Abstract
Abstract: Background
Monoclonal antibodies have become attractive clinical anti-cancer drugs in the last 3 decades due to their targeting specificity and suitable pharmacokinetic properties. Mesothelin is a tumor-associated antigen with limited expression in normal tissues. It is frequently over-expressed on the cell membrane of a number of epithelial malignancies (e.g. mesothelioma, pancreatic, ovarian, lung, triple negative breast and gastric cancers).
Methods
Mesothelin is validated as a suitable antibody target for cancer therapy. A number of novel antibody therapeutics targeting mesothelin in development are compared and their mechanisms of action are also discussed. Both basic science and clinical data are provided to give a complete veiw of how an agent is developed from bench to bedside.
Results
Novel antibody therapeutics, including unconjugated monoclonal antibodies, recombinant immunotoxins and antibody-drug conjugates, targeting mesothelin exert anti-tumor activities by different mechanisms of action. Based on the convincing preclinical data generated with these molecules, the antibody therapeutics have been brought into early clinical evaluation where initial promising results were obtained.
Conclusion
These antibody therapeutics directed against mesothelin are expected to have different safety profiles, based on their different mechanism of action. Further clinical development will reveal which of these molecules shows the best efficacy and widest therapeutic window and thus is best suited to bring benefit to the patients.
Keywords: Antibody (Ab), antigen, mesothelin, cancer, unconjugated antibody, chimeric monoclonal antibody, recombinant immunotoxin (RIT), antibody-drug conjugate (ADC), Amatuximab, SS1P, RG7787, Anetumab ravtansine, DM1 or DM4: maytansinoid tubulin inhibitors, Monomethyl auristatin E (MMAE), 7D9-MMAE, DMOT4039A, MDX-1204, BMS-986148
Introduction
Monoclonal antibodies have become attractive clinical anti-cancer drugs in the last 3 decades due to their targeting specificity and suitable pharmacokinetic properties. They are now a major focus for drug development for cancer therapy since rituximab (Rituxan) and trastuzumab (Herceptin) received market approval in the late 90s [1]. Treatment with unconjugated antibodies can yield significant clinical benefits, particularly in combination with chemotherapy, but many unconjugated antibodies lack sufficient therapeutic activity [2]. Radioimmunotherapy was developed to improve the therapeutic efficacy of antibodies. Radioisotopes are conjugated to monoclonal antibodies via a bifunctional chelator for targeted delivery of high doses of radiation to the site of tumors. This strategy led to two FDA-approved drugs anti-CD20 yttrium-90 ibritumomab tiuxetan (Y90-Zevalin) and anti-CD20 iodine-131 tositumomab (I131-Bexxar) for non-Hodgkin lymphoma in 2000 [3, 4]. Another improvement strategy is to arm monoclonal antibodies with cytotoxic agents, which can be either protein toxins to form recombinant immunotoxins (RIT) or potent small molecule toxophores to form antibody-drug conjugates (ADC). Therefore, antibody treatment modalities in cancer therapy include unconjugated antibodies, radioimmunotherapy, RITs and ADCs.
RIT is one of the active research areas in the field of cancer therapy [5]. At present, the two major challenges with this novel treatment modality are toxicity and immunogenicity. Adverse effects with incompletely understood pathogenesis such as vascular leak syndrome and hepatotoxicity [6] are observed as dose-limiting side effects of RITs in some patients, requiring careful dose titration. Single administration of a RIT to humans is shown to trigger an immune response in 88% patients [7]. Therefore, humanization of the RITs will be key for elimination of their immunogenicity [8].
Recently, great strides have been made in the field of ADCs, marked by the launch of two ADC drugs, the CD30-targeting brentuximab vedotin for Hodgkin's lymphoma in 2011 and systemic anaplastic large cell lymphoma in 2012 and anti-HER2 trastuzumab emtansine for HER2-positive metastatic breast cancer in 2013 [9, 10]. ADCs function by binding to the targeted surface antigen on cancer cells, where upon the ADCs are internalized and catabolized to release the toxophore or its metabolites which can kill the cancer cells. Therefore, ADCs need to target cell surface antigens which are readily internalized after ADC binding. For example, brentuximab vedotin, an anti-CD30 antibody conjugated to the anti-mitotic agent MMAE via a lysosomal cathepsin-cleavable dipeptide linker, binds to the CD30-positive lymphoma cells and is internalized into lysosomes which release MMAE from the ADC. Trastuzumab emtansine, in which the microtubule inhibitor DM1 is linked to the antibody via non-cleavable linker SMCC, binds to the HER-2 antigen and undergoes internalization followed by lysosomal degradation to release DM1. With over 40 new ADCs currently undergoing clinical trials, it is likely that more ADCs will be approved in the near future.
Discovery and validation of Mesothelin as cancer target
Mesothelin was originally described as the antigen recognized by the K1 monoclonal antibody that was generated after immunizing mice with the OVCAR-3 human ovarian carcinoma cell line [11]. The mesothelin gene was then cloned in 1996 [12]. The mesothelin cDNA contains an open reading frame of 1884-bp and encodes a 69-kDa precursor protein (628 amino acids). After glycosylation, the precursor is cleaved by furin at amino acid 288-293 to yield a 40-kDa protein and a smaller 32-kDa fragment that is released from the cell. This 32-kDa shed fragment is called megakaryocyte-potentiating factor (MPF). The 40-kDa protein is found on the cell surface and can be released by treatment with phosphatidylinositol-specific phospholipase C. This 40-kDa GPI-linked membrane-bound protein was named mesothelin because it is produced by normal mesothelial cells. Since malignant mesotheliomas and ovarian adenocarcinomas are derived from normal mesothelial cells, it is not surprising that mesothelin is associated with these malignant diseases.
The most common form of mesothelin is membrane-bound, but 2 variants were found: Variant-1 with an 8 amino acid insertion is also membrane bound. Variant-2 is shed and soluble due to the lack of GPI-anchor signal sequences [13]. Soluble mesothelin proteins are detectable in sera from patients with ovarian carcinoma [14] and may provide a useful new marker for diagnosis of ovarian carcinoma and/or monitoring its response to therapy along with CA125 (cancer antigen-125). Moreover, soluble mesothelin is elevated in the blood and effusions of patients with mesothelioma and the determination of mesothelin levels in these fluids has been approved by the US FDA primarily as a tool for monitoring patient response and progression [15, 16].
Little is known about the biological function of mesothelin. Mesothelin knock-out mice show no phenotype. Mesothelin is suspected to play a role in cellular adhesion, based on the observation that NIH3T3 cells transfected with the mesothelin gene became harder to detach from the culture plate [12]. It appears to be involved in cell adhesion and tumor metastasis via its interaction with CA125, a tumor antigen used for diagnosis of ovarian cancer. CA125 is also named MUC16. CA125/MUC16 is a type I transmembrane protein expressed on the cell surfaces of many epithelia, and its soluble form can be released into extracellular space. Rump et al. found that mesothelin is a CA125/MUC16-binding protein and that CA125 might contribute to the metastasis of ovarian cancer to the peritoneum by initiating cell attachment to the mesothelial epithelium via binding to mesothelin [17]. In addition, recent studies suggest that mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation [18].
Mesothelin is considered a differentiation antigen because its expression in normal tissue is limited to mesothelia, but mesothelin is abundantly expressed in a variety of tumors including mesothelioma, ovarian cancer, pancreatic cancer and lung cancer [19, 20]. Mesothelin can be detected by immunohistological methods on normal mesothelial cells lining the pleural, pericardial, and peritoneal surfaces but not in any vital organs. It is often highly expressed in many epithelial cancers. Differential over-expression of mesothelin in tumors and its role in cell adhesion and tumor metastasis make mesothelin a suitable target for cancer therapy.
