Abstract
Background & objectives:
There is scarcity of data on the frequency of malignancies in HIV infected individuals from India. The objective of this study was to determine the type and frequency of malignancies in HIV infected individuals attending a tertiary care hospital in north India.
Methods:
The study design included retrospective analysis of data of all HIV infected individuals registered in the Immunodeficiency clinic from December 2009 to December 2011 and a prospective analysis of HIV infected individuals registered from January 2012 to April 2013. The clinical details and treatment outcomes of all individuals diagnosed to have AIDS defining and non-AIDS defining malignancies were recorded.
Results:
Records of 2880 HIV infected individuals were reviewed. Thirty one (19 males, 12 females) individuals were diagnosed to have malignancy. AIDS defining malignancy was found in the form of non-Hodgkin's lymphoma in 12 individuals and cervical cancer in six women. Non-AIDS defining malignancies included Hodgkin's lymphoma (n=2); and chronic myelogenous leukaemia, carcinoma base of tongue, carcinoma larynx, carcinoma bronchus, sinonasal carcinoma, ovarian carcinoma, anal carcinoma, carcinoma urinary bladder, pleomorphic sarcoma, parathyroid adenoma, and renal cell carcinoma in one individual each. Mean CD4+cell count prior to ART initiation was 250 ± 195.6 (median: 187; range, 22-805) cells/μl and at the time of diagnosis of malignancy was 272 ± 202 (median: 202; range, 15-959) cells/μl. The mean CD4+ count of individuals with AIDS defining malignancy was significantly lower when compared with non-AIDS defining malignancy (P<0.001). Fourteen individuals were alive and on regular follow up, 15 had died and two cases were lost to follow up.
Interpretation & conclusions:
The frequency of malignancies in HIV infected patients at our centre was 1 per cent, with non-Hodgkin's lymphoma being the commonest. Further studies need to be done to document similar data from different parts of the country.
Keywords: AIDS defining malignancies, HAART, HIV, non-AIDS defining malignancy, non-Hodgkin's lymphoma
In June 2015, 15.8 million people living with HIV were receiving antiretroviral therapy (ART) globally. At the end of 2014, 36.9 million people globally were living with HIV. Of these, 25.8 million were from sub-Saharan Africa and five million from Asia and Pacific1. Although combination antiretrovirals have dramatically reduced morbidity and mortality from AIDS related conditions, but malignancies remain an important cause of morbidity and mortality in HIV infected individual2,3. There is a complex relationship between infectious agents, immunity and cancer. Most of the cancers that occur during the state of immunodeficiency are rare and are associated with various viral infections4. In this context, though the incidence of AIDS defining malignancies (ADM) has declined, as with other AIDS events, non-AIDS-defining malignancies (NADM) are now an important cause of mortality in HIV-infected individuals5. The incidence of NADM is higher among HIV-infected individuals than among the general population in the same geographic area6. It has been reported that such cancers contributed to 13 per cent of deaths during the HAART (highly active anti retroviral therapy) era as compared to less than 1 per cent in the pre-HAART era7. The burden of cancers in HIV individuals is increasingly being contributed by developing countries where the overall burden of HIV infection is high and is increasing8. Various studies have shown that cancers in resource limited regions mostly present in the advance stage of malignancy with limited therapeutic options resulting in poor survival for individuals with HIV associated malignancy8,9,10. Studies published from India have shown that despite availability of HAART, patients present in the advance stage and AIDS defining malignancies continue to be prevalent in the HAART era9,10,11. There are no published data on pattern of malignancies in HIV from north India. This study was undertaken to find out the type and frequencies of malignancies in HIV infected individuals presented to a tertiary care hospital in north India.
Material & Methods
This study was conducted in the Immunodeficiency clinic of the department of Internal medicine of Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. A retrospective and prospective analysis of the data of HIV infected patients registered in the clinic was done over a period of three years and three months.
Study design
Retrospective analysis: A comprehensive search of archival patient records was made. The search included all consecutive HIV seropositive patients enrolled in the immunodeficiency clinic of the department of Internal Medicine during a period of December 2009 to December 2011. All patients diagnosed to have a malignant neoplastic disease during the above mentioned period were selected for further analysis.
