
Autism Spectrum Disorder
- CGI
Clinical Global Impression
- CSF
Cerebrospinal Fluid
- DANVA
Diagnostic Analysis of Non-Verbal Accuracy Test
- fMRI
Functional Magnetic Resonance Imaging
- IU
International Units
- RBS
Repetitive Behavior Scale-Revised
- RMET
Reading the Mind in the Eyes Test
- RCT
Randomized, Controlled Trial
- SRS
Social Responsiveness Scale
- TD
Typical Development
- WHO-QOL
World Health Organization Quality of Life Scale
- Y-BOCS
Yale Brown Obsessive Compulsive Scale
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Am Psychiatr Publishers; 2013. [Google Scholar]
- 2.Weintraub K. The prevalence puzzle: Autism counts. Nature. 2011;479:22–24. doi: 10.1038/479022a. [DOI] [PubMed] [Google Scholar]
- 3.Lai M.C., Lombardo M.V., Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 4.Posey D.J., Erickson C.A., McDougle C.J. Developing drugs for core social and communication impairment in autism. Child Adolesc. Psychiatr. Clin. N. Am. 2008;17:787–801. doi: 10.1016/j.chc.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinrichs M., von Dawans B., Domes G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Lee H.J., Macbeth A.H., Pagani J.H., Young W.S., III Oxytocin: the great facilitator of life. Prog. Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LoParo D., Waldman I.D. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol. Psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- 8.Modahl C., Green L., Fein D., et al. Plasma oxytocin levels in autistic children. Biol. Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 9.Preti A., Melis M., Siddi S., Vellante M., Doneddu G., Fadda R. Oxytocin and autism: a systematic review of randomized controlled trials. J. Child Adolesc. Psychopharmacol. 2014;24:54–68. doi: 10.1089/cap.2013.0040. [DOI] [PubMed] [Google Scholar]
- 10.Yamasue H. Promising evidence and remaining issues regarding the clinical application of oxytocin in autism spectrum disorders. Psychiatry Clin. Neurosci. 2015;70(2):89–99. doi: 10.1111/pcn.12364. [DOI] [PubMed] [Google Scholar]
- 11.Domes G., Heinrichs M., Glascher J., Buchel C., Braus D.F., Herpertz S.C. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol. Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Kosfeld M., Heinrichs M., Zak P.J., Fischbacher U., Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 14.Labuschagne I., Phan K.L., Wood A., et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rimmele U., Hediger K., Heinrichs M., Klaver P. Oxytocin makes a face in memory familiar. J. Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viero C., Shibuya I., Kitamura N., et al. Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neurosci. Ther. 2010;16:e138–e156. doi: 10.1111/j.1755-5949.2010.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S., Fonagy P., Koos O., Dorsett K., Strathearn L. Maternal oxytocin response predicts mother-to-infant gaze. Brain Res. 2014;1580:133–142. doi: 10.1016/j.brainres.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strathearn L., Fonagy P., Amico J., Montague P.R. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guastella A.J., Mitchell P.B., Dadds M.R. Oxytocin increases gaze to the eye region of human faces. Biol. Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- 21.Baumgartner T., Heinrichs M., Vonlanthen A., Fischbacher U., Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Kirsch P., Esslinger C., Chen Q., et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green L., Fein D., Modahl C., Feinstein C., Waterhouse L., Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biol. Psychiatry. 2001;50:609–613. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- 24.Alabdali A., Al-Ayadhi L., El-Ansary A. Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J. Neuroinflammation. 2014;11:4. doi: 10.1186/1742-2094-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Ayadhi L.Y. Altered oxytocin and vasopressin levels in autistic children in Central Saudi Arabia. Neurosciences. 2005;10:47–50. [PubMed] [Google Scholar]
- 26.Tomova A., Husarova V., Lakatosova S., et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 27.Miller M., Bales K.L., Taylor S.L., et al. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: sex differences and associations with symptoms. Autism Res. 2013;6:91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker K.J., Garner J.P., Libove R.A., et al. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc. Natl. Acad. Sci. USA. 2014;111:12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taurines R., Schwenck C., Lyttwin B., et al. Oxytocin plasma concentrations in children and adolescents with autism spectrum disorder: correlation with autistic symptomatology. Atten. Defic. Hyperact. Disord. 2014;6:231–239. doi: 10.1007/s12402-014-0145-y. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson J.D., Ellerbeck K.A., Kelly K.A., et al. Evidence for alterations in stimulatory G proteins and oxytocin levels in children with autism. Psychoneuroendocrinology. 2014;40:159–169. doi: 10.1016/j.psyneuen.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldman R., Golan O., Hirschler-Guttenberg Y., Ostfeld-Etzion S., Zagoory-Sharon O. Parent-child interaction and oxytocin production in pre-schoolers with autism spectrum disorder. Br. J. Psychiatry. 2014;205:107–112. doi: 10.1192/bjp.bp.113.137513. [DOI] [PubMed] [Google Scholar]
- 32.McCullough M.E., Churchland P.S., Mendez A.J. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Szeto A., McCabe P.M., Nation D.A., et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jokinen J., Chatzittofis A., Hellstrom C., Nordstrom P., Uvnas-Moberg K., Asberg M. Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology. 2012;37:482–490. doi: 10.1016/j.psyneuen.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Kagerbauer S.M., Martin J., Schuster T., Blobner M., Kochs E.F., Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocrinol. 2013;25:668–673. doi: 10.1111/jne.12038. [DOI] [PubMed] [Google Scholar]