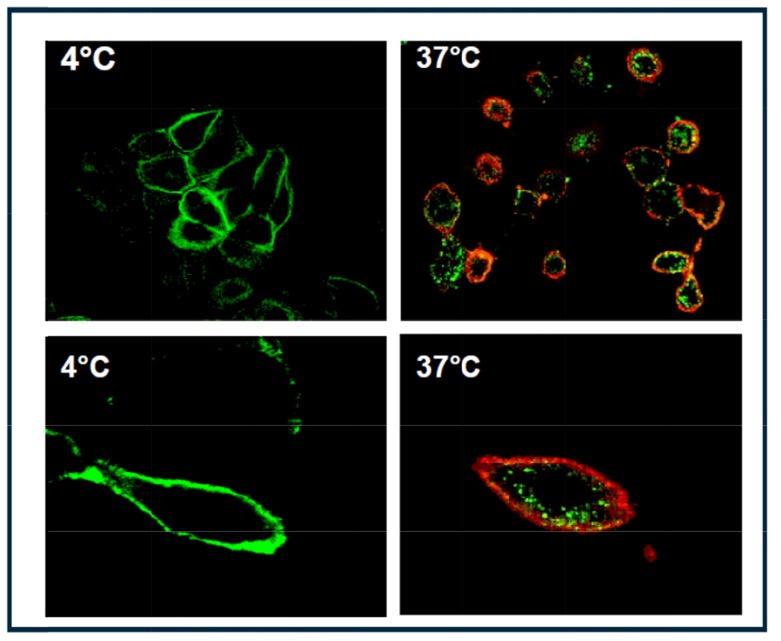
One prerequisite for RIT or ADC therapy is the internalization of the antibody upon target binding to effectively deliver the cytotoxic payload into cancer cells. To test whether mesothelin is an internalizing antigen, we developed a live-cell internalization assay [21]. Images by confocal microscopy (Fig. 1) demonstrated that mesothelin proteins were localized to the plasma membrane of NCI-H226 cells, the human lung squamous carcinoma and mesothelioma cell line from ATCC (catalog# CRL-5826), at 4°C, a nonpermissive temperature for internalization (left). At 37°C, the permissive temperature for internalization, mesothelin was internalized into vesicle structures of cytoplasm on antibody binding, moving from the cell membrane to the perinuclear region, most likely through endocytosis (right). These data clearly verified that mesothelin is an internalizing antigen. Hence, mesothelin-targeting via RIT or ADC approaches are viable clinical options for the treatment of mesothelin-positive cancers.
Fig. (1).
Confocal microscopic analyses of mesothelin internalization by NCI-H226 cancer cells that express the endogenous mesothelin. Live cells were incubated with anti-mesothelin antibody for 2 h at 4°C, and unbound antibody was removed by washes. Cells were then incubated with AlexaFluor 488–labeled secondary antibody (green) for 3 h at 4°C (left) or at 37°C (right). After incubation at 37°C, lipid bilayers were further stained by CM-DiI red dye (right). No internalization was observed at 4°C (left). Internalization was observed at 37°C (right).
Novel antibody therapeutics targeting mesothlin as cancer drugs in development
A number of novel antibody-based therapeutics targeting mesothelin have been developed and evaluated in clinical trials for solid tumors. They include unconjugated monoclonal antibody amatuximab by Morphotek/NCI; recombinant immunotoxins SS1P and RG7787 by NCI/Roche, and different antibody-drug conjugates (ADCs) by Bayer, Roche/ Genentech and BMS/Medarex. Here we discuss how these therapeutics are developed from bench to bedside, their mechanisms of action, in vitro and in vivo anti-proliferative activities as well as their clinical evaluations (Table 1).
Table 1.
Antibody-based therapeutics targeting mesothelin in solid tumors.
| mAb | RIT | ADC | ADC | ADC | |
|---|---|---|---|---|---|
| Names | amatuximab/MORAb-009 | SS1P & RG7787 | anetumab ravtansine/BAY 94-9343 | aMSLN-MMAE (h7D9.v3) | MDX-1204 |
| Company | Morphotek/NCI | NCI/Roche | Bayer | Roche/Genentech | BMS/Medarex |
| Ab | mouse-human chimeric | mouse-human chimeric | fully human | humanized | fully human |
| Ab generation | Panning mouse scFv phage display library | Panning human Fab phage display library | Hybridoma technology | Transgenic mice immunization | |
| KD | 1.5 nM | 1.5 nM | 10 nM | 0.23 nM | 2.4-5.9 nM |
| Cross-reactivity | monkey | monkey | none | none | monkey |
| Attachment site | none | Furin-cleavable site | Lysine | Cysteine | Lysine |
| Linker | reducible disulfide linker SPDB | lysosomal protease cleavable valine-citrulline dipeptide linker | |||
| Drug:Ab ratio | 3.2 | 3.5 | 1.4 | ||
| Toxophore/toxin | None | Bacterial toxin PE38 | DM4 maytansinoid | MMAE | Duocarmycin-related |
| MOA | Elicits ADCC | Inhibits protein synthesis | anti-mitotic by disrupting microtubules | alkylating DNA | |
| Inhibits cell adhesion | Induces apoptosis | Induces apoptosis | Induces apoptosis | ||
| In vitro IC50 | 100ng/mL in cell adhesion | 0.4 ng/mL in cell growth | 0.72-5.72 nM in cell growth | 0.32-20 nM in celll growth | |
| In vivo model | OV, PANC, Meso, Lung | OV,PANC,Meso,Lung,Bca,Gas | OV, PANC, Meso, NSCLC | OV, PANC, Meso, Lung | OV, PANC, NSCLC |
| Drug in clinical trial | amatuximab | SS1P; SS1(dsFv)PE38 | anetumab ravtansine | DMOT4039A | BMS-986148 |
| Identified # | Phase 2, NCT00738582 | Phase 1/2, NCT01362790 | Phase 1, NCT01439152 | Phase I, NCT01469793 | Phase 1/2a, NCT02341625 |
|
Estimated patient # |
89 | 75 | 141 | 71 | 204 |
| Adverse events | Experienced DLT | Immunogenecity, Pleuritis, CLS | Ocular toxicity | DLTs at 2.8 mg/kg: | No data yet |
| Grade4 transaminitis | hyperglycemia (Grade3) | ||||
| Grade3 serum sickness | hypophosphatemia (Grade 3) | ||||
Abbreviations:
Ab: antibody
KD: antibody-antigen binding affinity as measured by surface plasmon resonance
MOA: mechanism of action
ADCC: antibody-dependent cell-mediated cytotoxicity
In vitro IC50: Drug concentrations causing 50% inhibition of tumor cell growth or cell adhesion (amatuximab). IC50 depends on the expression levels of antigen on the surface of tumor cells.
OV: ovarian cancer; PANC: pancreatic cancer; Meso: mesothelioma; Lung: lung cancer; BCa: breaset cancer; Gas: gastric cancer; NSCLC: non-small cell lung carcinoma
DLT: dose-limiting toxicity
CLS: capillary leak syndrome
MTD: maximal tolerated dose
1. Development of An Unconjugated Monoclonal Antibody Targeting Mesothelin: Amatuximab (MORAb-009)
Amatuximab is a mouse-human chimeric IgG1k monoclonal antibody with a binding affinity of 1.5 nM for human mesothelin. It is derived from a mouse antibody isolated by panning a phage display library made from splenic mRNA of a mouse immunized with mesothelin cDNA on mesothelin-positive cells [22]. The affinity of this mouse single-chain anti-mesothelin antibody was optimized and its variable regions were cloned and grafted in frame with human IgG1 and kappa constant regions, yielding amatuximab.
Amatuximab can block the binding of mesothelin to the tumor antigen CA125/MUC16. The crystal structure of the complex between the mesothelin N-terminal fragment and amatuximab Fab at 2.6 Å resolution revealed that amatuximab recognizes a non-linear epitope that is contained in the first 64-residue fragment of mesothelin [23]. It has been shown that the N-terminal 64-residue fragment of mesothelin is also responsible for CA125 recognition [24] and that this interaction can be interrupted by amatuximab [25]. Thus, overlapping binding sites on mesothelin for both CA125 and amatuximab provides a basis for the therapeutic effect of the antibody.
