Abstract
Background & objectives:
The effectiveness of interleukin-2 (IL-2) and induced killer cells for non-small cell lung cancer (NSCLC) is controversial. This study evaluates the efficacy and safety of interleukin-2 and induced killer cells on NSCLC, so as to provide references for further clinical practice and research.
Methods:
Relevant randomized controlled trials (RCTs) were searched in Cochrane library (Issue 2, 2013), Web of Science (1980-March 2013), PubMed (1966-March 2013), China Knowledge Resource Integrated database (CNKI) (1994-March 2013), China Biology Medicine database (CBM) (1978-March 2013), VIP (1989-March 2013), and Wan Fang databases (1997-March 2013). There were no language restrictions. After independent quality assessment and data extraction by two authors, meta-analysis was conducted by RevMan 5.1 software.
Results:
Ten RCTs were included. Odds ratio (OR), 95% confidence intervals (CI), P value expressed as test group (interleukin-2 or induced killer cells combined chemotherapy) versus control group (chemotherapy alone), was 2.02 (1.24, 3.29; P=0.004) for disease control rate. Hazard ratios (HR) (95% CI; P value), expressed as test group (interleukin-2 or induced killer cells) versus control group, were 0.60 (0.46, 0.79; P=0.0003) for overall survival of postoperative treatment, and 0.77 (0.60, 0.99; P =0.04) for overall survival of combination with chemotherapy. Mean differences (MD) (95% CI; P value), expressed as test group (interleukin-2 or induced killer cells) versus control group (after treatment), were 11.32 (6.32, 16.33; P=0.00001) for NK cells, 11.79 (2.71, 20.86; P=0.01) for CD3+ cells, 14.63 (2.62, 26.64; P=0.02) for CD4+ cells, and -4.49 (-7.80, 1.18; P=0.008) for CD8+ cells.
Interpretation & conclusions:
The meta-analysis showed that IL-2 or induced killer cells combination therapy was efficacious in treating NSCLC and improved overall survival. Further analysis of trials having adequate information and data need to be done to confirm these findings.
Keywords: A systemic review and meta-analysis, activated killer cells, induced killer cells, interleukin-2, non-small cell lung cancer
Lung cancer was the most commonly diagnosed cancer and the leading cause of cancer death in 2012 all over the world; it accounted for 19 per cent of cancer deaths globally in 20121. Non-small cell lung cancer (NSCLC) accounts for approximately 85 per cent of all lung cancers2. Despite recent advances in surgery, radiotherapy, and chemotherapy, the prognosis of patients with lung cancer is poor3. Each stage of cancer development is particularly regulated by the immune system; however, complete activation of adoptive immune cells at the tumour stage may result in eradication of malignant cells, chronic activation of innate immune cells at the sites of premalignant growth may enhance tumour development4. Adoptive immunotherapies of various killer cells and cytokine have been reported, including lymphokine-activated killer cells (LAK)5, cytokine-induced killer cells (CIK)6, tumour infiltrating lymphocytes (TIL)7 and interleukin-2 (IL-2)8. However, their therapeutic efficacy is still controversial9,10. A meta-analysis of several randomized clinical trials (RCTs) has demonstrated immunotherapy in patients with advanced stage of NSCLC11. But it was the subgroup analysis of cytokine immunotherapy only, there were no data of IL-2 and induced killer cells or activated killer cells therapy for NSCLC. So we performed a systemic review and meta-analysis to evaluate the efficacy and safety of IL-2, induced killer cells or activated killer cells therapy for patients with NSCLC.
Material & Methods
Literature search strategy: A literature search was conducted in Cochrane library (Issue 2, 2013), Web of Science (1980-March 2013) and PubMed (1966-March 2013) which are English databases, and China Knowledge Resource Integrated database (CNKI) (1994-March 2013), China Biology Medicine database (CBM) (1978-March 2013), China Science and Technology Periodical database (1989-March 2013) and Wan Fang (1997-March 2013) which are Chinese databases. The keywords used were “induced killer cells” OR “tumour infiltrating lymphocytes”, OR “activated killer cells” OR “interleukin-2” AND “non-small cell lung cancer” AND “randomized controlled trials”. No language restrictions were applied. Various combinations of the keywords were applied.
Selection criteria: The inclusion criteria were as follows: (i) Study type: RCTs regardless of use of blinding; (ii) Patients with histologically diagnosed primary cancer of the NSCLC; (iii) Normal haematologic, renal, cardiac and hepatic function; and (iv) Comparison of IL-2 and induced killer cells or activated killer cells therapy versus control therapy.
The exclusion criteria were as follows: (i) Study type: case studies, review articles; (ii) Pre-existing autoimmune diseases; (iii) Prior immunotherapy was permitted; and (iv) Cannot extract the exact data.
