Abstract
Background
We conducted a phase I trial of cisplatin-pemetrexed-imatinib mesylate, an oral PDGFR inhibitor, in chemo-naive MPM patients.
Methods
A standard 3+3 dose-escalating trial was used with the endpoints of MTD, response rate, survival, safety/toxicity and tumor PDGFR levels.
Results
17 MPM patients were enrolled. The most common (any grade) side effects were nausea, fatigue, hypomagnesemia, and anemia. The MTD was established at dose level 3 (imatinib 600 mg) with the DLT nausea and vomiting. The median PFS was 7.9 months and median OS was 8.8 months. Sarcomatoid patients have worse PFS (p=0.01) and OS (p=0.009), while better ECOG PS 0–1 predicted for improved OS (p=0.001) and PFS (p=0.013). The 6 patients who completed all 6 treatment cycles had better OS (p=0.006), their median PFS was 9.6 months and OS was 22.4 months. In the translational studies, 14 patients had adequate tumor tissue that could be assessed for IHC and FISH. Patients with higher than median p- PDGFRα IHC expression had a better OS (p=0.013). When assessed as a continuous variable, higher p-PDGFRα in tumor cells correlated with an improved OS (p=0.045). None of the other 8 IHC biomarkers were predictive or prognostic for survival. Twelve patients had successful PDGFRB FISH results, but none met the criteria of ≥ 4 copies of PDGFRB gene, thus a correlation with clinical outcomes could not be done.
Conclusions
Cisplatin-pemetrexed-imatinib mesylate has clinical benefit in some MPM patients but was not well-tolerated. Further investigation into alternative anti-angiogenic agents, including PDGFRα inhibitors is warranted.
Keywords: imatinib mesylate, pemetrexed, cisplatin, mesothelioma, PDGFR
Introduction
Unresectable malignant pleural mesothelioma (MPM) is a challenging disease with few treatment options. The median survival ranges between 9–12 months with systemic chemotherapy response rates occurring between 3% to 30%.1 The current standard of care frontline systemic chemotherapy is cisplatin-pemetrexed, which showed superiority over cisplatin monotherapy in a randomized trial of 456 unresectable patients.2 This study reported improvement in the combination arm in median overall survival (12.1 months vs. 9.3 months; p=0.02) and response rate (41.3% vs. 16.7%, p<0.0001).2 Although this regimen was an advance in the field, the survival statistics remain dismal and novel agents and new methods of drug delivery are needed to optimize treatment for this disease.
Imatinib mesylate (STI-571, Gleevec, Novartis Pharma AG, Basel, Switzerland) is a 2-phenylaminopyrimidine, a highly selective inhibitor of platelet derived growth factor receptor (PDGF-Rα,β).3,4 The PDGF/PDGF-R pathway activates several downstream signaling cascades (i.e. c-Src, phospholipase C-γ, phosphotidylinositol-3-kinase/AKT, vascular endothelial growth factor, and Grb2/Sos) that ultimately induce cell proliferation, cell morphology changes, and anti-apoptosis.5 MPM tumor cells express protein PDGF-BB and PDGF-Rβ and PDGF has been shown to increase cell proliferation in vitro by murine xenografts with potential autocrine stimulation.6–11 It is theorized that the PDGF pathway has autocrine stimulation in malignant mesothelioma cells.7,9 In addition, inhibition of PDGF/PDGF-R pathway enhances tumor vascular uptake of chemotherapy by decreasing tumor interstitial fluid pressure (IFP).12–15
Although prior studies16,17 of single agent imatinib mesylate in MPM largely failed to show significant response rates, preclinical mesothelioma and solid tumor studies report synergistic benefit with the combination of imatinib mesylate and chemotherapy.12,18,19 Early solid tumor phase I trials also reported anecdotal responses with the combination of imatinib and chemotherapy in MPM.20 We hypothesized that inhibition of PDGF pathway in combination with cisplatin-pemetrexed in chemo-naive MPM patients would show a clinical benefit and embarked on a phase I clinical trial. For the correlative studies, we saught to identify any predictive biomarkers from the PDGFR pathway by immunohistochemistry and fluorescent in situ hybridization on the baseline tumor tissue. We postulated that overexpression of PDGFR pathway proteins or PDGFRB gene amplification would correlate to better clinical outcomes.