- 36.Wu N., Li Z., Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. J. Affect. Disord. 2012;138:468–472. doi: 10.1016/j.jad.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Takayanagi Y., Yoshida M., Bielsky I.F., et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andari E., Duhamel J.R., Zalla T., Herbrecht E., Leboyer M., Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl. Acad. Sci. USA. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoki Y., Watanabe T., Abe O., et al. Oxytocin's neurochemical effects in the medial prefrontal cortex underlie recovery of task-specific brain activity in autism: a randomized controlled trial. Mol. Psychiatry. 2015;20:447–453. doi: 10.1038/mp.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoki Y., Yahata N., Watanabe T., et al. Oxytocin improves behavioural and neural deficits in inferring others' social emotions in autism. Brain. 2014;137:3073–3086. doi: 10.1093/brain/awu231. [DOI] [PubMed] [Google Scholar]
- 41.Auyeung B., Lombardo M.V., Heinrichs M., et al. Oxytocin increases eye contact during a real-time naturalistic social interaction in males with and without autism. Transl. Psychiatry. 2015;5:e507. doi: 10.1038/tp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domes G., Heinrichs M., Kumbier E., Grossmann A., Hauenstein K., Herpertz S.C. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol. Psychiatry. 2013;74:164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Domes G., Kumbier E., Heinrichs M., Herpertz S.C. Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with asperger syndrome. Neuropsychopharmacology. 2014;39:698–706. doi: 10.1038/npp.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon I., Vander Wyk B.C., Bennett R.H., et al. Oxytocin enhances brain function in children with autism. Proc. Natl. Acad. Sci. USA. 2013;110:20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guastella A.J., Einfeld S.L., Gray K.M., et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol. Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe T., Abe O., Kuwabara H., et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: a randomized trial. JAMA Psychiatry. 2014;71:166–175. doi: 10.1001/jamapsychiatry.2013.3181. [DOI] [PubMed] [Google Scholar]
- 47.Hollander E., Bartz J., Chaplin W., et al. Oxytocin increases retention of social cognition in autism. Biol. Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 48.Hollander E., Novotny S., Hanratty M., et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- 49.Anagnostou E., Soorya L., Brian J., et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res. 2014;1580:188–198. doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 50.Kosaka H., Munesue T., Ishitobi M., et al. Long-term oxytocin administration improves social behaviors in a girl with autistic disorder. BMC Psychiatry. 2012;12:110. doi: 10.1186/1471-244X-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tachibana M., Kagitani-Shimono K., Mohri I., et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J. Child Adolesc. Psychopharmacol. 2013;23:123–127. doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- 52.Munesue T., Yokoyama S., Nakamura K., et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci. Res. 2010;67:181–191. doi: 10.1016/j.neures.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Anagnostou E., Soorya L., Chaplin W., et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol. Autism. 2012;3:16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dadds M.R., MacDonald E., Cauchi A., Williams K., Levy F., Brennan J. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J. Autism Dev. Disord. 2014;44:521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- 55.Guastella A.J., Gray K.M., Rinehart N.J., et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J. Child Psychol. Psychiatry. 2015;56:444–452. doi: 10.1111/jcpp.12305. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe T., Kuroda M., Kuwabara H., et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138:3400–3412. doi: 10.1093/brain/awv249. [DOI] [PubMed] [Google Scholar]
- 57.Yatawara CJ, Einfeld SL, Hickoe IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. doi: 10.1038/mp.2015.162. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bales K.L., Perkeybile A.M., Conley O.G., et al. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol. Psychiatry. 2013;74:180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldman M.B., Gomes A.M., Carter C.S., Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl.) 2011;216:101–110. doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- 60.Scheele D., Kendrick K.M., Khouri C., et al. An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology. 2014;39:2078–2085. doi: 10.1038/npp.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bakermans-Kranenburg M.J., van IJzendoorn M.H. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl. Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang S.W., Barter J.W., Ebitz R.B., Watson K.K., Platt M.L. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc. Natl. Acad. Sci. USA. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dal Monte O., Noble P.L., Turchi J., Cummins A., Averbeck B.B. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS One. 2014;9:e103677. doi: 10.1371/journal.pone.0103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Modi M.E., Connor-Stroud F., Landgraf R., Young L.J., Parr L.A. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology. 2014;45:49–57. doi: 10.1016/j.psyneuen.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Striepens N., Kendrick K.M., Hanking V., et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macdonald K., Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front. Neurosci. 2013;7:35. doi: 10.3389/fnins.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leng G., Ludwig M. Intranasal Oxytocin: Myths and Delusions. Biol. Psychiatry. 2015;79(3):243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Leng G., Ludwig M. Reply To: Intranasal oxytocin mechanisms can be better understood, but its effects on social cognition and behavior are not to be sniffed at. Biol. Psychiatry. 2016;79(8):e51–e52. doi: 10.1016/j.biopsych.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 69.Quintana D.S., Woolley J.D. Intranasal oxytocin mechanisms can be better understood, but its effects on social cognition and behavior are not to be sniffed at. Biol. Psychiatry. 2016;79(8):e49–e50. doi: 10.1016/j.biopsych.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 70.Huang H., Michetti C., Busnelli M., et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39:1102–1114. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]