Prospective analysis: All consecutive HIV seropositive patients referred to the Immunodeficiency clinic from January 2012 to April 2013 were screened during routine investigations at baseline and during routine follow up visits for occurrence of any malignant neoplastic disease. All patients with confirmed malignancy were included in the study.
The enrollment of patients was based on availability of histopathology report from tissue biopsy, confirming presence of a malignant tumour. Data of all consecutive patients diagnosed to have a malignancy during the retrospective and prospective time periods were extracted and analyzed. Various parameters noted included demographic details, mode of HIV acquisition, duration of HIV seropositivity at the time of diagnosis of malignancy, data related to occurrence of opportunistic infection, co-infection in the form of hepatitis B, hepatitis C and syphilis. CD4+ cell counts at the time of HIV diagnosis and at the time of diagnosis of malignancy were noted. Details of antiretroviral therapy given, stage of disease and treatment offered for the malignancy were recorded. Mean duration of follow up in the clinic was noted. Further records were analyzed to review the outcome of these individuals. The study was approved by the ethical committee of the institute.
Statistical analysis: Statistical analysis was performed using SPSS software (Version 20, SPSS, Inc., Chicago, USA). Descriptive data were expressed as mean ± SD (range) and/or median, depending on distribution of data. Paired t test was used to compare CD4+ cell counts at baseline and at the time of diagnosis of malignancy. One sample t test was used to compare CD4+ cell counts of ADM and NADM groups.
Results
During the study period, records of 2880 HIV infected individuals registered in the Immunodeficiency clinic of the department were reviewed. Thirty one (19 males, 12 females) (amounting to a frequency of 1%) were diagnosed with malignancy. Eighteen had AIDS defining malignancy and 13 had non-AIDS defining malignancies. The mean age was 39 ± 12.3 (16-77) yr. Modes of HIV acquisition were heterosexual, perinatal and blood transfusion in 28, two and one individuals, respectively. Only one individual was identified with hepatitis B virus co-infection. Four were current smokers and alcoholic and five had documented history of alcohol abuse in the past while two had documented history of tobacco intake (Table I).
Table I.
Characteristics of HIV infected individuals (n=31) diagnosed with malignancy
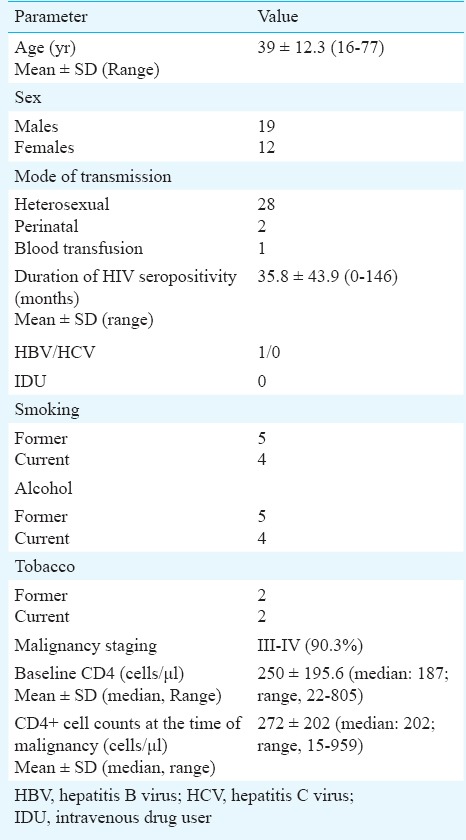
Median duration of HIV seropositivity at the time of malignancy diagnosis was 22 (range, 0-146) months. Twenty eight (93.3%) patients presented in the advanced stage of malignancy. Mean CD4+ cell counts prior to ART initiation was 250 ± 195.6 (median: 187; range 22-805) cells/μl and at the time of diagnosis of malignancy was 272 ± 202 (median: 202; range 15-959). There was no significant difference in the CD4+ cell count at baseline and at the time of diagnosis of malignancy. Mean CD4+ cell counts at the time of diagnosis of ADM were 285.8 ± 277.8 (range 15-959) and at the time of diagnosis of NADM were 308 ± 207 (range 128-722) cells/μl. The mean CD4+ cell count of individuals with ADM was significantly lower than with NADM (P<0.001). Thirteen were already receiving first line ART at the time of diagnosis of malignancy. The median duration of ART in these were 26 (range 3-96) months. ADM was found in the form of non-Hodgkin's lymphoma (NHL) in 12 individuals and cervical cancer in six women. Nine had nodal presentation and one individual each had primary site as CNS, cheek and stomach. Of the individuals diagnosed with NHL, nine were of B cell lineage and three were of T cell lineage. Six were identified with diffuse large B cell lymphoma (DLBCL), one each had Burkitt's lymphoma, CNS lymphoma and small cell lymphoma. Three had anaplastic large cell lymphoma (ALCL). Six women had invasive cervical carcinoma. No case of Kaposi's sarcoma was identified. Thirteen had NADM. These included Hodgkin's lymphoma in two and chronic myelogenous leukaemia, carcinoma base of tongue, carcinoma larynx, carcinoma bronchus, sinonasal carcinoma, ovarian carcinoma, anal carcinoma, carcinoma urinary bladder, pleomorphic sarcoma, parathyroid adenoma, and renal cell carcinoma in one individual each.