In vitro, amatuximab prevents adhesion of mesothelin-expressing tumor cells to CA125/MUC16-positive cells and can also elicit antibody-dependent cell-mediated cytotoxicity (ADCC) on mesothelin-bearing tumor cells [25]. In vivo, amatuximab as a single agent exerts modest anti-tumor activity against mesothelin-transfected A431-tumor xenografts, presumably via ADCC and inhibiting the mesothelin/CA125 mediated cell adhesion. This anti-tumor effect was markedly increased in combination with chemotherapeutic agents such as gemcitabine or paclitaxel [25].
Amatuximab cross-reacts with monkey mesothelin but does not bind rodent species (mouse and rat). Its staining patterns in human and in monkey tissues were similar, with staining observed only in mesothelia. Therefore, the potential toxicity was analyzed in monkeys. In a 23-day toxicology study, amatuximab was dosed to monkeys Q3D repeatedly 7 times at 2 mg/kg and 15 mg/kg. There was no adverse effect of amatuximab observed in monkeys. Pharmacokinetic analysis showed a dose-dependent increase in amatuximab AUC and Cmax and its half-life was estimated to be between 11.9 and 14.2 days [25].
Biodistribution and dosimetry of 111In-amatuximab in 6 patients with mesothelin-expressing cancers (4 with malignant mesothelioma and 2 with pancreatic adenocarcinoma) was recently evaluated using Single Photon Emission Computed Tomography-Computed Tomography (SPECT-CT) imaging [26]. 111In-amatuximab targeted mesothelin-expressing cancer cells, with a clinically meaningful uptake in both primary tumors and metastatic sites of both tumor types, although a higher uptake was noted in mesothelioma than pancreatic cancer. This is the first study to show tumor localization of an anti-mesothelin antibody in humans. 111In-amatuximab was well tolerated with a favorable dosimetry profile.
Hassan et al. conducted a Phase I clinical trial of amatuximab in 24 patients with advanced mesothelin-expressing cancers, including 13 mesothelioma, 7 pancreatic cancer, and 4 ovarian cancer [27]. Amatuximab was generally well tolerated by patients, albeit with frequent drug-related hypersensitivity reactions (grade 1-2) and dose-dependent incidence of immunogenicity. The single-agent maximum tolerated dose (MTD) of amatuximab given once weekly is 200 mg/m2. At the 400 mg/m2 dose level, 2 subjects experienced drug-related dose-limiting toxicities (grade 4 transaminitis and grade 3 serum sickness) [27]. The same single-agent MTD of 200 mg/m2 was reported in the Phase 1 study of amatuximab given weekly in 17 Japanese patients with mesothelioma or pancreatic cancer [28]. Amatuximab exposure in plasma increased in a dose-dependent fashion, with a half-life of approximately 10 days. This, together with the pharmacokinetic analysis in Cynomolgus monkeys mentioned above, supported the dosing regimen where amatuximab is given on 2 consecutive weeks (days 1 and 8) followed by one or two weeks off to minimize the risk of adverse events such as pericarditis and pleuritis. Although no objective partial tumor responses were reported in these studies, stable disease was observed [27, 28].
The antitumor activity and safety of amatuximab in combination pemetrexed and cisplatin was evaluated in a single-arm Phase II study in patients with unresectable malignant pleural mesothelioma (MPM) [29]. Amatuximab (5 mg/kg) was administered on days 1 and 8 with pemetrexed (500 mg/m2) and cisplatin (75 mg/m2) on day 1 of 21-day cycles for up to 6 cycles. Patients with clinical benefit (partial tumor response [PR] or stable disease [SD]) received amatuximab maintenance until disease progression. 89 patients received a median of five cycles (range, 1–6) of combination treatment, and 56 (63%) of patients received amatuximab maintenance. Combination therapy resulted in no overlapping toxicities, with 11 (12.4%) patients experiencing
Data are extracted from References #25; 27; 30; 38; 44; 46; 48 and 49.
amatuximab-related hypersensitivity reactions. The anti-tumor efficacy results for amatuximab included 33 (40%) patients with PR and 42 (51%) with SD. Due to the favorable safety profile and preliminary anti-tumor efficacy of amatuximab, confirmatory Phase II trials are ongoing at the time of preparation of this manuscript.
2. Development of Recombinant Immunotoxins (RIT) Targeting Mesothelin: SS1P and RG7787
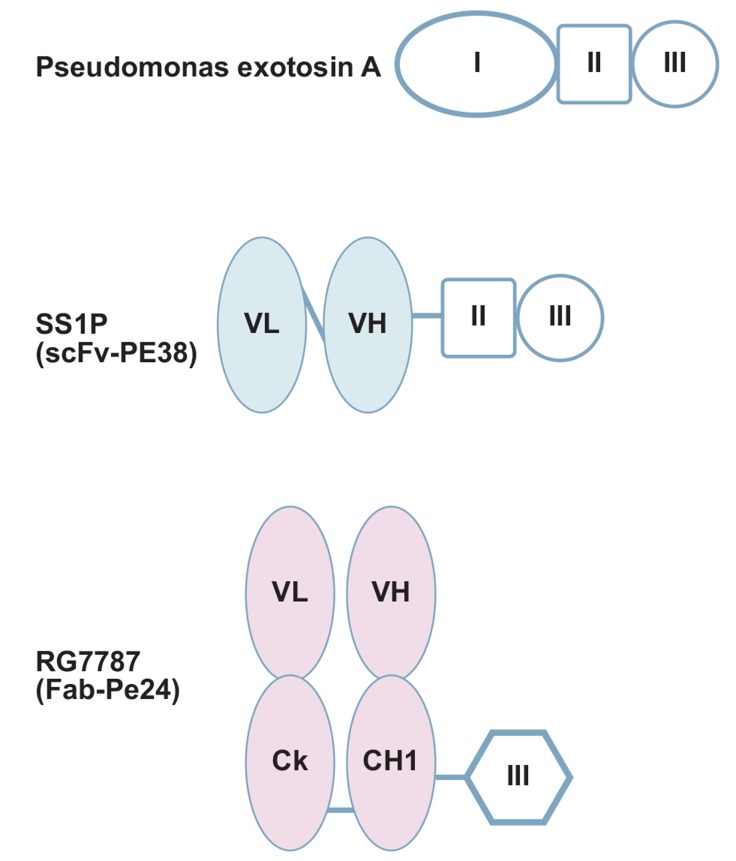
SS1P (anti-mesothelin dsFv-PE38) is a recombinant immunotoxin (RIT) engineered for the targeted elimination of cancer cells that express mesothelin [30]. It consists of a murine anti-mesothelin disulfide-stabilized single-chain Fv fragment (targeting moiety) linked to PE38 (effector moiety), the protein-synthesis-inhibiting domain of Pseudomonas exotoxin A (PE) (Fig. 2).
Fig. (2).
Domain structures of SS1P and RG7787. Pseudomonas exotoxin A (PE) contains binding domain I (oval), domain II (square) and catalytic domain III (circle). Immunotoxin SS1P is composed of murine anti-mesothelin disulfide-stabilized single-chain Fv fragment (light blue) linked to PE38 (domain II and domain III). New immunotoxin RG7787 consists of humanized anti-mesothelin Fab (pink) and deimmunized PE24 domain III.
After dsFv binding to mesothelin on the cancer cell surface, SS1P is internalized via receptor-mediated endocytosis, and traffics via the endocytic compartment and Golgi to the endoplasmic reticulum (ER). During this process, the toxin becomes separated from the dsFv by the action of furin. PE is subsequently transferred to the cytosol, where it ADP-ribosylates and inactivates elongation factor-2 (EF-2). This terminates protein synthesis and induces programmed cell death. Since the effector moiety is a toxin, not a drug, there is no concern of multiple-drug resistance (MDR) for SS1P. On the other hand, SS1P has the potential risk of immunogenicity and toxic reactions.
Since the same scFv (ss1Fv) as in amatuximab was used to create SS1P [22], the monovalent binding-affinity, recognition epitope, and species cross-reactivity of SS1P are identical to antibody amatuximab. However, SS1P has a size of 63-kDa and is rapidly cleared by the kidneys. This size has been deliberately chosen to limit vascular leak syndrome which is a common and severe adverse off-target effect of the PE toxin.