Data extraction and critical appraisal: To reduce the bias and to improve the reliability, two authors checked all relevant studies independently. Data on the following characteristics were also extracted by all three authors: the clinical outcomes used to evaluate efficacy of immunotherapy in advanced NSCLC were (i) overall survival (OS); (ii) complete response (CR), partial response (PR), stable disease (SD), the total effective rate (CR+PR) according to World Health Organization (WHO) and International Union Against Cancer Criteria12, the disease control rate or clinical benefit (CR + PR+ SD); (iii) toxicity (adverse events) was measured according to the WHO criteria13; and (iv) variation of natural killer (NK) cells, T-cell subgroups. OS was defined as the period from the treatment date to the date of death or the day of the last follow up visit. The disease control rate (DCR) or clinical benefit was defined as the rate of the sum total of CR, PR and SD.
The quality of studies was appraised independently using the following criteria: (i) the methods for generation sequence; (ii) whether blinding was reported; (iii) whether there was proper concealment of allocation; (iv) whether the groups were similar at baseline in terms of prognostic features; (v) whether there were incomplete outcome data; and (vi) whether there was selective outcome reporting. In case of studies with inadequate information to determine the above-mentioned assessment criteria, additional data were obtained direct from the authors.
Discrepancies between the two authors were resolved by discussion and consensus with the third author. The final results were reviewed by all investigators to avoid bias.
Statistical analysis: We estimated the combined odds ratio (OR) or hazard ratios (HR) for test cases and control cases, and expressed continuous data as weighted mean differences (WMD) with 95% confidence intervals (CIs) or as standardized weighted mean differences (SMD) if outcomes were conceptually the same but measured in different ways in the different trials. HR and CIs were calculated according to Cox proportional hazards modelling and a combined HR>1 suggested a higher risk of poor survival. The method by Tierney et al14 was used to extract data when studies did not have direct information. Statistical heterogeneity assumption among studies was checked using the x2-based Q-test15. When I2 (describes the percentage of variation across studies that is due to heterogeneity rather than chance) was not more than 50 per cent, pooled outcomes and 95% CIs were calculated using Mantel-Haenszel method with fixed-effect models16. When significant heterogeneity (P<0.1, I2>50%) among the studies was detected, a random-effect model17 (Der Simonian and Laird method) was adopted. If necessary, sensitivity analysis was also performed to evaluate the influence of individual studies on the final effect. All P-values were two-sided. All the statistical analyses were performed using RevMan 5.1 software (The Cochrane Collaboration, Oxford, United Kingdom, 2011).
Results
The original search identified 526 articles in electronic databases. Of these, 476 studies were excluded after review of the title and abstract, because these were duplicate documents, irrelevant studies and review articles. The remaining 50 articles were read in full, independently by two investigators, to assess their accordance with the predefined inclusion criteria. Finally, 10 articles were considered eligible for inclusion in the meta-analysis. Nine articles18,19,20,21,22,23,24,25,26 were published in English and one article27 was published in Chinese. The study flow diagram is shown in Fig. 1, and the characteristics of eligible studies are summarized in Table I.
Fig. 1.
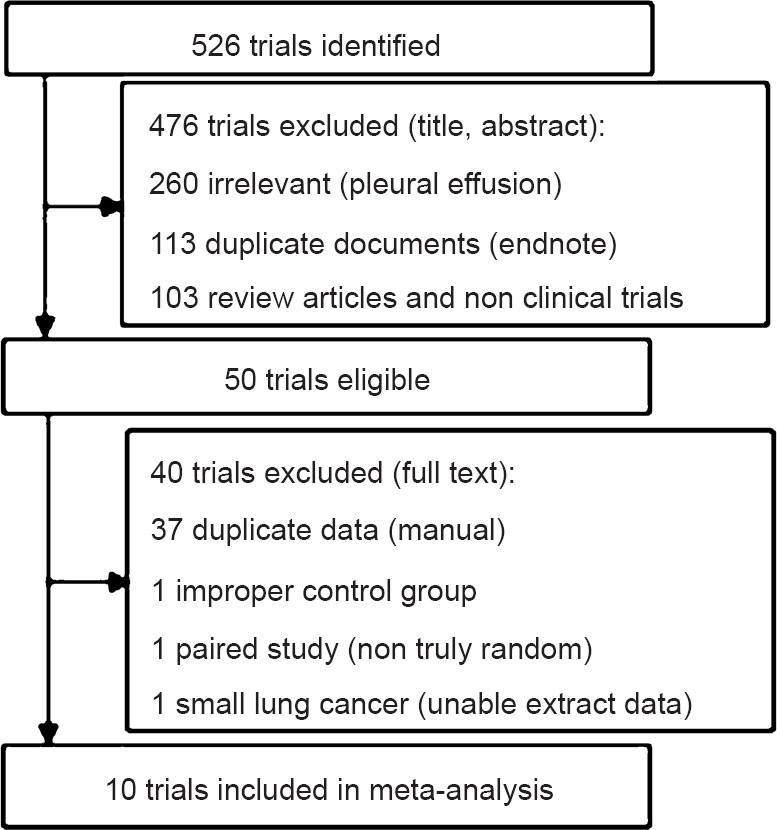
Flow chart showing article selection.