Materials and Methods
A standard phase I 3+3 study design (see Table 1) evaluated daily imatinib mesylate with every 4 week (1 cycle = 28 days) intravenous cisplatin-pemetrexed for a maximum of 6 cycles of therapy. Imatinib mesylate was given orally daily 1 week before the cisplatin and pemetrexed was administered. Cisplatin and pemetrexed was given every 28 days (+/− 4 days). Patients continued on this regimen until disease progression, unacceptable side effects, or withdrawal of consent. Patients received a maximum of 6 cycles of cisplatin and pemetrexed. Patients will stop taking imatinib mesylate 28 days (+/− 4 days) after completion of 6 cycles of therapy or immediately if they develop progressive disease.. There was no maintenance therapy given on this trial. Eligible patients had unresectable MPM (any histology), chemo-naive, ECOG PS 0–2. The primary endpoint was maximum tolerated dose (MTD); secondary endpoints included response rate (RR), progression-free and overall survival, safety/toxicity, and tumor biomarker results. The dose-limiting toxicity (DLT) was defined by National Cancer Institute Common Toxicity Criteria Version 3. Dose-limiting toxicities (DLT) are defined as (1) febrile neutropenia (fever > grade 2 with grade 4 neutropenia and requiring IV antibiotics); (2) grade 4 neutropenia (ANC < 500/μL) for more than seven days duration; (3) grade 4 thrombocytopenia; (4) grade 3 or 4 non-hematologic toxicity (except alopecia). The MTD was the highest dose level in which 6 patients had ≤ 2 instances of DLT. No intra-patient dose escalations were allowed. Clinical responses were assessed by RECIST21 every 8 weeks by CT scans. Progression-free survival, overall survival, and response duration were estimated using Kaplan-Meier method. Log-rank tests were used to conduct univariate analyses for categorical variables and Cox proportional hazards models were used for univariate analysis of continuous biomarkers. Adjustment for covariates in a multivariable model was not done due to small sample size.
Table 1.
The standard “3+3” design was applied with 6 pre-defined dose levels of imatinib mesylate, cisplatin and pemetrexed
| Dose levels | −2 | −1 | 0 | +1 | +2 | +3 |
|---|---|---|---|---|---|---|
| Imatinib mesylate (mg) | 300 | 300 | 300 | 300 | 400 | 600 |
| Cisplatin (mg/m2) | 60 | 60 | 60 | 75 | 75 | 75 |
| Pemetrexed (mg/m2) | 350 | 400 | 500 | 500 | 500 | 500 |
Baseline tumor tissue specimens were obtained and evaluated for immunohistochemistry (IHC) biomarkers (PDGFRα, PDGFRβ, p-PDGFRα, p-PDGFRβ) in the tumor, nucleus, stroma and fluorescence in situ hybridization (FISH) for PDGFRβ gene amplification. The methodology for the IHC and FISH has been previously published.22,23
Immunohistochemistry
We performed standard IHC studies of 3 biomarkers and localized them to the stromal, nucleus, and membrane: PDGFRβ, p-PDGFRβ, and PDGFRα. The histology sections were incubated with primary antibodies PDGFR-β (P-20, dilution 1:300, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), p-PDGFRβ (Tyr 1021, dilution 1:400, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 65 min, and PDGFRα (dilution 1:100, Cell Signaling Technology, Danvers, MA) for 90 min, at room temperature. Tissue sections were then incubated with the secondary antibody (EnVision Dual Link+; DAKO, Carpinteria, CA) for 30 min, after which diaminobenzidine chromogen was applied for 5 min. Tumor malignant cells and stroma