In vitro, SS1P is a potent anticancer agent as a single agent [31]. The IC50 of SS1P in inhibiting the growth of cultured A431/K5 tumor cells is 0.4 ng/ml (as comparison, paclitaxel: 7 ng/ml).
In vivo, SS1P at 0.2 mg/kg as a single agent showed a clear anti-tumor activity against mesothelin-expressing tumor xenografts [31]. This effect was markedly increased in combination with gemcitabine or paclitaxel, when tumor-bearing mice received a single dose of paclitaxel, followed by SS1P 24 hours later. The 24-hour interval was chosen because 24 hours after paclitaxel administration, there appears to be a decrease in interstitial fluid pressure in solid tumors [32] and a concomitant increase in immunoconjugate uptake [33].
SS1P as a single agent was first tested as a bolus (30-minute) I.V. injection in a phase I clinical trial of 34 patients with advanced mesothelin-expressing solid cancers that failed standard therapy (20 mesotheliomas, 12 ovarian and 2 pancreatic) [7]. The first 17 patients received SS1P QOD x6 doses (at 8/12/18/25 μg/kg) and the other 17 were treated with SS1P QOD x3 doses (at 25/35/45/60 μg/kg). The MTD of SS1P given QOD x6 was 18 μg/kg. The DLT was Grade 3 hypersensitivity (urticarial) and Grade 3 vascular leak syndrome. Because all the DLTs were observed after the fourth dose of SS1P, the protocol was amended to allow treatment of patients with 3 dose regimen, which allowed further dose escalation. The MTD of SS1P given QOD x3 was 45 μg/kg/dose. Pleuritis was the DLT observed in 2 of 2 patients at the 60 μg/kg/dose level and 1of 6 patients at the 45 μg/kg/dose level.
At the MTD of 45 μg/kg, SS1P as a single agent showed minor responses in 4 and disease stabilization in 19 patients [7]. The anti-tumor efficacy would have been limited by neutralizing antibodies against PE38, which developed in the majority (88%) of patients in the first treatment cycle. The half-life of SS1P in plasma at the MTD was approximately 8 hours [7].
SS1P as a single agent was subsequently tested as continuous infusion over 10 days in a phase I trial of 24 patients with advanced mesothelin-expressing solid cancers (16 mesotheliomas, 7 ovarian and 1 pancreatic) [34]. The DLT with this regimen was vascular leak syndrome, immunogenicity rate was high (75% of patients after the first cycle) and the anti-tumor activity was modest at the MTD of 25 μg/kg/d ×10. Overall, continuous infusion of SS1P had no significant benefit over the bolus injection in patients with refractory solid cancers.
In a Phase 1 study of advanced refractory malignant mesothelioma, Hassan et al. have shown that T- and B-lymphocyte-depleting immunosuppressive treatment with pentostatin and cyclophosphamide before and concurrent with SS1P has markedly delayed the development of neutralizing antibodies against SS1P given as single agent by bolus IV injection [35]. Although more treatment cycles were given with immunosuppression, this intervention did not appear to increase the anti-tumor activity of SS1P.
The preclinical potential for synergistic anti-tumor activity of SS1P with chemotherapy [31] was recently corroborated by a preliminary report from an ongoing Phase 1 study of SS1P given together with standard recommended doses of cisplatin/pemetrexed combination in the front-line treatment of 24 chemotherapy-naïve patients with advanced pleural mesothelioma [36]. Intra-patient dose escalation of SS1P was performed in 10 μg/kg increments from 25 μg/kg to 55 μg/kg given as bolus IV injection. SS1P in combination with cisplatin/pemetrexed was safe and well tolerated in this preliminary assessment with Grade 3 hypoalbuminemia, back pain and hypotension being the main toxicities associated with SS1P at the MTD of 45 μg/kg. Albeit in a small number of 13 evaluable patients, the combination exhibited significant anti-tumor activity at the MTD [10 (77%) had a partial response and 1 (7.7%) had stable disease); further, decreases from baseline in serum mesothelin, megakaryocyte potentiating factor (MPF) and CA125 levels correlated well with objective tumor response. The concurrent administration of chemotherapy did not reduce the immunogenicity, as SS1P-neutralizing antibodies were found in 90% of patients after 1 cycle of treatment. Modification of the SS1P structure is being considered to minimize its immunogenicity and allow for more extended administration.
The clinical use of SS1P is currently limited by its immunogenicity and a dose-limiting vascular leak syndrome (VLS). In an attempt to overcome these limitations, Weldon et al. reengineered SS1P with improved properties [37]. The new molecule, SS1-LR/GGS/8M, has cytotoxic activity comparable to SS1P on several mesothelin-expressing cell lines and remarkably improved activity on primary cells from patients with mesothelioma. In a mouse xenograft tumor model, high doses of SS1-LR/GGS/8M elicit anti-tumor activity superior to the activity of SS1P at its maximum-tolerated dose. In addition, SS1-LR/GGS/8M has greatly decreased ability to cause VLS in a rat model and reduced antigenicity or reactivity with antibodies to the sera of patients previously treated with SS1P.
To minimize immunogenicity, Hollevoet et al. also re-engineered the targeting moiety from mouse dsFv to humanized Fab and de-immunized the effector moiety PE24 to generate a new immunotoxin RG7787 [huSS1(Fab)-LR-GGS-LO10-PE24] [38]. Differences in domain structure between SS1P and RG7787 are shown in (Fig. 2). PE24 in RG7787 consists only of the catalytic domain III of PE, whereas SS1P contains the highly immunogenic wildtype 38-kD fragment (PE38) comprised of domain II and III of PE. Replacing domain II of PE in the RG7787 fusion protein by a furin cleavable peptide linker eliminates B-cell as well as major T-cell epitopes.
In vitro, RG7787 produced >95% cell killing of the HCC70 and SUM149 human breast cancer cell lines with IC50 < 100 pM [39]. RG7787 was also effective against gastric cancer cell lines MKN28, MKN45, and MKN74 with activity in the subnanomolar range. Furthermore, RG7787 was significantly more active than SS1P in GUMC108, KLM-1, and Panc 3.014 cells. The cell line GUMC108 was most sensitive, with RG7787 killing >99% of the cells [38].
In a nude mouse model, RG7787 treatment resulted in a statistically significant 41% decrease in volumes of HCC70 xenograft tumors (P < 0.0001) and an 18% decrease in MKN28 tumors (P < 0.0001) [39]. Pretreatment with paclitaxel (50 mg/kg i.p.) enhanced efficacy, producing 88% and 70% reduction in tumor volumes for HCC70 and MKN28, respectively, a statistically significant improvement over paclitaxel alone (P < 0.0001 for both). In a subcutaneous KLM-1 xenograft mouse model, 3 × 2.5 mg/kg RG7787 QOD combined with 1 × 50 mg/kg paclitaxel induced near-complete responses, with all tumors regressing below 5 mm3 within 30 days after therapy was initiated (>95% decrease) and no significant growth increase for at least another 3 weeks. RG7787 alone gave limited but significant regressions, and paclitaxel by itself arrested tumor growth [38].
To mimic the serum half-life of RG7787 as closely as possible to the serum half-life of SS1P, the targeting moiety is switched from a scFv to a Fab fragment to compensate the loss of molecular size of effector moiety caused by the elimination of domain II of PE (Fig. 2). Indeed, pharmacokinetic studies in rodents and monkeys demonstrated an identical half-life [39]. Interestingly, despite very comparable pharmacokinetic properties of the two molecules, RG7787 was found to be up to 10-fold better tolerated in different animal species. For instance, in female Wistar Furth rats, SS1P at 2 mg/kg caused massive hepatotoxicity and VLS, whereas even a 5-fold higher dose of RG7787 did not.