Table I.
Characteristics of the selected studies (n=10) in the meta-analysis
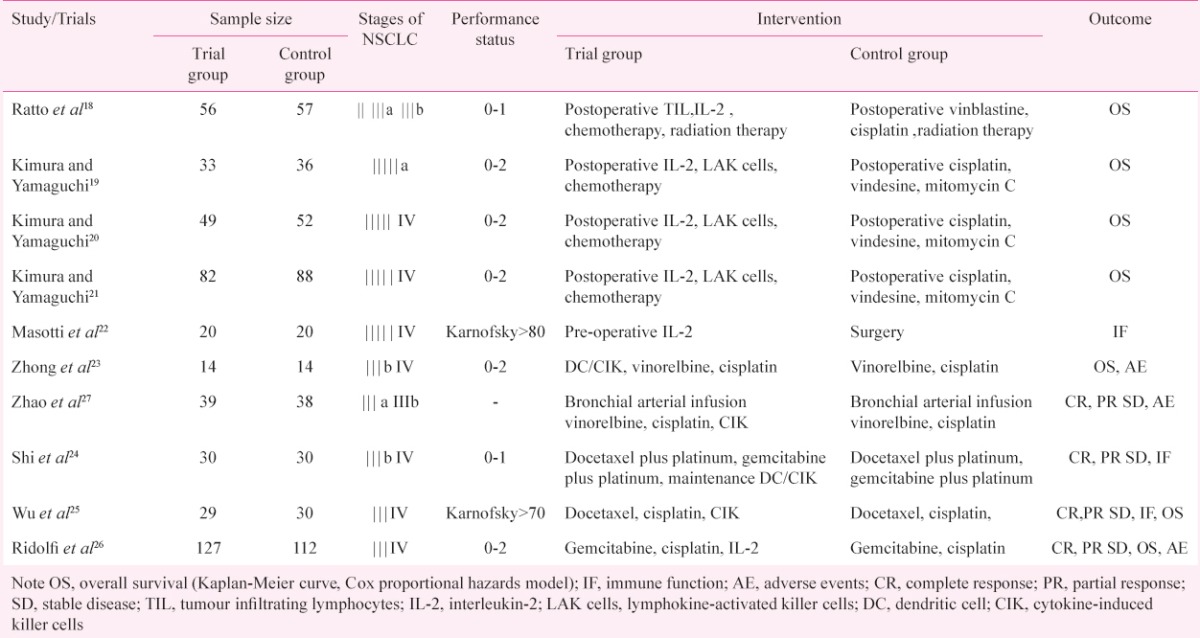
Quality of trials: The baseline characteristics of each trial were comparable. All the trials were RCTs, but six20,22,24,25,26 did not mention the sequence for generation of randomization, only two19,21 trials used a sealed envelope for allocation concealment, and none of trials mentioned the using of blinding.
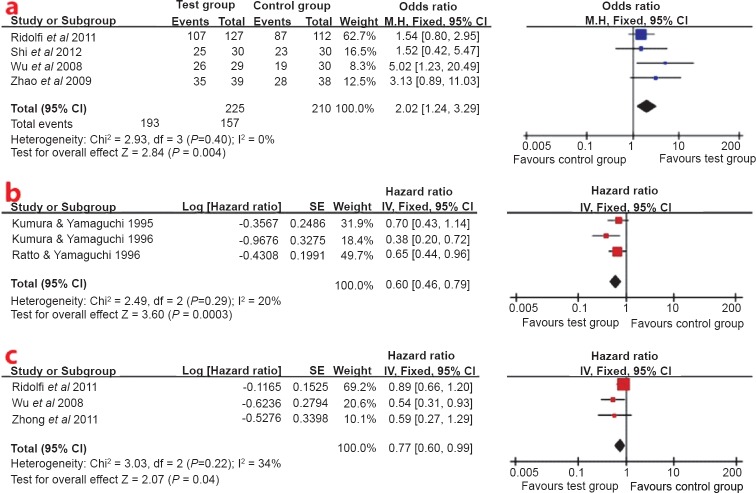
Disease control rate: Interleukin-2 or induced killer cells plus chemotherapy showed a better disease control rate (OR 2.02; 95% CI: 1.24-3.29, P<0.05) than chemotherapy alone. There was no heterogeneity of the RCTs24,25,26,27. The fixed effects model was used to pool the result (Fig. 2a).
Fig. 2.
(a). Forest plot of disease control rate for interleukin-2 or induced killer cells plus chemotherapy vs. chemotherapy alone, (b) Forest plot of overall survival for postoperative treatment, (c) Forest plot of overall survival for combination with chemotherapy.