were evaluated using the same methodology for PDGFRβ, p-PDGFRβ, and PDGFRα protein expressions. Briefly, for each marker, two experienced thoracic pathologists (I.I.W. and J.F.) examined both the intensity and extent of immunostaining by light microscopy using a ×20 magnification objective. Immunoreactivity of all antibodies was detected in the cytoplasm22 of malignant cell and stroma fibroblast, and p- PDGFRβ was detected in both the cytoplasm and nucleus. Cytoplasmic or nuclear expressions were quantified using a four-value intensity score (0, none; 1+, weak; 2+, moderate; and 3+, strong) and the percentage (0–100%) of the extent of reactivity. A final expression score was obtained by multiplying the intensity and reactivity extension values (range, 0–300).22
Fluorescence in situ hybridization (FISH) for analysis of gene copy number gain (CNG)
In addition, PDGFRB gene copy CNG was evaluated in tumor tissue. We analyzed the gene copy number per cell using a dual-color FISH assay. The PDGFRB probe was prepared from the Vysis LSI CSF1R probe (5q33-34) SpectrumOrange (Abbott Molecular, Illinois). A similarly probe Vysis LSI D5S23, D5S721 SpectrumGreen Probe (Abbott Molecular, Illinois) mapping to chromosome 5p was used as an internal control. The 4 μm thick sections were incubated for two hours to overnight at 56°C, deparaffinized in Citri-Solv (Fisher, Waltham, MA) and washed in 100% ethanol. The slides were sequentially incubated in 2x Saline-sodium Citrate Buffer (SSC) at 75°C for 18 to 23 min, digested in 0.5mg/ml proteinase K/2xSSC at 45°C for 18 to 23 min, washed in 2xSSC for 5 min, and dehydrated in ethanol. DNA denaturation was performed for 15 min at 85°C and hybridization was allowed to occur at 37°C for 36–48 hours. Chromatin was counterstained with 4’,6-diamidino-2-phenyldole (0.3 μg/ml in Vectashield mounting medium, Vector Laboratories). Gene copy number analysis was done in approximately 50 nuclei per tumor in at least four areas, and the selection of the area was guided by H&E-stained section. PDGFRB gene copy number was evaluated by three ways: a) gene amplification, defined as presence of loose or tight gene cluster or PDGFRB gene to centromeric probe 5 ratio ≥2; b) copy number gain (CNG) defined at 2 levels: ≥ 4 copies in > 10% cells and in > 40% of cells as previously published.23
Results
There were 17 chemo-naive mesothelioma patients enrolled on this phase I trial from September 2006 until April 2009. Patient demographics are listed in Table 2. The adverse events by dose level are listed in Table 3. The most common (any grade) side effects associated with this regimen were nausea, fatigue, hypomagnesemia, and anemia. Grade 3 toxicities included anemia (n=4), neutropenia (n=2), vomiting (n=2), and one incident each of hypomagnesemia, hypophosphatemia, and leukopenia were reported. There was one grade 4 anemia event reported. There were no treatment related deaths that occured. The MTD was established at dose level 3 with the DLT of nausea and vomiting.
Table 2.
Patient Demographics.
| Patient Characteristic | N patients (n=17) | % patients |
|---|---|---|
|
| ||
| Age | ||
| Median | 61 | |
| Range | 48–79 | |
|
| ||
| Gender | ||
| Female | 3 | 18% |
| Male | 14 | 82% |
|
| ||
| Ethnicity | ||
| Caucasian | 11 | 65% |
| Hispanic | 2 | 11% |
| Asian/Indian | 1 | 6% |
| African-American | 2 | 11% |
| Native American Indian | 1 | 6% |
|
| ||
| ECOG PS | ||
| 0 | 3 | 18% |