In conclusion, RG7787 has high cytotoxic activity on cancer cell lines as well as on primary patient cells. In vivo, this novel RIT gives durable near-complete tumor responses when combined with paclitaxel. RG7787 merits further evaluation for the treatment of triple-negative breast and gastric cancers [39]. A clinical phase I trial assessing safety, MTD and immunogenicity of RG7787 under the sponsorship of Roche and the NCI is currently under way.
3. Development of Antibody-drug Conjugates (ADCs) Targeting Mesothelin
A number of ADCs targeting mesothelin have been produced and are at various stages of clinical development. ADCs comprise whole IgGs covalently linked to cytotoxic drugs that are orders of magnitude more potent than chemotherapeutics. ADCs like prodrugs are inert in circulation and require internalization by the targeted cancer cells so that the antibody-linker is cleaved in order to release the active drug. Three pharmaceutical companies (Bayer, Roche/ Genentech, BMS/Medarex) are currently conducting clinical trials on anti-mesothelin ADCs. Although still in early clinical development, some promising results are observed.
3a. Anetumab Ravtansine (BAY 94-9343) By Bayer
At Bayer, fully human anti-mesothelin antibodies were identified by panning the HuCAL Gold human Fab-phage display library from MorphoSys [40]. Among 22 specific binders, the Fab MF-T was selected to move forward. MF-T has a mesothelin-binding affinity of 10 nM by surface plasmon resonance (SPR) and has been used for immunohistochemical evaluation of xenograft tumor sections and primary human cancer samples. MF-T also shows specific binding to NCI-H226 cancer cells by FACS. Importantly, when fluorescently-labeled MF-T was injected into tumor-bearing mice, it demonstrated higher accumulation in mesothelin-high tumors than in mesothelin-low tumors [40]. MF-T appears to be specific for human mesothelin. MF-T does not bind to mouse, rat, or monkey mesothelin.
The binding epitope of MF-T on mesothelin appears to be different from that of amatuximab or SS1P. It has been shown that the binding properties of MF-T are not altered by the presence of CA125 [41]. Thus, MF-T binding epitope does not overlap with the CA125 binding epitope on mesothelin. A detailed epitope mapping of antibody MF-T is still ongoing.
In collaboration with ImmunoGen, MF-T was conjugated (via lysine residues of the antibody) to the maytansinoid tubulin inhibitor DM4 through a hindered disulfide linker (SPDB) to produce the ADC anetumab ravtansine (BAY94-9343) [40]. Maytansinoids, of which maytansine is the parent compound, are a class of anti-mitotic agents that are 100- to 1000- fold more cytotoxic than conventional cancer chemotherapeutics agents [42]. This combination of a linker and toxophor has been shown to elicit bystander cytotoxic activity due to the formation of active maytansinoid metabolites that are able to diffuse from antigen-positive cancer cells into the neighboring cells [43]. The bystander killing activity of ADC is beneficial when its penetration into a solid tumor is limited or antigens are heterogeneously expressed on human tumors. Once anetumab ravtansine is bound and internalized by a tumor cell, degradation of the anetumab ravtansine linker in lysosomes releases cell-permeable DM4 metabolites. These DM4 metabolites induce cell-cycle arrest and subsequent cell death.
Anetumab ravtansine demonstrated potent and selective cytotoxicity on mesothelin-expressing cells with nanomolar IC50 (e.g. 0.71 nM for HT-29/meso; 1.59 nM for MIAPaCa-2/meso and OVCAR-3; and 5.72 nM for NCI-H226) [40]. While anetumab ravtansine showed antiproliferative activity in all mesothelin-positive tumor cell lines, mesothelin-negative tumor cells were not affected unless the ADC was present in micromolar concentrations. Moreover, anetumab ravtansine did not affect non-dividing mesothelin-positive primary peritoneal mesothelial cells, in accordance with DM4 mode of action, i.e. inhibition of microtubule dynamics. This data could suggest that its safety/toxicity profile would differ from that of SS1P. SS1P is toxic to all antigen-positive cells (ie. both dividing and non-dividing cells) due to the mode of action of PE38 (i.e. inhibiting protein synthesis and inducing programmed cell death).
The efficacy of anetumab ravtansine was evaluated in ovarian and mesothelioma tumor xenograft models as well as in patient-derived tumor models for ovarian cancer, pancreratic cancer, mesothelioma [40]. Treatment of HT-29/meso tumors with 11.2 mg/kg ADC (related to 0.224 mg/kg DM4) (Q3D x3) resulted in complete tumor eradication. Furthermore, anti-tumor activity in the endogenously mesothelin-expressing OVCAR3 tumor model was observed. Treatment with 2.8 mg/kg ADC Q3Dx3 inhibited tumor growth, and in 4 out of 6 animals complete tumor eradication was detected. The anti-tumor efficacy of the ADC correlated with the amount of mesothelin expressed and was generally superior to that of standard-of-care regimen resulting in complete tumor eradication in most of the tumor models. Notably, treatment with 0.2 mg/kg S-methyl-DM4 together with 11 mg/kg antibody MF-T did not affect tumor growth, indicating that antibody-directed delivery of cytotoxic drug to cancer cells by ADC is essential.
The bystander effect of ADC was demonstrated using a xenograft model with different proportions of mesothelin-positive and -negative cells within the inoculated tumors [40]. Anetumab ravtansine not only inhibited tumor growth, but also induced tumor regression even when only 20% of the cells within the tumor were mesothelin-positive. This strongly suggests that, in addition to the enhanced permeability and retention effect observed in the mesothelin-negative cells, the bystander effect contributes to the antitumor activity of anetumab ravtansine. Similar results have been reported on other ADCs previously by Kovtun and colleagues [43].
These preclinical data validate mesothelin as a cancer antigen for an ADC as therapeutic approach. Anetumab ravtansine is currently in phase 1 clinical evaluation for its safety, pharmacokinetics and tumor response in patients with advanced solid tumors, with a particular focus on patients with mesothelioma [44, 45].
Anetumab ravtansine was given IV (q3w) in 77 cancer patients: 45 patients in 10 dose escalation cohorts from 0.15 to 7.5 mg/kg (21 mesothelioma, 9 pancreatic, 5 breast, 4 ovarian, 6 other), and 32 patients in 2 expansion cohorts (12 mesothelioma and 20 ovarian); 38 patients were treated at MTD in escalation and expansion cohorts (16 mesothelioma, 21 ovarian, 1 breast). Dose-limiting toxicity (DLT) was observed at 7.5 mg/kg (DLTs: 1 patient with G2 keratitis and G3 neuropathy, 1 patient with G4 keratitis and G2 neuropathy). Anetumab ravtansine MTD was 6.5 mg/kg (DLT: G3 AST increase). No drug-related deaths and few drug-related SAEs (7 total and 5 at MTD) were reported. Seventeen of 38 (45%) patients total or 7 of 16 (44%) mesothelioma patients at MTD had drug-related AE requiring dose reduction (G1-4 keratitis, G2-3 neuropathy, G3 fatigue, anorexia, asthenia, diarrhea, AST increase). LFT increases were the most common drug-related laboratory abnormality at MTD: AST in 7 patients (2 G3), ALT in 6 patients (no G3), alkaline phosphatase in 4 patients (one G3) and bilirubin increase in 1 patient (no G3). There were no drug-related G3 hematological abnormalities at any dose. Fourteen of 38 (37%) patients total or 4 of 16 (25%) mesothelioma patients at MTD had G1-4 keratitis (worst G3-4 in 3 pts, blurred vision in 10, dose reduction in 8, dose delay in 11, all fully reversible). Anetumab ravtansine at the MTD showed a PR in 6 patients (19%) and SD in 18 patients (47%) overall. At the MTD, all drug-related adverse events were reversible and non-life-threatening but required dose reduction in about half of patients, most commonly due to G1-4 keratitis and G2-3 peripheral neuropathy. Given this benefit-risk ratio, the recommended phase II dose of anetumab ravtansine in second line treatment of advanced mesothelioma is 6.5 mg/kg IV q3w.