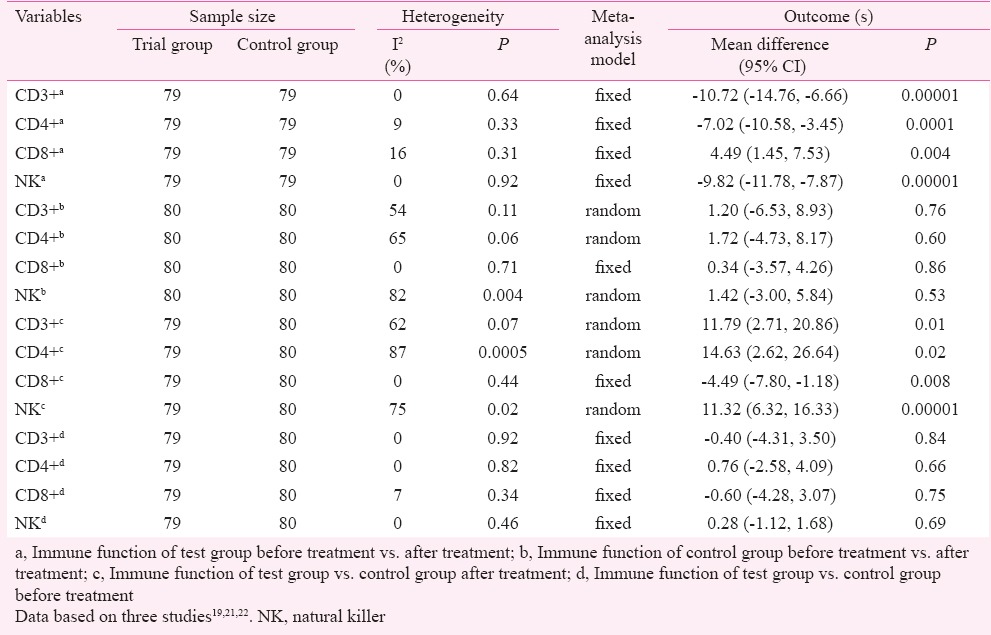
Immune function: Three studies22,24,25 reported variation of immune function in trial and control groups before and after treatment. In the trial group, the proportion of NK cells and T-cell subgroups CD3+ and CD4+ significantly increased and the proportion of CD8+ cells decreased before and after treatment, (P<0.05) (Table IIa). The mean differences (MD) (95% CI) were -9.82 (-11.78,-7.87) for NK cells, -10.72 (-14.76,-6.66) for CD3+ cells, -7.02 (-10.58,-3.45) for CD4+ cells and 4.49 (1.45, 7.53) for CD8+ cells, respectively. There were no obvious changes in the control group (Table IIb). The proportion of NK cells and T-cell subgroups CD3+ and CD4+ significantly increased and the proportion of CD8+ cells was decreased after treatment for test group vs. control group (P<0.05) (Table IIc). The MD (95% CI) were 11.32 (6.32, 16.33) for NK cells, 11.79 (2.71, 20.86) for CD3+ cells, 14.63 (2.62, 26.64) for CD4+ cells and -4.49 (-7.80, 1.18) for CD8+ cells, respectively. There were no obvious changes for test group vs. control group before treatment (Table IId). Although a random-effect model was adopted in pooled significant heterogeneity studies, in order to prove robust results of high heterogeneity outcomes, sensitivity analyses were conducted. When the influence of a single study on the overall meta-analysis estimate was investigated by omitting one study at a time, no significant heterogeneity (P>0.1, I2<50%) among the studies was detected. Exclusion of one study22 did not alter the main outcomes of analysis (CD3+b), with a range from 1.20 (95% CI: -6.53-8.93, P=0.76) to -0.13 (95% CI: -4.45-4.19, P=0.95). Exclusion of one study22 did not alter the main outcomes of analysis (CD4+b), with a range from 1.72 (95% CI: -4.73-8.17, P=0.60) to -0.14 (95% CI: -3.29-3.01, P=0.93). Exclusion of one study22 did not alter the main outcomes of analysis (NKb), with a range from 1.42 (95% CI: -3.00-5.84, P=0.53) to -1.15 (95% CI: -2.81-0.51, P=0.17). Exclusion of one study22 did not alter the main outcomes of analysis (CD3+c), with a range from 11.79 (95% CI: 2.71-20.86, P=0.01) to 10.15 (95% CI: -5.68-14.62, P=0.0001). Exclusion of one study22 did not alter the main outcomes of analysis (CD4+c), with a range from 14.63 (95% CI: 2.62-26.64, P=0.002) to 7.75 (95% CI: 4.35-11.15, P=0.001). Exclusion of one study22 did not alter the main outcomes of analysis (NKc), with a range from 11.32 (95% CI: 6.32-16.33, P=0.00001) to 8.88 (95% CI: 6.71-11.05, P=0.00001).