Of the 31 patients diagnosed with malignancies, 14 individuals were alive and doing well on ART, 15 died and two were lost to follow up. Mean duration of follow up of these 14 individuals after the diagnosis of malignancy was 37.5 ± 25.0 (range 4 - 90) months. Of the surviving individuals, 12 were stable on first line ART, one was on second line ART and one was receiving salvage therapy.
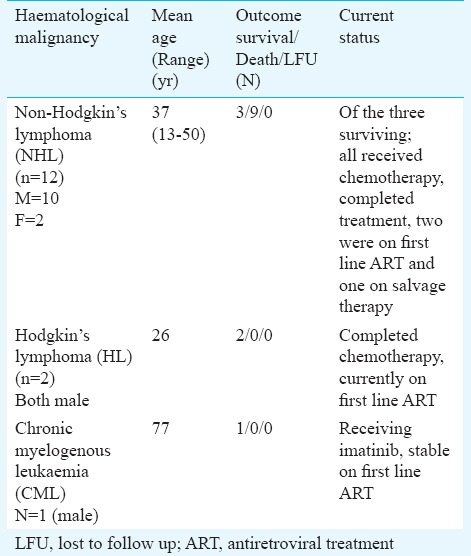
Outcomes in individuals diagnosed with haematological malignancy are shown in Table II. Of the 15 individuals diagnosed with haematological malignancy, six were alive (3/12 NHL, 2 HL, 1 CML) (Table II). All the three surviving persons among NHL group had completed chemotherapy, two of them were on first line ART (stavudine+ lamivudine+nevirapine) and one was receiving salvage ART (tenofovir+lamivudine+darunavir boosted with ritonavir + raltegravir). The mean follow up in ART centre was of 35.6 months after the diagnosis of malignancy. Both patients with Hodgkin's lymphoma had completed chemotherapy and were on first line ART with mean follow up of 27.5 months. The patient diagnosed with CML was receiving imatinib and is currently on first line ART (tenofovir + lamivudine + nevirapine) with consistent follow up of 43 months (Table II).
Table II.
Outcomes of HIV-infected individuals with haematological malignancies (n=15)
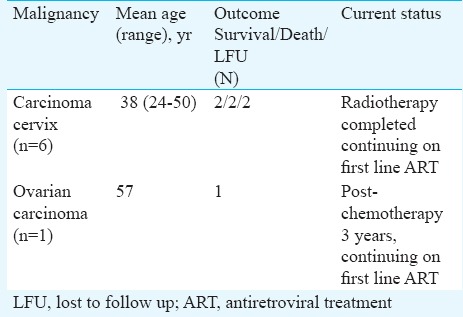
Outcomes of the women diagnosed with gynaecological malignancies are shown in Table III. Of the six women diagnosed with cervical cancer, only two were at regular follow up in the clinic, completed radiotherapy and were on first line ART with a mean follow up of 51 months. The woman who was diagnosed with ovarian cancer completed chemotherapy and was doing well on first line ART with a follow up of 45 months after completion of chemotherapy (Table III).
Table III.
