Abstract
Background & objectives:
Loss of function of adenomatous polyposis coli (APC) has been reported in cancer. The two promoters of APC, 1A and 1B also have roles in cancer. But, the epigenetic role of APC promoters is not yet clear in gallbladder cancer (GBC) and gallstone diseases (GSD). We undertook this study to determine the epigenetic role of APC in GBC and GSD.
Methods:
Methylation-specific (MS)-PCR was used to analyze the methylation of APC gene. The expression of APC gene was studied by semi-quantitative PCR, real-time PCR and immunohistochemistry (IHC) in GBC, GSD and adjacent normal tissues.
Results:
Of the two promoters, APC 1A promoter was found methylated in 96 per cent GBC (P=0.0155) and 80 per cent GSD (P=0.015). Exon 1 was downregulated in grade II (P=0.002) and grade III (P=0.0001) of GBC, while exon 2 was normally expressed. Scoring analysis of IHC revealed 0 or negativity in 34.48 per cent (P=0.057) and 1+ in 24.14 per cent (P=0.005) GBC cases suggesting loss of APC expression.
Interpretation & conclusions:
The present findings indicate epigenetic silencing of APC in advanced GBC. The methylation pattern, followed by expression analysis of APC may be suggested for diagnostic, prognostic and therapeutic purposes in GBC in future.
Keywords: Adenomatous polyposis coli, epigenetics, gallbladder cancer, gallstone disease, tissue microarray
Gallbladder cancer (GBC) is a common neoplasm with high incidence in central India1. Due to lack of suitable diagnostic early biomarkers and its location inside the body, the success rate of chemotherapy and radiotherapy treatments has remained a major problem. Therefore, its early diagnosis is important in disease management. Adenomatous polyposis coli (APC) is a tumour suppressor gene, located on chromosome 5q212,3. Multiple forms of APC transcripts were found to be expressed in tissue specific manner, and its two transcripts have origin from exon 1A and 1B, respectively4,5,6,7. Differential methylation patterns of 1A and 1B are involved in cancers of gastric, skin, breast and lung7,8,9. Both somatic10 and germline11 mutations of APC play a key role in familial adenomatous polyposis (FAP) and colorectal cancer. Inactivation of 1B has been observed in FAP due to deletion mutation in the promoter region12. Lower frequency of APC promoter methylation was earlier reported in Chilean GBC patients13,14. But, the actual role of both promoters is not yet elucidated in GBC. Based on our preliminary observations, we hypothesize that APC might be functionally inactive in GBC due to promoter methylation. Here, we report which promoter(s) of APC, 1A, 1B or both, is responsible for loss of expression of this gene in GBC and GSD (gallstone disease), that provides a significant clue on its role in the pathogenesis of GBC.
Material & Methods
Gallbladder tissue samples were collected from Cancer Hospital and Research Institute (CHRI), Gwalior, India. Prior to collection of samples, a written informed consent was obtained from the patients. The study protocol was approved by the Institutional Ethics Committee of Jiwaji University, Gwalior, India. Freshly resected tissues were transported from the Department of Pathology, CHRI, to the Centre for Genomics, Jiwaji University in an ice pack (-10°C). All GBC and GSD samples, finally diagnosed by fine needle aspiration cytology (FNAC) and histopathological examination, were included in the study. Epigenetic study was done in 50 GBC, 30 GSD and corresponding adjacent normal tissues (ANT). For expression analysis at RNA level, 20 GBC, 20 GSD tissues and 20 ANT were selected. For immunohistochemical analysis on tissue microarray (TMA), archival 138 gallbladder cases (88 GBC, 31 GSD and 19 adjacent normal tissues) were included in the study. Tissue samples were collected during January 2009 - December 2013, and stored at -70°C until use.
Genomic DNA isolation and bisulphite modification: Genomic DNA was isolated from the gallbladder tissues by manual phenol-chloroform-isoamyl alcohol method15. The quality and quantity of the DNA was checked by UV-VIS Spectrophotometer (Shimadzu Scientific Instruments, Japan). One μg of genomic DNA was used for bisulphite modification (Zymo Research, USA); 100 per cent methylated standard gold DNA (Zymo Research, USA) was used as positive control and the reaction was set with unmodified genomic DNA as negative control in methylation specific PCR (MS-PCR).