Table II.
Immune function of interleukin-2 and induced killer cells for non-small cell lung cancer
Overall survival: No significant heterogeneity was detected for the two subgroups which were defined by therapeutic categories (Fig. 2b, c). A fixed-effect model was used for overall survival analysis. The overall survival analysis showed that test group significantly increased overall survival at the end of follow up compared with the control group (P<0.05). The HR (95% CI) were 0.60 (0.46, 0.79) for the postoperative treatment (Fig. 2b), 0.77 (0.60, 0.99) for combination with chemotherapy (Fig. 2c).
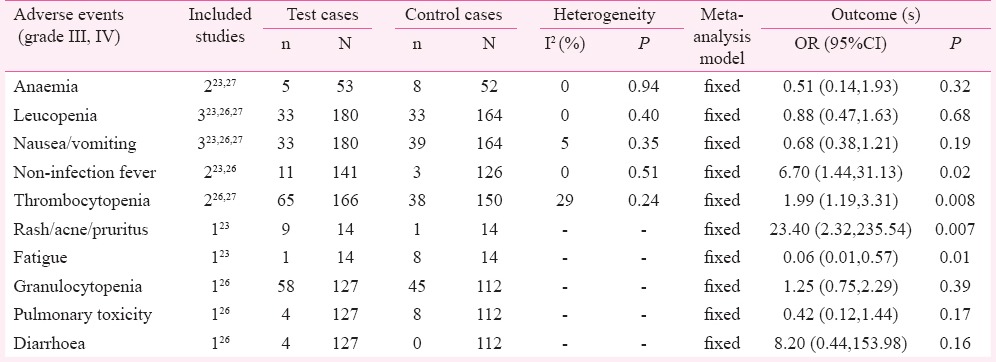
Adverse events: Interleukin-2 and induced killer cells combination with chemotherapy showed no difference in anaemia, leucopenia, nausea/vomiting, granulocytopenia, pulmonary toxicity and diarrhoea; the ORs (95% CI) were 0.51 (0.14, 1.93), 0.88 (0.47, 1.63), 0.68 (0.38, 1.21), 1.25 (0.75, 2.29), 0.42 (0.12, 1.44), and 8.20 (0.44, 153.98), respectively. Interleukin-2 and induced killer cells combination with chemotherapy showed increase in non-infection fever, thrombocytopenia and rash (acne, pruritus) (P<0.05). The ORs (95% CI) were 6.70 (1.44, 31.13), 1.99 (1.19, 3.31) and 23.40 (2.32, 235.54), respectively; and a decrease in fatigue (OR 0.06; 95% CI: 0.01-0.57; P<0.05) (Table III).
Table III.
Adverse events of test group vs. control group
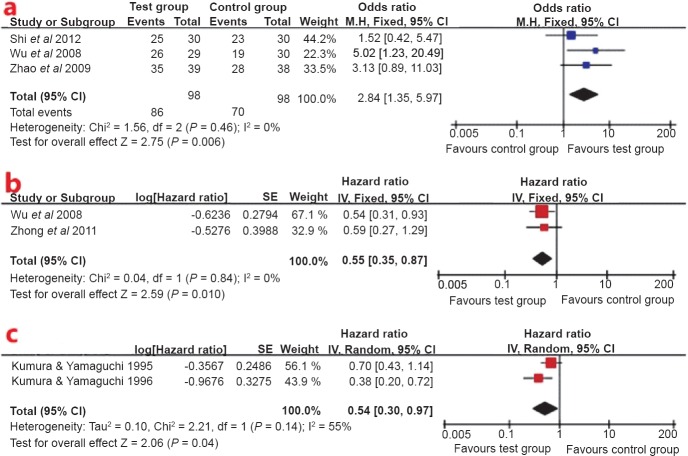
Subgroup analysis: Because one study22 did not present DCR and OS data for IL-2, two studies24,27 did not present OS data for CIK cell, and one study21 presented duplicate OS data for LAK cell; only the LAK cell and CIK cell groups were subjected to subgroup analysis. There was no heterogeneity in the RCTs24,25,27, so the fixed effect model was used to pool the result (Fig. 3a). CIK cell plus chemotherapy showed a better disease control rate (OR 2.84; 95% CI: 1.35-5.97; P<0.05) than chemotherapy alone. Because no significant heterogeneity was found across studies23,25, the fixed effect model was applied to perform meta-analysis (Fig. 3b). Pooled data from these studies showed that the OS was significantly in favour of CIK cell therapy (HR 0.55; 95% CI: 0.35-0.87, P<0.05). But owing to significant heterogeneity across studies19,20, the random effect model was applied to perform meta-analysis (Fig. 3c). Pooled data from these studies showed OS was significantly in favour of LAK cell therapy (HR 0.54; 95% CI: 0.30-0.97, P<0.05).