3b. ADC h7D9.v3-mc-vc-PAB-MMAE By Roche/Genentech
At Genentech, an anti-mesothelin ADC was made using the ADC technology license from Seattle Genetics [46]. The mesothelin-binding and internalizing humanized antibody 7D9 was conjugated to the drug MMAE (monomethyl auristatin E, a synthetic analog of the naturally occurring microtubule-disrupting agent, dolastatin) with a lysosomal protease-cleavable valine-citrulline dipeptide linker.
Mouse anti-human mesothelin monoclonal antibodies were produced using hybridoma technology. The antibody 7D9 with a mesothelin-binding affinity of 0.23 nM as measured by SPR was selected for conjugation because it internalized to lysosomes to a great extent [46]. Internalization, however, was relatively slow, with little uptake before 6 hours and significant surface signal remaining after overnight incubation.
As a conjugation site reduced cysteines of the IgG were chosen. Chimeric 7D9 antibody was first conjugated via the protease-sensitive linker maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl (mc-vc-PAB) to MMAE. Upon internalization the dipeptide linker valine-citrulline is recognized and cleaved by the lysosomal enzyme cathepsin and then free MMAE is released from the ADC. It has been reported that the intracellular concentration of released MMAE from ADCs could reach >400 nM within 24 hours of treatment in antigen-positive cancer cells [47]. MMAE potently inhibits tubulin polymerization leading to apoptotic cell death. Moreover, MMAE, being membrane permeable, is able to exert cytotoxic activity on neighboring antigen-negative cancer cells.
To compare different linkers and drugs in the ADC 7D9-mc-vc-PAB-MMAE, the 7D9 antibody was also conjugated to the relatively impermeable auristatin MMAF, and to uncleavable maleimidocaproyl-MMAF (mc-MMAF) [46]. Conjugation of both prodrugs did not affect 7D9 binding, and all 3 ADCs exhibited anti-proliferative activity against OVCAR3 cancer cells in vitro, but their efficacy differed significantly in vivo [46].
In vivo, cleavable 7D9-mc-vc-PAB-MMAE was more efficacious than both MMAF ADCs against OVCAR3 tumors [46]. Their in vivo potency is ranked as follows: 7D9-mc-vc-PAB-MMAE>7D9-mc-vc-PAB-MMAF>7D9-mc-MMAF. The uncleavable maleimidocaproyl-MMAF (mc-MMAF) was least potent. This may be due to its lysosomal metabolite Cys-mc-MMAF having a slower diffusion into the cytoplasm to exert cytotoxic effects than the free MMAF or MMAE resulting from the valine-citrulline cleavage. The most potent ADC is 7D9-mc-vc-PAB-MMAE since its lysosomal metabolite, the free MMAE, has the ability to kill neighboring cells. In contrast, the free MMAF has poor membrane permeability and is lacking a bystander effect. Therefore, the humanized 7D9 antibody (h7D9.v3) was conjugated to MMAE via the protease-sensitive cleavable linker mc-vc-PAB and selected for development.
The h7D9.v3 antibody retained high affinity for mesothelin binding, ranging from 0.4 to 3 nM as measured by Scatchard analysis of 0.5+ to 3+ mesothelin-expressing cells. The unconjugated antibody (h7D9.v3) had no effect on cancer cell proliferation up to 100 nM on any cell lines tested. In contrast, the free MMAE kills cancer cells with an IC50 in the range of 0.17-0.2 nM. The ADC h7D9.v3-mc-vc-PAB-MMAE inhibited the proliferation of mesothelin-expressing cells with an IC50 of 0.32 nM to 20 nM depending on the levels of mesothelin on cancer cells [46].
A phase I study of ADC DMOT4039A [ADC composed of a humanized IgG1 anti-mesothelin antibody and monomethyl auristatin E (MMAE)] is ongoing in patients with unresectable pancreatic (PANC) or platinum-resistant ovarian cancer (OV) [48]. DMOT4039A (0.2-2.8 mg/kg) was given q3w to cancer patients. Anti-tumor response was evaluated per RECIST 1.1, and serum CA19-9 or CA125. Tumor MSLN expression was determined by IHC. As of 5 Dec 2013, 49 patients have enrolled (30 PANC; 19 OV) and received a median of 3 (range 1-14) cycles of DMOT4039A. The MTD was 2.4 mg/kg, q3w, with 2 DLTs at the maximum dose level of 2.8 mg/kg: hyperglycemia (G3) and hypophosphatemia (G3). ADC exposure was dose-proportional over all dose levels. Four patients (8%) had a confirmed partial response (PR). One PANC patient without RECIST response showed > 50% decrease from baseline in CA19-9 levels; 7 OV patients showed > 50% decrease in CA125. Mesothelin expression was IHC 2+/3+ in 64% of PANC patients and 91% of OV patients, in part due to diagnostic selection for enrollment in expansion cohorts. All patients with OV with RECIST/CA125 responses were IHC 2/3+. ADC DMOT4039A, at 2.4 mg/kg q3w, showed clinical activity in both PANC and OV cancers expressing mesothelin levels at IHC 2/3+, with a tolerable safety profile.
3c. ADC MDX-1382/duocarmycin By BMS/Medarex
At Medarex, fully human anti-mesothelin monoclonal antibodies have been generated in transgenic mice [49, 50], selected for high binding affinity and their ability to be rapidly internalized for drug delivery [49]. Recombinant soluble human mesothelin-His tag protein (40-kDa) was purified and used as the immunogen to immunize the transgenic mice intraperitonealy and subcutaneously. Hybridomas producing human monoclonal antibodies recognizing mesothelin protein were identified by ELISA screening. Three antibodies (3C10, 6A4, 7B1), all hIgG1/kappa were selected. They bind mesothelin protein with an affinity of ~5 nM as measured by SPR. All three antibodies bind effectively to cell-surface human mesothelin as determined by FACS [49]. They show EC50s in sub nM range for binding to 4 different cancer cell lines (OVCAR3, NCI-H226, CFPAC-1, and KB). The 6A4 antibody was internalized by all four cell lines. This antibody exhibits the ability to inhibit the interaction of mesothelin with CA125, indicating that its binding blocks the mesothelin-CA125 interaction region. It was also shown that the 6A4 monoclonal antibody exhibits ADCC activity against a cell line expressing mesothelin on its cell surface [49].
In 2008, Medarex disclosed that “fully human anti-mesothelin antibody drug conjugate demonstrates anti-tumor effects in human lung cancer models.” in the Second AACR Centennial Conference on Translational Cancer Medicine [51]. The anti-mesothelin antibodies were conjugated to DNA alkylating agents and tested in vivo. A single dose of 0.3 μmole/kg of ADC resulted in complete tumor regression in the NCI-H226 xenograft model of lung cancer & mesothelioma. The dose as μmole/kg is likely calculated using molecular weight of SMOL drug not the antibody (150,000Da).
In 2009, a detailed description of the ADC was presented at the AACR Conference [52]. The ADC MDX-1204 consists of a fully human anti-mesothelin antibody (MDX-1382) conjugated to a potent DNA alkylating agent related to duocarmycin (MED2460) via a cleavable valine-citrulline dipeptide linker. MED-2460 is a small molecule which itself is composed of the releasable prodrug MED-2284, a cleavable di-peptide linker, and a maleimide reactive group intended to facilitate protein attachment at -SH group. This ADC shows anti-tumor efficacy in xenograft models of ovarian, lung and pancreatic cancer at doses as low as 0.3 μmole/kg. Since MDX-1382 cross-reacts with monkey mesothelin (but not mouse mesothelin) with similar affinity and tissue distribution as in humans, ADC toxicity was assessed in monkeys. This ADC was well tolerated in Cynomolgus monkeys and did not result in overt clinical or histological signs of toxicity when dosed intravenously at 0.4 μmole/kg.
The duocarmycin class of DNA minor groove-binding alkylating agents represents synthetic and highly potent toxophors. They bind to the minor groove of DNA and subsequently cause irreversible alkylation of DNA. This disrupts the nucleic acid architecture, which eventually leads to cell death. They are able to exert their mode of action at any phase in the cellular cycle, whereas tubulin inhibitors will only kill dividing cells.