Methylation-specific PCR (MS-PCR): Methylation-specific PCR was carried as described by Herman et al16, with slight modification. The master mix was composed of 1.5 mM MgCl2, 200 μM dNTPs, 10 pmol each of forward and reverse primers and 1U of Hotstart Taq polymerase (Qiagen, Germany). The primer sequences of APC 1A promoter were - AMF (Promoter IA, methylated, forward): 5’-TGTTTTGCGGATTTTTTTC-3’, AMR: 5’-GCAATAAAACACAAAACCCCG-3’ and AUMF (IA, unmethylated, forward) : 5’-GTGTTTTATTGTGGAGTGTGGGTT-3’, AUMR: 5’-CCAATCAACAAACTCCCAACAA-3’, and APC 1B promoter are BMF (Promoter IB, methylated, forward): 5’-TGTTTAGGTAGTAATGGTTTAC-3’, BMR: 5’-TAAAACCTATTATACGCAAACG-3’ and (Promoter 1B, unmethylated, forward): 5’-GGTTGTTTAGGTAGTAATGGTTTAT-3’, BUR: 5’-AAACTAAAACCTATTATACACAAACA-3’7. The PCR products were separated on 10 per cent polyacrylamide gel, followed by silver staining. Gel images were acquired by gel documentation system (BioRad, USA).
Total RNA and cDNA preparation: Frozen tissues (25 mg), stored in RNA Later Solution (Qiagen, Germany), were taken for total RNA preparation using RNAeasy Fibrous Tissue kit (Qiagen, Germany). QIAshredder spin column (Qiagen, Germany) was used for tissue homogenization. Quality control of RNA was done by Bio-analyzer 2100 (Agilent, USA). cDNA was prepared from 750 ng of total RNA by using QuantiTect Reverse Transcription Kit (Qiagen, Germany), following the manufacturer's instructions. Working aliquots of cDNAs were prepared in the ratio of 1:30 and kept frozen until further use.
Semi-quantitative reverse transcriptase PCR (RT-PCR): Two μl of cDNA sample (working) was used in 25 μl reaction mix for RT-PCR. The reaction master mix contained 1× Taq PCR buffer, 4 pmol of each primer, 2 mM MgCl2, 200 μM dNTPs and 1U Taq Polymerase (Fermentas, USA). β-actin gene was used as internal control and reaction set without template as negative control. Primer sequences of APC exon 1A were - forward: 5’-GGAGACAGAATGGAGGTGC-3’ and reverse: 5’- CAACTGATCATATGAAGCTGCAGCCAT-3’, and for exon 1B forward: 5’-GCGAGCAGGAGCTGCGT-3’, and reverse: 5’- CAACTGATCATATGAAGCTGCAGCCAT- 3’7. The primer sequences of beta actin were forward: 5’-CCAGAGCAAGAGAGGTATCC-3’, and reverse: 5’-CTGTGGTGGTGAAGCTGTAG-3’17. Equal amount of PCR products (5 μl) were separated on 10 per cent polyacrylamide gel, followed by silver staining15. In densitometric analysis, after loading the gel to the Image Lab software (Gel Doc XR+, BioRad, USA), lanes were manually marked and adjusted respective to their positions. Band detection sensitivity programme detected the correct bands on the gel against a 100 bp ladder (Fermentas, USA).
Real-time PCR: Two μl of diluted cDNA, each from GBC and GSD, was used for real-time PCR to estimate the normalized relative fold expression ΔΔCT of exon 1 (1A) and exon 2 (1B) in the GBC and GSD tissues with respect to their adjacent normal tissues. Average of CT values of APC exon 1 and exon 2 were 29.65 and 29.21 in GBC, 28.65 and 28.71 in GSD, respectively. The CT value of beta-actin was in 28.05 and 27.34 in GBC and GSD, respectively. Each reaction was set in duplicate. The primers used in the semi-quantitative PCR, were also used in real-time PCR (CFX96 BioRad, USA). Power SYBR green PCR mix (Applied Biosystem, USA) was used for real time PCR. CFX manager software (BioRad, USA) was used for quantitative analysis.