| 1 | 11 | 65% |
| 2 | 3 | 18% |
|
| ||
| Histology | ||
| Epitheliod | 7 | 41% |
| Biphasic | 5 | 29% |
| Sarcomatoid | 5 | 29% |
Table 3.
Adverse events by grade.
| ADVERSE EVENT | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| ALOPECIA | 1 | 0 | 0 | 0 |
| ANEMIA | 5 | 5 | 4 | 1 |
| ANOREXIA | 6 | 1 | 0 | 0 |
| CONSTIPATION | 0 | 1 | 0 | 0 |
| DEHYDRATION | 3 | 4 | 0 | 0 |
| DIARRHEA | 3 | 1 | 0 | 0 |
| DIZZINESS | 0 | 1 | 0 | 0 |
| EDEMA | 7 | 0 | 0 | 0 |
| EPISTAXIS | 1 | 0 | 0 | 0 |
| FATIGUE | 3 | 4 | 0 | 0 |
| GASTRITIS | 0 | 1 | 0 | 0 |
| HEARING DEFICIT | 0 | 1 | 0 | 0 |
| HYPERURICEMIA | 1 | 0 | 0 | 0 |
| HYPOCALCEMIA | 3 | 0 | 0 | 0 |
| HYPOKALEMIA | 4 | 0 | 0 | 0 |
| HYPOMAGNESEMIA | 8 | 5 | 1 | 0 |
| HYPONATREMIA | 3 | 0 | 0 | 0 |
| HYPOPHOSPHATEMIA | 1 | 2 | 1 | 0 |
| INFECTION | 1 | 0 | 0 | 0 |
| LYMPHOPENIA | 1 | 0 | 0 | 0 |
| MUSCLE WEAKNESS | 3 | 1 | 0 | 0 |
| NAUSEA | 7 | 24 | 0 | 0 |
| NEUTROPENIA | 6 | 4 | 3 | 0 |
| OCULAR/VISUAL CHANGES | 2 | 0 | 0 | 0 |
| PAIN | 2 | 1 | 0 | 0 |
| PROTEINURIA | 1 | 0 | 0 | 0 |
| RASH | 1 | 1 | 0 | 0 |
| TASTE ALTERATION | 1 | 0 | 0 | 0 |
| THROMBOCYTOPENIA | 2 | 1 | 0 | 0 |
| VOMITING | 2 | 8 | 2 | 0 |
| WEIGHT LOSS | 1 | 0 | 0 | 0 |
Best response by RECIST criteria, showed 1 PR, 3 minor responses, 7 SD, and 3 PD. Three patients were not evaluable for a response as they did not complete re-staging studies. Six patients completed all 6 cycles of therapy, 1 completed 5 cycles, 2 completed 4 cycles of treatment, 1 completed 3 cycles of therapy. The remaining 7 patients completed 2 or fewer cycles of therapy. The median number of cycles was 4, range 1–6. Patients were removed from the study for the following reasons: 5 disease progression, 1 renal insufficiency, 2 withdrew due to treatment intolerance, 1 withdrew for social reasons, 1 had debility, and 1 was hospitalized for pneumonia with his treatment held and exceeded the amount of time allowed to be off the imatinib mesylate.
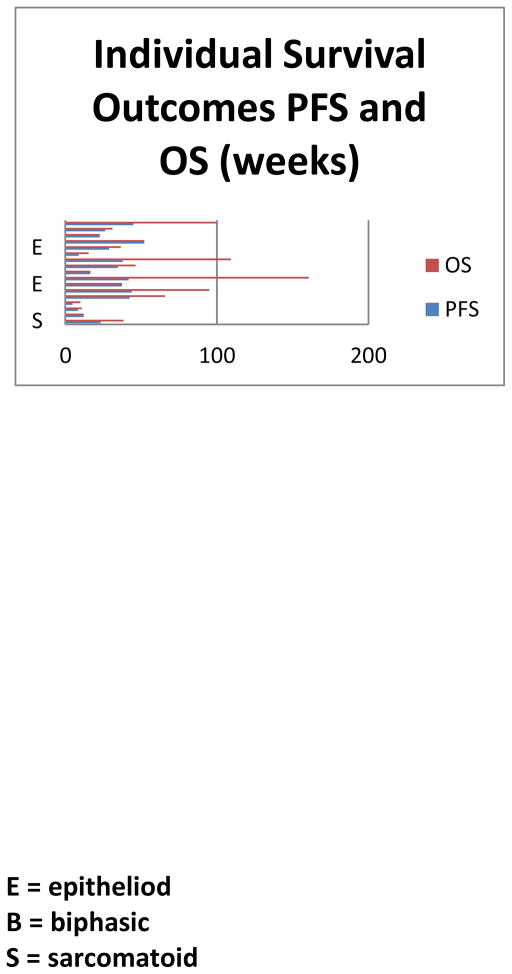
For the 17 patients on trial, the median PFS was 7.9 months and median OS was 8.8 months (see Figure 1). Patients with sarcomatoid histology had a worse PFS (p=0.01) and OS (p=0.009) compared to epitheliod and biphasic histology patients. Gender and race were not significant in predicting survival outcomes, but improved ECOG PS 0 or 1 predicted for improved OS (p=0.001) and PFS (p=0.013). In the 6 patients who completed 6 cycles of therapy, their median PFS was 9.6 months and OS was 22.4 months (Figure 1). Patients who were able to complete 6 cycles of treatment had a better OS compared to those who were not able to complete treatment (p=0.006). The histology of these 6 patients included 4 epitheliod and 2 biphasic mesothelioma.
Figure 1.
Individual Survival outcomes for progression-free and overall survival and designation of histologic subtype.
In the translational studies, fourteen patients had adequate tumor tissue that could be assessed for IHC and FISH. In the IHC results, when assessing results by above and below the median, patients with higher than median p- PDGFRα IHC expression in tumor cells had a better OS (p=0.013). When assessed as a continuous variable, higher p-PDGFRα in tumor cells correlated with an improved OS (p=0.045). Patients with higher p-PDGFRα in tumor cells were more likely to have completed 6 cycles of triplet therapy. None of the other 8 IHC biomarkers were predictive or prognostic for survival.