Fig. 3.
(a). Forest plot of disease control rate for cytokine induced killer (CIK) cell plus chemotherapy vs. chemotherapy alone, (b) Forest plot of overall survival for CIK cell therapy, (c) Forest plot of overall survival for lymphokine-activated killer (LAK) cell therapy.
In the funnel plot analysis of publication biases, the horizontal axis of the plot was the OR effect estimate and the vertical axis of the plot was the standard error (SE) of the log (OR) (Fig. 4a), and in Fig. 4b the horizontal axis of the plot was the HR effect estimate and the vertical axis of the plot was the standard error (SE) of the log (HR). The shape of the funnel plot was approximately symmetrical in disease control rate and overall survival, so no publication bias was detected in meta-analysis.
Fig. 4.
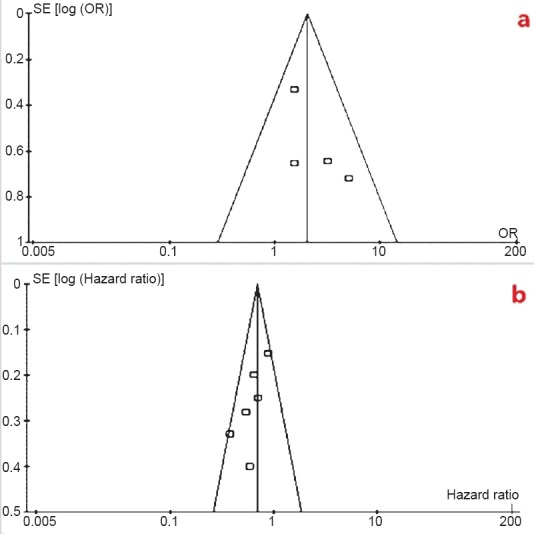
(a). Funnel plot analysis of publication biases for disease control rate, (b) Funnel plot analysis of publication biases for overall survival.
Discussion
IL-2, a T-lymphocyte activation and growth factor, is a cytokine with most important functions in the physiology of cell-mediated immunity28. Its other biologic function includes the amplification of antibody dependent cytotoxicity, proliferative responses, cytokine production, and interferon production29,30. LAK cells are cytotoxic effector lymphocytes whose cytolytic activities are not restricted by major histocompatibility complex (MHC) and have the ability to kill tumour cells and NK-resistant tumour cell lines31. Tumour infiltrating lymphocytes (TILs) represent part of the host immune response to human malignancy and contain an enriched population of cells with both cytotoxic and helper functions that are reactive to the autologous tumour32. CIK cells exhibit potent, non-MHC-restricted cytolytic activities against susceptible tumour cells of both autologous and allogeneic origins6. Dendritic cells (DCs) are the most potent antigen-presenting cells in the immune system, and are responsible for the initiation of both innate and adoptive immune responses. Cytotoxicity has been shown to be significantly increased, after DCs stimulated CIK cells33.
Primary lung cancers are treated with a combination of surgery, radiotherapy and chemotherapy. Combining cancer immunotherapy with such standard oncology treatments has been recognized as a potentially important approach34. Li et al35 showed that the CD4 + CD25 + regulatory T cells decreased the anti-tumour activity of CIK cells of lung cancer patients. But two studies10,36 showed that CIK cell immunotherapy could improve the antitumour effect by stratifying analysis in NSCLC patients. Hontscha et al37 have reported that CIK cells may prevent recurrence and improve quality of life and progression-free survival rates in patients with cancer. So we evaluated the efficacy and safety of IL-2 and induced killer cells depending on subgroups of therapeutic categories for NSCLC, and sensitivity analysis on the various efficacy parameters with alternative exclusions of one of the trials supported the conclusions.
The results of the meta-analysis showed that IL-2 and induced killer cells plus chemotherapy significantly improved the disease control rate. But Ridolfi et al26 showed no relevant difference in disease control rate after the addition of IL-2 to chemotherapy. Low-dose IL-2 (no induced killer cells) may activate cell-mediated immunity very slowly, and does not translate into clinical benefit quickly. The results of the meta-analysis showed IL-2 and induced killer cells significantly increased overall survival at the end of follow up for the postoperative treatment, but firm conclusion could not be drawn whether IL-2 and induced killer cells combination with chemotherapy could increased overall survival. So we excluded a single study26 (low-dose IL-2 only), and the overall combined risk outcome showed that IL-2 and induced killer cells combination with chemotherapy increased overall survival. Subgroup analysis of therapeutic category (LAK cell, CIK cell) was performed, the results showed that CIK cell therapy had a better disease control rate and overall survival, and LAK cell therapy showed a better overall survival. From a clinical point of view it would be interesting to know about histological subgroup analysis of NSCLC, but there was lack of actual data in studies included. All of these will be needed to provide sufficient data to calculate the effect sizes in future research.