Immunohistochemistry (IHC): Tissue microarray (TMA) of 138 archival gallbladder tissues in duplicate cores was commercially developed. These samples from gallbladder cancer patients included grade I=13, grade II=32, grade III=35, poorly differentiated adenocarcinoma (PDA)=4, squamous cell carcinoma (SCC)=2, colloidal carcinoma (CoC)=1, mucinous carcinoma (MC)=1, GSD=31 and adjacent normal tissues (ANT)=19. One core each of kidney, ovary, cervix, liver, stomach, colon, salivary and breast were included as internal tissue controls. Vectastain Universal ABC kit (Vector laboratories, USA) was used for immunohistological staining. For tissue staining, the protocol given in the kit was followed, but with slight modification as standardised in our laboratory. The primary antibody against APC (Santa Cruz Biotechnology Inc., USA; Catalogue number sc-896) was used at a dilution of 1:500. Nuclei were counterstained with haematoxylin. Images were acquired and analysed in a fluorescence microscope (DM 4000, Leica, Germany). Scoring and analysis were done according to America Society of Clinical Oncology/College of American Pathologists Guidelines Recommendations18.
Statistical analysis: For methylation analysis, Student's t test was performed to determine the differences in the methylation status of tumour and control samples using Graph pad Prism version 519. Linear regression analysis was performed to check the association of methylation with GBC and GSD. For RNA and protein analyses, Student's t test was performed to test the significance of difference in APC expression.
Results
In a total of 80 patients (50 gallbladder cancer and 30 gallstone diseases), almost 80 per cent of the gallbladder cases possessed either gallstones or chronic inflammation (chronic cholecystitis). Most of the patients (>70%) included in this study were females (n=49) from the age group 40-50 yr, and had adenocarcinoma type of gallbladder. Mean age of GBC patients was 47 ± 8.5 yr (Table).
Table.
Details of the patients recruited for methylation specific PCR (MSPCR) and semi-quantitative PCR
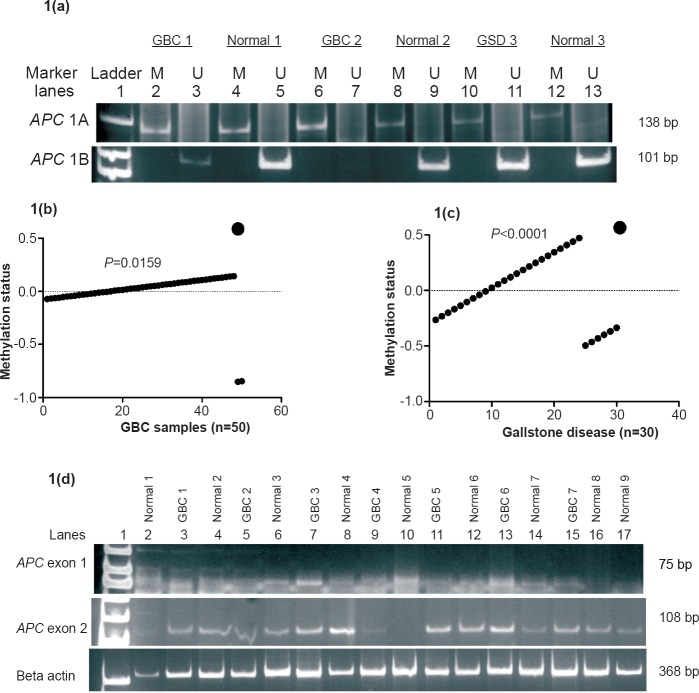
Methylation status of promoter 1A and 1B in gallbladder cancer: MSP analysis showed the presence of methylated alleles of APC exon 1 (1A) in 46 (96%; P=0.0155) GBC cases (Fig. 1a). Linear regression analysis revealed significant association of APC promoter 1A methylation with GBC (P=0.0159) (Fig. 1b). A few samples of adjacent normal tissues of GBC (n=10) also displayed methylation. Both methylation and unmethylation were observed in 12 GBC and five adjacent normal tissues. 1B promoter region was unmethylated. The DNA samples from 30 cancerous tissues showed methylated allele of 1A (AM), but none of the cancerous samples examined, showed unmethylated 1A (AU) allele (Table). Most tumour cells, which had origin at the body region of gallbladder and metastasized in hepatic cells, showed higher frequency of methylation. None of the samples showed presence of methylated alleles of 1B (BU).