Outcomes of HIV-infected individuals with gynecological malignancies (n=7)
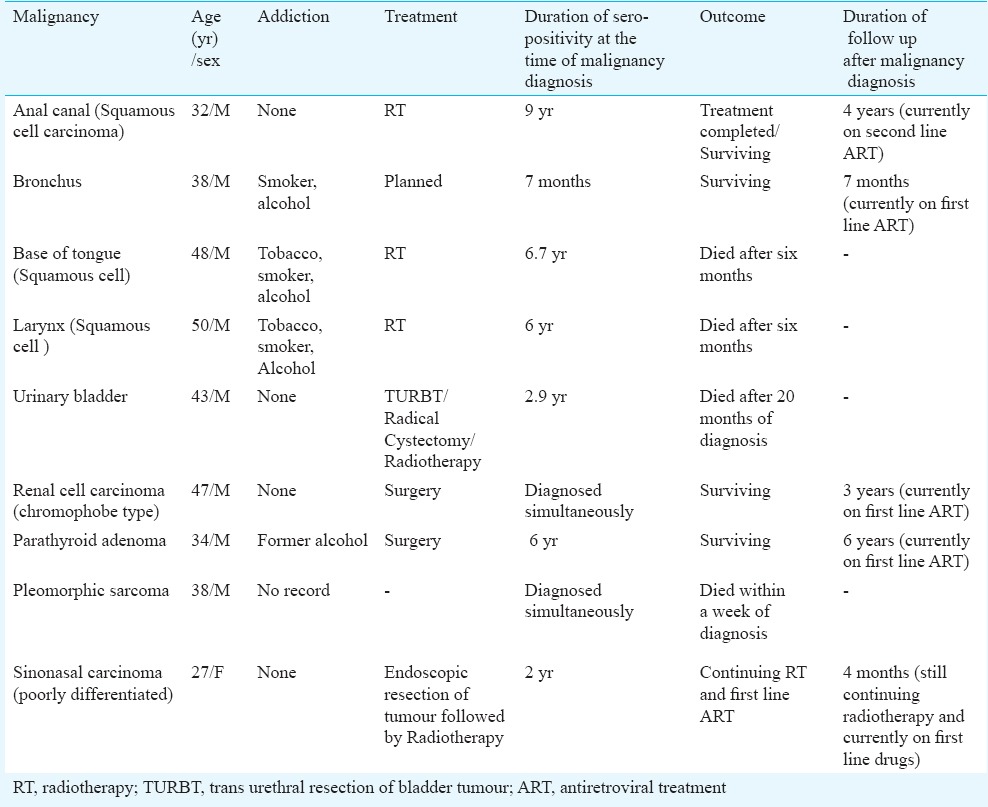
The detailed description of individuals diagnosed with other non AIDS defining cancer with probable risk factors found, treatment offered for malignancy and outcome is shown in Table IV. Of the nine patients, those who were diagnosed with carcinoma anal canal, renal cell carcinoma, parathyroid adenoma, sinonasal carcinoma had completed their respective modality of tumour treatment and were stable on antiretrovirals with a mean follow up of 41 months. ART was started in patient with carcinoma bronchus (Table IV). Individuals diagnosed with carcinoma base of tongue, larynx, pleomorphic sarcoma of chest did not survive.
Table IV.
Outcome of other non-AIDS defining malignancies
Discussion
In this study we analyzed the frequency of occurrence of ADM and NADM amongst patients enrolled in immunodeficiency clinic of a single tertiary care hospital in north India. This study revealed the frequency of occurrence of malignancies among HIV seropositive patients to be 1 per cent at our centre. AIDS defining malignancies were seen more frequently than non-AIDS defining malignancies with non-Hodgkin's lymphoma being the commonest type. HIV infection has been associated with increased risk of cancers12. Immunosuppression and longer life expectancy due to effective HAART have been postulated as factors associated with occurrence of non-AIDS defining malignancies13.
Role of baseline CD4+ cell count (i.e. CD4+ count prior to the initiation of antiretrovirals) in the diagnosis of malignancy is controversial14,15. Biggar et al16 reported that the risk of cancer was inversely related to CD4+ count for all AIDS defining cancers except cervical cancer, where no relationship between CD4+ and cancer incidence was noted. In the present study, there was no significant difference between the baseline CD4+count and CD4+count at the time of malignancy. However, mean CD4+cell count was significantly lower in patients with AIDS defining malignancy when compared with non-AIDS defining malignancy. The mechanisms by which depressed immunity could increase the risk of cancers are not clear, except for that in Kaposi's sarcoma and most subtypes of NHL, where it is associated with a low CD4+ count17. Powles et al18 have highlighted that in the HAART era there is substantial decline in the ADM as compared to NADM.