Low lymphocyte count has a negative independent prognostic value for survival in advanced NSCLC38 and low lymphocyte percentage has the same unfavourable prognostic value in patients with solid tumours39. Our meta-analysis showed that the proportion of NK cells and T-cell subgroups CD3+ and CD4+ significantly increased and the proportion of CD8+ cells decreased in IL-2 and induced killer cells treatment group. But we found significant heterogeneity which could be explained by therapeutic category of pre-operative IL-2 administration22. Exclusion of one study22 did not alter the main outcomes of our analysis. When no significant heterogeneity among the studies was detected, sensitivity analysis indicated that our results were statistically reliable.
Interleukin-2 and induced killer cells combination with chemotherapy did not show significant toxic reactions (adverse events), including anaemia, leucopenia, nausea/vomiting. But there were limited data on other adverse events to perform a meta-analysis and, therefore, no firm conclusion could be drawn.
This systematic review and meta-analysis had some limitations: First, some RCTs20,21,22,23,24,25,26 included in our meta-analysis were not adequate as these did not report the detailed method of random sequence generation and concealment of allocation, and all trials did not mention the use of blinding. Second, publication bias is a wide phenomenon for all forms of meta-analysis40,41. Although we searched several databases and no publication bias was detected in our funnel plot analysis, publication bias might still be a limitation. Third, the standard preparation process of biological drugs, the combination treatment strategies, the efficacy of different cell types and other issues (dose strategy, timing, individual therapy of histological types, the long-term survival rate) mentioned above should be further explored with a large sample and rigorous clinical research. Fourth, there were limited data of detail adverse events to perform a meta-analysis.
Despite these limitations of this meta-analysis, the current evidence showed that IL-2 or induced killer cells combination therapy was efficacious in treating NSCLC and improved overall survival.
Acknowledgment
This study was supported by the Research Program for Health of Gansu Province of China (No.GSWST09-06), the Natural Science Foundation of Gansu Province of China (No. 1010RJZA162) and the Postgraduate Business Program of Evidence-Based Medicine Center of Lanzhou University (No. 2010LDEBM-A).
Footnotes
Conflicts of Interest: None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Gridelli C, Rossi A, Maione P, Ferrara ML, Castaldo V, Sacco PC. Vaccines for the treatment of non-small-cell lung cancer: a renewed anticancer strategy. Oncologist. 2009;14:909–20. doi: 10.1634/theoncologist.2009-0017. [DOI] [PubMed] [Google Scholar]
- 3.Yasumoto K, Hanagiri T, Takenoyama M. Lung cancer-associated tumor antigens and the present status of immunotherapy against non-small-cell lung cancer. Gen Thorac Cardiovasc Surg. 2009;57:449–57. doi: 10.1007/s11748-008-0433-6. [DOI] [PubMed] [Google Scholar]
- 4.de Visser K, Coussens L. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol. 2006;13:118–37. doi: 10.1159/000092969. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg S. Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J Natl Cancer Inst. 1985;75:595–603. [PubMed] [Google Scholar]
- 6.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–49. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–21. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 8.Ardizzoni A, Bonavia M, Viale M, Baldini E, Mereu C, Verna A, et al. Biologic and clinical effects of continuous infusion interleukin-2 in patients with non-small cell lung cancer. Cancer. 1994;75:1353–60. doi: 10.1002/1097-0142(19940301)73:5<1353::aid-cncr2820730508>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Shablak A, Hawkins RE, Rothwell DG, Elkord E. T cell based immunotherapy of metastatic renal cell carcinoma: modest success and future perspective. Clin Cancer Res. 2009;15:6503–10. doi: 10.1158/1078-0432.CCR-09-1605. [DOI] [PubMed] [Google Scholar]
- 10.Li R, Wang C, Liu L, Du C, Cao S, Yu J, et al. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012;61:2125–33. doi: 10.1007/s00262-012-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Zou ZH, Xia HL, He JX, Zhong NS, Tao AL. Strengths and weaknesses of immunotherapy for advanced non-small-cell lung cancer: A meta-analysis of 12 randomized controlled trials. PLoS One. 2012;7:e32695. doi: 10.1371/journal.pone.0032695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JO, Lee SI, Song SY, Kim K, Kim WS, Jung CW, et al. Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Jpn J Clin Oncol. 2003;33:533–7. doi: 10.1093/jjco/hyg093. [DOI] [PubMed] [Google Scholar]