Bristol-Myers Squibb Company initiated in June-2015 a phase I/IIa study of a mesothelin-directed ADC (BMS-986148) in subjects with select advanced solid tumors (mesothelioma, non-small cell lung cancer, ovarian cancer, pancreatic cancer and gastric cancer) [53]. This study is to determine the safety, tolerability, pharmacokinetics, immunogenicity, anti-tumor activity and pharmacodynamics of BMS-986148 (NCT Identifier: NCT02341625). It is not clear if BMS-986148 is related to MDX-1204.
Conclusion
Mesothelin has been validated as a suitable antibody target for cancer therapy. A number of novel antibody-based therapeutics targeting mesothelin have been developed in recent years to treat solid tumors. They are in various stages of preclinical and clinical development.
The unconjugated antibody against mesothelin (amatuximab) has a favorable safety profile, but its therapeutic efficacy (mainly via antibody effector function ADCC and cell adhesion inhibition) is not profound as a single agent. Nevertheless, combination of amatuximab with chemotherapy show clinical activity in phase II trials. This finding encourages to perform additional phase II studies of combination therapy of amatuximab with various chemotherapeutic agents in different solid tumors.
As demonstrated in preclinical and clinical studies, both RITs and ADCs targeting mesothelin are more potent in anti-tumor activity than unconjugated antibody due to their different mechanism of action and the nature of the conjugated cytotoxic toxins or drugs. A combination of RIT or ADC with chemotherapy could further improve their anti-tumor activities.
The therapeutic efficacy of RIT or ADC is determined by many complex factors, besides cytotoxic potency of the payload conjugated to the antibody. These factors include antibody binding kinetics, antigen expression density, antigen recycling or resynthesis rate, tumor-penetrating capacity, internalization efficiency, linker chemistry, conjugate processing and retention in tumors, payload release kinetics, bystander effects of free drug or metabolites and etc. Compared to RITs like SS1P immunotoxin, ADCs appear to have the following advantages. 1) ADCs appear to be less immunogenic, allowing multiple dosing cycles and enabling maximal efficacy; 2) ADCs have longer circulating half-lives than RITs, thus increasing therapeutic exposure and resulting in less frequent dosing; 3) Anti-mitotic activity of toxophors in ADCs like DM4 and MMAE confers cytotoxicity on dividing cancer cells over non-dividing cells, so that ADCs should have a wider therapeutic window than RITs.
On the other hand, some adverse effects are associated with clinically validated anti-mitotic agents delivered as ADCs. The main toxicities with ADCs conjugated to microtubule inhibitors (MMAE or DM1) were observed in the bone marrow, as evidenced by decreases in neutrophils, lymphocyte, reticulocytes, and platelets, and in liver [54, 55].
Ocular toxicity was also reported with ADCs containing DM4 [56]. Although the mechanism(s) of ocular toxicity is unknown, similar findings of corneal epitheliopathy with microcystic appearance have been described in association with another ADC, SGN-75, which is a humanized anti-CD70 antibody conjugated to the monomethyl auristatin F (MMAF), via a maleimidocaproyl (mc) linker. In this study, the ocular toxicity correlated with exposure of SGN-75 ADC but not that of the free cytotoxic molecule, Cys-mc-MMAF [57]. While some patients may have experienced a significant shift in visual acuity, the ocular toxicity was reversible for most patients in both cases (DM4-ADC and MMAF-ADC) and manageable with supportive care, including steroid eye drops and dose delays.
In conclusion, novel antibody therapeutics (mAbs, RITs, ADCs) targeting mesothelin exert anti-tumor activities by different mechanisms of action. Based on the convincing preclinical data generated with these molecules, the antibody therapeutics have been brought into early clinical evaluation. There, first promising results were obtained. Based on their different mechanisms of action these antibody therapeutics are expected to have different safety profiles. Further clinical development will reveal which of these molecules shows the best efficacy and widest therapeutic window and thus is best suited to bring benefit to the patients.
ACKNOWLEDGEMENTS
The authors thank Bing Liu (a current employee of Bayer Pharmaceuticals) for her technical support for Figure 1 of this article.
CONFLICT OF INTEREST
All authors are employees of Bayer Pharmaceuticals during writing this manuscript.
REFERENCES
- 1.Jarboe J., Gupta A., Saif W. Therapeutic human monoclonal antibodies against cancer. Methods Mol. Biol. 2014;1060:61–77. doi: 10.1007/978-1-62703-586-6_4. [DOI] [PubMed] [Google Scholar]
- 2.Weiner G.J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer. 2015;15(6):361–370. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiseman G.A., White C.A., Stabin M., et al. Phase I/II 90Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) radioimmunotherapy dosimetry results in relapsed or refractory non-Hodgkin's lymphoma. Eur. J. Nucl. Med. 2000;27(7):766–777. doi: 10.1007/s002590000276. [DOI] [PubMed] [Google Scholar]
- 4.Kaminski M.S., Estes J., Zasadny K.R., et al. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96(4):1259–1266. [PubMed] [Google Scholar]
- 5.Madhumathi J., Devilakshmi S., Sridevi S., Verma R.S. Immunotoxin therapy for hematologic malignancies: where are we heading? Drug Discov. Today. 2015 doi: 10.1016/j.drudis.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Alewine C., Hassan R., Pastan I. Advances in Anticancer Immunotoxin Therapy. Oncologist. 2015;20:176–185. doi: 10.1634/theoncologist.2014-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan R., Bullock S., Premkumar A., et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin. Cancer Res. 2007;13(17):5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 8.Shan L., Liu Y., Wang P. Recombinant Immunotoxin Therapy of solid tumors: challenges and strategy. J. Basic Clin. Med. 2013;2(2):1–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Leal M., Sapra P., Hurvitz S.A., et al. Antibody–drug conjugates: an emerging modality for the treatment of cancer. Ann. N. Y. Acad. Sci. 2014;1321:41–54. doi: 10.1111/nyas.12499. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton G.S. Antibody-drug conjugates for cancer therapy: The technological and regulatory challenges of developing drug-biologic hybrids. Biological. 2015;43(5):318–332. doi: 10.1016/j.biologicals.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Chang K., Pai L.H., Batra J.K., Pastan I., Willingham M.C. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res. 1992;52:181–186. [PubMed] [Google Scholar]
- 12.Chang K., Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho M., Onda M., Wang Q.C., Hassan R., Pastan I., Lively M.O. Mesothelin is shed from tumor cells. Cancer Epidemiol. Biomarkers Prev. 2006;15:1751. doi: 10.1158/1055-9965.EPI-06-0479. [DOI] [PubMed] [Google Scholar]