In our study AIDS defining malignancies were found to be higher than non-AIDS defining malignancies. Agarwal et al19 studied 35 HIV positive individuals with lymphoma in a large cancer hospital from Mumbai and found 24 patients with NHL, seven Hodgkin's and four plasmocytomas. We identified six cases with DLBCL along with one case of CNS lymphoma, one Burktt's lymphoma and one small cell lymphoma. Powari et al20 reviewed 40 CNS NHLs from our hospital from 1985-1999 but none of the 14 patients tested for HIV were found to be infected. We have earlier reported 14 haematological malignancies in 5100 HIV patients registered at our centre in five years (2004-2009) and found NHL in 10 and Hodgkin's in two21. Chitale22 has reported that NHL perhaps affects 2 per cent of HIV infected individuals in India. Another study from a tertiary care hospital in north India reported frequency of malignancies in HIV infected adult patients to be about 1 per cent (26/2598)23. They found 16 cases with NHL and three carcinoma cervix.
Six women in the present study had cervical cancer. Cervical cancer appears to be higher in Indian HIV infected women9. Joshi et al11 reported dysplasia in 6.3 per cent of 287 HIV women screened. Previously, we reported dysplasia in 14 of 136 and one woman with invasive cancer in HIV infected women screened for cervical cancer on routine PAP (papanicolaou) smear examination24.
NADMs which have been consistently found to be more commonly among HIV-infected persons include Hodgkin's lymphoma, carcinoma skin, lung, squamous cell carcinoma of anus, and oral cavity/pharynx cancers. Prostate and breast cancers appear to occur less frequently25. Factors like smoking, alcohol use, and oncogenic virus co-infection, including human papilloma virus (HPV) or hepatitis B and C viruses have been identified as traditional cancer risk factors26,27,28,29. There was history of smoking and tobacco intake in both individuals with carcinoma base of tongue and laryngeal carcinoma in our study.
In conclusion, the frequency of malignancies in HIV infected individuals in our centre was 1 per cent. Of these, ADMs outnumbered NADMs. Non-Hodgkin's lymphoma was the most common malignancy. Further studies are required for risk factor assessment, and to determine the outcome of various screening strategies for early cancer diagnosis in this population.
Acknowledgment
Authors acknowledge the National AIDS Control Organization for providing free antiretroviral therapy and base line investigations to HIV/AIDS patients.
Footnotes
Conflicts of Interest: None.
References
- 1.UNAIDS facts sheet. 2015. [accessed on m0 arch 15, 2016]. Available from: www.unaids.org/sites/default/files/media_asset/20150901_Factsheet_2015_en.pdf .
- 2.Achenbach CJ, Cole SR, Kitahata MM, Casper C, Willi JH, Mugavero MJ, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011;25:691–700. doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d’ArminioMonforte A, et al. Decline in the AIDS and death rates in the EuroSIDA Study: an observational study. Lancet. 2003;362:22–9. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 4.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS related immunosuppression in adults. JAMA. 2001;285:1736–45. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 5.Mocroft A, Brettle R, Kirk O, Blaxhult A, Parkin JM, Antunes F, et al. for the EuroSIDA study group. Changes in the cause of death among HIV positive subjects across Europe: results from the Euro SIDA s0 tudy. AIDS. 2002;16:1663–71. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 6.Patel P, Hanson D, Sullivan P, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet F, Lewden C, May T, Heripret L, Jougla E, Bevilacqua S, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101:317–24. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- 8.Casper C. The increasing burden of HIV associated malignancies in Resource limited regions. Annu Rev Med. 2011;62:157–70. doi: 10.1146/annurev-med-050409-103711. [DOI] [PubMed] [Google Scholar]
- 9.Dhir AA, Sawant S, Dikshit RP, Parikh P, Srivastava S, Badwe R, et al. Spectrum of HIV/AIDS related cancers in India. Cancer Causes Control. 2008;19:147–53. doi: 10.1007/s10552-007-9080-y. [DOI] [PubMed] [Google Scholar]