There were 12 patients with successful PDGFRβ FISH results, but none met the criteria of ≥ 4 copies of PDGFRB gene or had gene amplification. Thus a correlation with clinical outcomes could not be done.
Discussion
This study was undertaken to demonstrate the feasibility of treating mesothelioma patients with triplet therapy regimen including a PDGFR inhibitor. The MTD was established at dose level 3 (cisplatin 75 mg/m2, pemetrexed 500 mg/m2, imatinib mesylate 600 mg). In the efficacy analysis, patients who completed 6 cycles of therapy had a significant PFS (9.6 months) and OS benefit (22.4 months). The demographic analysis showed that these patients were more likely to have a better ECOG PS 0-1 and none had sarcomatoid histology. It is therefore difficult to claim that this regimen had significant efficacy as this subgroup of patientsare more likely to have improved survival outcomes anyway.
This triplet regimen was tolerable in good performance status patients, but a significant proportion of patients chose to remove themselves from this study or had unacceptable side effects requiring study discontinuation. Seven patients received ≤ 2 cycles of this triplet regimen. Also post-study, 4 patients received no subsequent therapy, 4 patients received 1 or 2 cycles of additional single agent therapy, 2 patients were unknown, and the remaining seven received 2 or more lines of subsequent treatment. This emphasizes the difficulty of treating MPM patients and future studies should account for the fact that MPM patients are less likely to receive salvage therapy.
The tumor tissue results were not helpful in identifying predictive biomarkers since the study was small and the baseline tissue specimens were not prospectively obtained. However, it is plausible that higher expression of p-PDGFRα on tumor cells may identify a population of patients who have a better OS with systemic treatment, but this should be studied in a prospective manner with a larger sample size. Unfortunately as well, none of the 12 patients with adequate tissue for FISH had a positive result, and correlations could not be done.
Our initial hypothesis that PDGFR inhibition has a synergistic role with systemic chemotherapy, has been well-supported by data from preclinical models.12 However, in our trial, PDGFR inhibition with chemotherapy was difficult to tolerate and led to insufficient treatment. In the small number of patients who received adequate therapy, the triplet regimen had more of a stabilizing effect rather than improved response. Thus, it may be a more effective strategy to target PDGFR in the maintenance setting or to study chemotherapy in combination with other multi-targeted anti-angiogenic inhibitors. SWOG 0905 is a phase I/II trial for chemo-naive patients with unresectable or metastatic malignant pleural mesothelioma. S0905 combines cisplatin-pemetrexed +/− cediranib, a dual PDGFR and VEGFR inhibitor and is currently open for enrollment in the randomized phase II portion which has a maintenance arm of cediranib versus placebo.
In conclusion, cisplatin-pemetrexed-imatinib mesylate was tolerable in good performance status patients and was able to prolong survival outcomes in patients who were able to complete 6 cycles of therapy. However, this study is significantly limited as there were small numbers of patients and tumor specimens to evaluate. Also, this trial is limited as it did not include a maintenance therapy treatment arm, since it was designed and conducted prior to this paradigm shift in thoracic oncology. It is unlikely that this triplet regimen with imatinib mesylate will be developed further. SWOG 0905 with PDGFR/VEGFR inhibition is the evolution from this clinical trial. Future translational studies should elaborate on the prognostic role of PDGFRα expression on mesothelioma tumor cells.