- 13.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:1–16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 17.der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Ratto GB, Zino P, Mirabelli S, Minuti P, Aquilina R, Fantino G, et al. A randomized trial of adoptive immunotherapy with tumor-infiltrating lymphocytes and interleukin-2 versus standard therapy in the postoperative treatment of resected non small cell lung carcinoma. Cancer. 1996;78:244–51. doi: 10.1002/(SICI)1097-0142(19960715)78:2<244::AID-CNCR9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Kimura H, Yamaguchi Y. Adjuvant chemo-immunotherapy after curative resection of Stage II and IIIA primary lung cancer. Lung Cancer. 1996;14:301–14. doi: 10.1016/0169-5002(96)00555-7. [DOI] [PubMed] [Google Scholar]
- 20.Kimura H, Yamaguchi Y. Adjuvant immunotherapy with interleukin 2 and lymphokine-activated killer cells after noncurative resection of primary lung cancer. Lung Cancer. 1995;13:31–44. doi: 10.1016/0169-5002(95)00478-j. [DOI] [PubMed] [Google Scholar]
- 21.Kimura H, Yamaguchi Y. A phase III randomized study of interleukin-2 lymphokine-activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma. Cancer. 1997;80:42–9. [PubMed] [Google Scholar]
- 22.Masotti A, Morandini G, Ortolani R, Fumagalli L. Phase-II randomized study of pre-operative IL-2 administration in operable NSCLC. Lung Cancer. 1998;20:191–202. doi: 10.1016/s0169-5002(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhong R, Teng J, Han B, Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother. 2011;60:1497–502. doi: 10.1007/s00262-011-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi SB, Ma TH, Li CH, Tang XY. Effect of maintenance therapy with dendritic cells: cytokine-induced killer cells in patients with advanced non-small cell lung cancer. Tumori. 2012;98:314–9. doi: 10.1177/030089161209800306. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 2008;28:3997–4002. [PubMed] [Google Scholar]
- 26.Ridolfi L, Bertetto O, Santo A, Naglieri E, Lopez M, Recchia F, et al. Chemotherapy with or without low-dose interleukin-2 in advanced non-small cell lung cancer: results from a phase III randomized multicentric trial. Int J Oncol. 2011;39:1011–7. doi: 10.3892/ijo.2011.1099. [DOI] [PubMed] [Google Scholar]
- 27.Zhao G, Huang Y, Ye L, Duan L, Zhou Y, Yang K, et al. Therapeutic efficacy of traditional vein chemotherapy and bronchial arterial infusion combining with CIKs on III stage non-small cell lung cancer. Zhong guo Fei Ai Za Zhi. 2009;12:1000–4. doi: 10.3779/j.issn.1009-3419.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Lange JR, Raubitschek AA, Pockaj BA, Spencer WF, Lotze MT, Topalian SL, et al. A pilot study of the combination of interleukin-2-based immunotherapy and radiation therapy. J Immunother. 1992;12:265–71. doi: 10.1097/00002371-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–85. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treisman J, Hwu P, Minamoto S, Shafer GE, Cowherd R, Morgan RA, et al. Interleukin-2-transduced lymphocytes grow in an autocrine fashion and remain responsive to antigen. Blood. 1995;85:139–45. [PubMed] [Google Scholar]
- 31.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–41. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiteside TL, Miescher S, Hurlimann J, Moretta L, von Fliedner V. Separation, phenotyping and limiting dilution analysis of T-lymphocytes infiltrating human solid tumors. Int J Cancer. 1986;37:803–11. doi: 10.1002/ijc.2910370602. [DOI] [PubMed] [Google Scholar]
- 33.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 34.Bradbury PA, Shepherd FA. Immunotherapy for lung cancer. J Thorac Oncol. 2008;3:S164–70. doi: 10.1097/JTO.0b013e318174e9a7. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Yu JP, Cao S, Wei F, Zhang P, An XM, et al. CD4 +CD25 + regulatory T cells decreased the antitumor activity of cytokine-induced killer (CIK) cells of lung cancer patients. J Clin Immunol. 2007;27:317–26. doi: 10.1007/s10875-007-9076-0. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Wang C, Yu J, Cao S, Wei F, Zhang W, et al. Dendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgery. Cytotherapy. 2009;11:1076–83. doi: 10.3109/14653240903121252. [DOI] [PubMed] [Google Scholar]
- 37.Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC) J Cancer Res Clin Oncol. 2011;137:305–10. doi: 10.1007/s00432-010-0887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25–32. [PubMed] [Google Scholar]
- 39.Maltoni M, Pirovano M, Nanni O, Marinari M, Indelli M, Gramazio A, et al. Biological indices predictive of survival in 519 Italian terminally ill cancer patients. Italian Multicenter Study Group on Palliative Care. J Pain Symptom Manage. 1997;13:1–9. doi: 10.1016/s0885-3924(96)00265-5. [DOI] [PubMed] [Google Scholar]
- 40.[No authors listed]. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–85. [PubMed] [Google Scholar]
- 41.Nordmann AJ, Kasenda B, Briel M. Meta-analyses: what they can and cannot do. Swiss Med Wkly. 2012;142:w13518. doi: 10.4414/smw.2012.13518. [DOI] [PubMed] [Google Scholar]