- 14.Scholler N., Fu N., Yang Y.I., Ye Z., Goodman G.E., Hellström K.E., Hellström I. Soluble member(s) of the mesotheline-megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc. Natl. Acad. Sci. USA. 1999;96:11531–11536. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollevoet K., Reitsma J.B., Creaney J., et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta-analysis. J. Clin. Oncol. 2012;30:1541–1549. doi: 10.1200/JCO.2011.39.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creaney J., Dick I.M., Robinson B.W. Discovery of new biomarkers for malignant mesothelioma. Curr Pulmonol Rep. 2015;4(1):15–21. doi: 10.1007/s13665-015-0106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rump A., Morikawa Y., Tanaka M., et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004;279(10):9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 18.Chen S.H., Hung W.C., Wang P., Paul C., Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci. Rep. 2013;3:1870. doi: 10.1038/srep01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan R., Bera T., Pastan I. Mesothelin: a new target for immunotherapy. Clin. Cancer Res. 2004;10(12 Pt 1):3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 20.Ho M., Bera T.K., Willingham M.C. Mesothelin expression in human lung cancer. Clin. Cancer Res. 2007;13(5):1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X.Y., Liu H.L., Liu B., Willuda J., Siemeister G., Mahmoudi M., Dinter H. Tomoregulin internalization confers selective cytotoxicity of immunotoxins on prostate cancer cells. Transl. Oncol. 2008;1(2):102–109. doi: 10.1593/tlo.08124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury P.S., Viner J.L., Beers R., Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc. Natl. Acad. Sci. USA. 1998;95:669–674. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J., Tang W.K., Esser L., Pastan I., Xia D. Recognition of mesothelin by the therapeutic antibody MORAb-009: structural and mechanistic insights. J. Biol. Chem. 2012;287(40):33123–33131. doi: 10.1074/jbc.M112.381756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko O., Gong L., Zhang J., Hansen J.K., Hassan R., Lee B., Ho M. A binding domain on mesothelin for CA125/MUC16. J. Biol. Chem. 2009;284(6):3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan R., Ebel W., Routhier E.L., et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 26.Lindenberg L., Thomas A., Adler S., et al. Safety and biodistribution of 111In-amatuximab in patients with mesothelin expressing cancers using single photon emission computed tomography-computed tomography (SPECT-CT) imaging. Oncotarget. 2015;6(6):4496–4504. doi: 10.18632/oncotarget.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan R., Cohen S.J., Phillips M., et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin. Cancer Res. 2010;16:6132–6138. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujisaka Y., Kurata T., Tanaka K., et al. Phase I study of amatuximab, a novel monoclonal antibody to mesothelin, in Japanese patients with advanced solid tumors. Invest. New Drugs. 2015;33(2):380–388. doi: 10.1007/s10637-014-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan R., Kindler H.L., Jahan T., et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin. Cancer Res. 2014;20(23):5927–5936. doi: 10.1158/1078-0432.CCR-14-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiter Y., Pastan I. Antibody engineering of recombinant Fv immunotoxins for improved targeting of cancer: disulfide-stabilized Fv immunotoxins. Clin. Cancer Res. 1996;2(2):245–252. [PubMed] [Google Scholar]
- 31.Zhang Y., Xiang L., Hassan R., et al. Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin. Cancer Res. 2006;12(15):4695–4701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]
- 32.Griffon-Etienne G., Boucher Y., Brekken C., Suit H.D., Jain R.K. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59(15):3776–3782. [PubMed] [Google Scholar]
- 33.Holden S.A., Lan Y., Pardo A.M., Wesolowski J.S., Gillies S.D. Augmentation of antitumor activity of an antibody-interleukin 2 immunocytokine with chemotherapeutic agents. Clin. Cancer Res. 2001;7(9):2862–2869. [PubMed] [Google Scholar]
- 34.Kreitman R.J., Hassan R., FitzGerald D.J., Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin. Cancer Res. 2009;15(16):5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan R., Miller A.C., Sharon E., et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci. Transl. Med. 2013;5(208):208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan R., Sharon E., Thomas A., et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer. 2014;120(21):3311–3319. doi: 10.1002/cncr.28875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weldon J.E., Xiang L., Zhang J., et al. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol. Cancer Ther. 2013;12(1):48–57. doi: 10.1158/1535-7163.MCT-12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollevoet K., Mason-Osann E., Liu X.F., Imhof-Jung S., Niederfellner G., Pastan I. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol. Cancer Ther. 2014;13(8):2040–2049. doi: 10.1158/1535-7163.MCT-14-0089-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alewine C., Xiang L., Yamori T., Niederfellner G., Bosslet K., Pastan I. Efficacy of RG7787, a next-generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol. Cancer Ther. 2014;13(11):2653–2661. doi: 10.1158/1535-7163.MCT-14-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golfier S., Kopitz C., Kahnert A., et al. Anetumab ravtansine - a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol. Cancer Ther. 2014;13(6):1537–1548. doi: 10.1158/1535-7163.MCT-13-0926. [DOI] [PubMed] [Google Scholar]
- 41.Kahnert A., Light D., Schneider D., et al. Anti-mesothelin antibodies and uses thereof. US Patent 12/744,849. 2008 [Google Scholar]
- 42.Kupchan, et al. Maytansine, a novel anti-leukemic ansa macrolide from Maytenus ovatus. J. Am. Chem. Soc. 1972;94(4):1354–1356. doi: 10.1021/ja00759a054. [DOI] [PubMed] [Google Scholar]
- 43.Kovtun Y.V., Audette C.A., Ye Y., et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66:3214–3221. doi: 10.1158/0008-5472.CAN-05-3973. [DOI] [PubMed] [Google Scholar]
- 44.Bendell J., Blumenschein G., Zinner R., et al. First-in-human phase I dose escalation study of a novel anti-mesothelin antibody drug conjugate (ADC), BAY 94-9343, in patients with advanced solid tumors.; Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6-10; Washington, DC. 2013. [Google Scholar]
- 45.Hassan, et al. Phase I Study of Anti-Mesothelin Antibody Drug Conjugate Anetumab Ravtansine (ID 1574; Oral presentation; 16th World Conference on Lung Cancer WCLC 2015 in Denver; September 6 - 9, 2015; Colorado. [Google Scholar]
- 46.Scales S.J., Gupta N., Pacheco G., et al. An anti-mesothelin-monomethyl auristatin e conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol. Cancer Ther. 2014;13(11):2630–2640. doi: 10.1158/1535-7163.MCT-14-0487-T. [DOI] [PubMed] [Google Scholar]
- 47.Okeley NM, Miyamoto JB, Zhang X, et al. Intracellular activation of SGN-35, a potent anti-CD30 actibody-drug conjugate. Clin Cancer Res. 2010;1693:888–97. doi: 10.1158/1078-0432.CCR-09-2069. [DOI] [PubMed] [Google Scholar]
- 48.Weekes C.D., Lamberts L.E., Borad M.J., et al. A phase I study of DMOT4039A, an antibody-drug conjugate (ADC) targeting mesothelin (MSLN), in patients (pts) with unresectable pancreatic (PC) or platinum-resistant ovarian cancer (OC). J. Clin. Oncol. 2014;32:5s. doi: 10.1158/1535-7163.MCT-15-0693. [DOI] [PubMed] [Google Scholar]
- 49.Terrett JA, Pogue SL, Toy K, Yang L, Rao-Naik C, Chen B. Human antibodies that bind mesothelin, and uses thereof. WO 2009045957. A1, PCT/US2008/078123. 2009.
- 50.Lonberg N. Human antibodies from transgenic animals. Nat. Biotechnol. 2005;23(50):1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- 51.Pogue S., Chen X., Alexander S., et al. Fully human antimesothelin antibody drug conjugate demonstrates antitumor effects in human lung cancer models.; Second AACR Centennial Conference on Translational Cancer Medicine; 2008. [Google Scholar]
- 52.Rao C., Huber M., Vemuri K., Zhang Q., Chen B., Phillips J. Efficacy and toxicity of an anti-mesothelin antibody drug conjugate.; Proc Am Assoc Cancer Res; 2009. [Google Scholar]
- 53.Bononi A., Napolitano A., Pass H.I., Yang H., Carbone M. A Phase I/IIa Study of BMS-986148 in Subjects With Select Advanced Solid Tumors. https://clinicaltrials.gov/show/NCT02341625 .
- 54.Poon K.A., Flagella K., Beyer J., et al. Preclinical safety profile of trastuzumab emtansine (T-DM1): Mechanism of action of its cytotoxic component retained with improved tolerability. Toxicol. Appl. Pharmacol. 2013;273:298–313. doi: 10.1016/j.taap.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Li D., Poon K.A., Yu S.F., et al. DCDT2980S, an anti- CD22-monomethyl auristatin E antibody-drug conjugate, is a potential treatment for non-Hodgkin lymphoma. Mol. Cancer Ther. 2013;12:1255–1265. doi: 10.1158/1535-7163.MCT-12-1173. [DOI] [PubMed] [Google Scholar]
- 56.Younes A., Kim S., Romaguera J., et al. Phase I multidose-escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J. Clin. Oncol. 2012;30(22):2776–2782. doi: 10.1200/JCO.2011.39.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tannir N.M., Forero-Torres A., Ramchandren R., et al. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Invest. New Drugs. 2014;32(6):1246–1257. doi: 10.1007/s10637-014-0151-0. [DOI] [PubMed] [Google Scholar]