Acknowledgments
Supported by a grant from Novartis investigator initiated mechanism, National Institute of Health 5 K12 CA088084 05(PP-9), and Aileen M. Dillon and Lee M. Bourg Mesothelioma Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baas P. Chemotherapy for malignant mesothelioma: from doxorubicin to vinorelbine. Semin Oncol. 2002;29:62–9. doi: 10.1053/sonc.2002.30231. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 3.Buchdunger E, Cioffi CL, Law N, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–45. [PubMed] [Google Scholar]
- 4.Savage DG, Antman KH. Imatinib mesylate--a new oral targeted therapy. N Engl J Med. 2002;346:683–93. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 5.Pietras K, Sjoblom T, Rubin K, et al. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3:439–43. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 6.Roberts F, Harper CM, Downie I, et al. Immunohistochemical analysis still has a limited role in the diagnosis of malignant mesothelioma. A study of thirteen antibodies. Am J Clin Pathol. 2001;116:253–62. doi: 10.1309/XL6K-8E62-9FLD-V8Q8. [DOI] [PubMed] [Google Scholar]
- 7.Nowak AK, Lake RA, Kindler HL, et al. New approaches for mesothelioma: biologics, vaccines, gene therapy, and other novel agents. Semin Oncol. 2002;29:82–96. doi: 10.1053/sonc.2002.30234. [DOI] [PubMed] [Google Scholar]
- 8.Langerak AW, van der Linden-van Beurden CA, Versnel MA. Regulation of differential expression of platelet-derived growth factor alpha- and beta-receptor mRNA in normal and malignant human mesothelial cell lines. Biochim Biophys Acta. 1996;1305:63–70. doi: 10.1016/0167-4781(95)00196-4. [DOI] [PubMed] [Google Scholar]
- 9.Prins JB, Langerak AW, Dirks RP, et al. Identification of regulatory sequences in the promoter of the PDGF B-chain gene in malignant mesothelioma cell lines. Biochim Biophys Acta. 1996;1317:223–32. doi: 10.1016/s0925-4439(96)00060-9. [DOI] [PubMed] [Google Scholar]
- 10.Ascoli V, Scalzo CC, Facciolo F, et al. Platelet-derived growth factor receptor immunoreactivity in mesothelioma and nonneoplastic mesothelial cells in serous effusions. Acta Cytol. 1995;39:613–22. [PubMed] [Google Scholar]
- 11.Dorai T, Kobayashi H, Holland JF, et al. Modulation of platelet-derived growth factor-beta mRNA expression and cell growth in a human mesothelioma cell line by a hammerhead ribozyme. Mol Pharmacol. 1994;46:437–44. [PubMed] [Google Scholar]
- 12.Pietras K, Rubin K, Sjoblom T, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–84. [PubMed] [Google Scholar]
- 13.Pietras K, Ostman A, Sjoquist M, et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61:2929–34. [PubMed] [Google Scholar]
- 14.Sleeman BD, Nimmo HR. Fluid transport in vascularized tumours and metastasis. IMA J Math Appl Med Biol. 1998;15:53–63. [PubMed] [Google Scholar]
- 15.Zlotecki RA, Baxter LT, Boucher Y, et al. Pharmacologic modification of tumor blood flow and interstitial fluid pressure in a human tumor xenograft: network analysis and mechanistic interpretation. Microvasc Res. 1995;50:429–43. doi: 10.1006/mvre.1995.1069. [DOI] [PubMed] [Google Scholar]
- 16.Porta C, Mutti L, Tassi G. Negative results of an Italian Group for Mesothelioma (G.I.Me.) pilot study of single-agent imatinib mesylate in malignant pleural mesothelioma. Cancer Chemother Pharmacol. 2007;59:149–50. doi: 10.1007/s00280-006-0243-4. [DOI] [PubMed] [Google Scholar]
- 17.Mathy A, Baas P, Dalesio O, et al. Limited efficacy of imatinib mesylate in malignant mesothelioma: a phase II trial. Lung Cancer. 2005;50:83–6. doi: 10.1016/j.lungcan.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Bertino P, Piccardi F, Porta C, et al. Imatinib mesylate enhances therapeutic effects of gemcitabine in human malignant mesothelioma xenografts. Clin Cancer Res. 2008;14:541–8. doi: 10.1158/1078-0432.CCR-07-1388. [DOI] [PubMed] [Google Scholar]
- 19.Bertino P, Porta C, Barbone D, et al. Preliminary data suggestive of a novel translational approach to mesothelioma treatment: imatinib mesylate with gemcitabine or pemetrexed. Thorax. 2007;62:690–5. doi: 10.1136/thx.2006.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali Y, Lin Y, Gharibo MM, et al. Phase I and pharmacokinetic study of imatinib mesylate (Gleevec) and gemcitabine in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5876–82. doi: 10.1158/1078-0432.CCR-07-0883. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Behrens C, Feng L, Kadara H, et al. Expression of interleukin-1 receptor-associated kinase-1 in non-small cell lung carcinoma and preneoplastic lesions. Clin Cancer Res. 16:34–44. doi: 10.1158/1078-0432.CCR-09-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varella-Garcia M, Diebold J, Eberhard DA, et al. EGFR fluorescence in situ hybridisation assay: guidelines for application to non-small-cell lung cancer. J Clin Pathol. 2009;62:970–7. doi: 10.1136/jcp.2009.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]