- 10.Venkatesh KK, Saghayam S, Devaleenal B, Poongulali S, Flanigan TP, Mayer KH, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49:176–80. doi: 10.4103/0019-509X.98947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi SN, Gopalkrishna V, Kumar BK, Dutta S, Nyaynirgune P, Thakar M, et al. Cervical squamous intraepithelial changes and huma papillomavirus infection in women infected with human immunodeficiency virus in Pune, India. J Med Virol. 2005;76:470–5. doi: 10.1002/jmv.20385. [DOI] [PubMed] [Google Scholar]
- 12.Albini L, Calabresi A, Gotti D, Ferraresi A, Festa A, Donato F, et al. Burden of non-AIDS-defining and non-virus-related cancers among HIV-infected patients in the combined antiretroviral therapy era. AIDS Res Hum Retroviruses. 2013;29:1097–104. doi: 10.1089/aid.2012.0321. [DOI] [PubMed] [Google Scholar]
- 13.Silverberg MJ, Chun Chao C, Leyden WA, Xu L, Tang B, Horberg MA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–45. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbaro G, Barbarini G. HIV infection and cancer in the era of highly active antiretroviral therapy. Oncol Rep. 2007;17:1121–6. [PubMed] [Google Scholar]
- 15.Crum-Cianflone NF, Burgi A. Cancer among HIV-infected persons. Infect Dis Clin Pract. 2006;14:258–65. [Google Scholar]
- 16.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. HIV/AIDS Cancer Match Study. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–72. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 17.Ledergerber B, Telenti A, Egger M. Risk of HIV related Kaposi's sarcoma and non Hodgkin's lymphoma with potent antiretroviral therapy: prospective cohort study. BMJ. 1999;319:23–4. doi: 10.1136/bmj.319.7201.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, et al. Highly sctive antiretroviral therapy and the incidence of non-AIDS defining cancers in people with HIV infection. J Clin Oncol. 2009;27:884–90. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal B, Ramanathan U, Lokeshwas N, Nair R, Gopal R, Bhatia K, et al. Lymphoid neoplasms in HIV-positive individuals in India. J Acquir Immune Defic Syndr. 2002;29:181–3. doi: 10.1097/00042560-200202010-00012. [DOI] [PubMed] [Google Scholar]
- 20.Powari M, Radotra B, Das A, Banerjee AK. A study of primary central nervous system lymphoma in northern India. Surg Neurol. 2002;57:113–6. doi: 10.1016/s0090-3019(01)00675-9. [DOI] [PubMed] [Google Scholar]
- 21.Sachdeva RK, Sharma A, Wanchu A, Malhotra P, Varma S. Hematological malignancies in HIV positive individuals in North India. Leuk Lymphoma. 2011;52:1597–600. doi: 10.3109/10428194.2011.574756. [DOI] [PubMed] [Google Scholar]
- 22.Chitale AR. Cancer and AIDS. Indian J Pathol Microbiol. 2005;48:151–60. [PubMed] [Google Scholar]
- 23.Sharma SK, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: Initial experience in the HAART era. Indian J Med Res. 2015;142:563–7. doi: 10.4103/0971-5916.171283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagga R, Wanchu A, Rajwanshi A, Gupta KR, Prasad GRV, Gopalan S, et al. Papanicolaou smear abnormalities HIV-infected women in North India. Asia-Pacific J Clin Oncol. 2005;1:77–80. [Google Scholar]
- 25.Pantanowitz L, Dezube BJ. Evolving spectrum and incidence of non-AIDS-defining malignancies. Curr Opin HIV AIDS. 2009;4:27–34. doi: 10.1097/COH.0b013e32831a7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–22. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frazer IH, Medley G, Crapper RM, Brown TC, Mackay IR. Association between anorectal dysplasia, human papillomavirus and human immunodeficiency virus infection in homosexual men. Lancet. 1986;2:657–60. doi: 10.1016/s0140-6736(86)90168-6. [DOI] [PubMed] [Google Scholar]
- 28.Mendy ME, Welzel T, Lesi OA, Hainaut P, Hall AJ, Kuniholm MH, et al. Hepatitis B viral load and risk for liver cirrhosis and hepatocellular carcinoma in the Gambia, West Africa. J Viral Hepat. 2010;17:115–22. doi: 10.1111/j.1365-2893.2009.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, et al. Randomized trial of effects of interferon-alpha on incidence of hepatocellular carcinomain chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–5. doi: 10.1016/s0140-6736(95)91739-x. [DOI] [PubMed] [Google Scholar]