Fig. 1.
(a). Polyacrylamide gel (silver stained) showing MS-PCR products of methylated (M) and unmethylated (U) 1A and 1B promoters of APC in gallbladder cancer (GBC), gallbladder disease (GSD) tissues and ANT (adjacent normal tissues). Lane 7 showed faint band for unknown reason. (1b) Linear regression analysis of APC methylation and GBC. (1c) Linear regression analysis of APC methylation and GSD. (1d) Polyacrylamide gel (silver stained) showing semi-quantitative PCR products of exon 1, exon 2 of APC and beta actin gene in GBC, GSD and ANT (adjacent normal tissues) tissues of gallbladder. Lane 10 of APC exon 2 did not show amplification of unknown reason.
Methylation status of promoter 1A and 1B in gallstone diseases: Of the 30 GSD samples, 24 (80%; P=0.015) showed methylated allele of 1A promoter region (Fig. 1a). Regression analysis showed a positive association of APC 1A promoter methylation in GSD (P<0.0001) (Fig. 1c). While only five adjacent normal tissues showed methylation, 25 adjacent normal tissues displayed no methylation. Out of 24 GSD, only two samples showed both methylated and unmethylated alleles of 1A. Only six GSD samples possessed unmethylated allele of 1A (AU), while all 30 GSD and adjacent normal tissues showed absence of methylated alleles of 1B (BU).
Expression status (transcription) of exon 1 (1A) and exon 2 (1B) in gallbladder cancer: Semi-quantitative and quantitative RT-PCRs were carried out to estimate the levels of exon 1 and exon 2 transcripts. Comparative banding pattern and densitometric analyses (after semi-quantitative PCR) revealed downregulation of exon 1 and normal transcription of exon 2 in both GBC and GSD (see Fig. 1d). On densitometric analysis, exon 1 was quantified to be about 17.6 U (or ng/μl) in grade I (25.5 U in adjacent normal tissues from grade I), 12.96 U in grade II (29.4 U in adjacent normal tissues from grade II), and 11.5 U in grade III (25 U in adjacent normal tissues from grade III) GBC tissues (Fig. 2a). The downregulation of 1A exon in grade II GBC samples was found to be significant. Real-time PCR analysis also revealed < 3 unit fold expression of exon1 in GBC grade II (P=0.002) and < 1 unit fold expression in grade III (P=0.0001) tissues with respect to their adjacent normal tissues (Fig. 3a, 3b).
Fig. 2a.
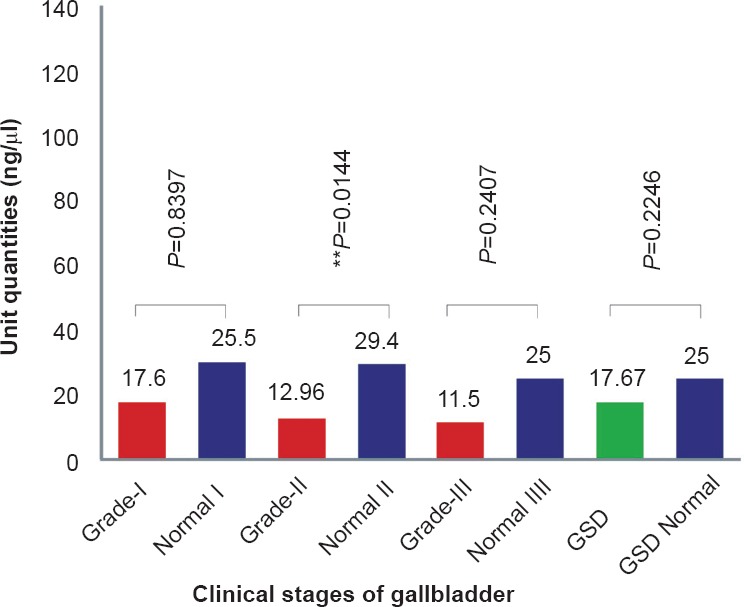
Semi-quantitative PCR analysis showing the estimated concentrations or expression level (U or ng/μl) of APC exon1 in GBC, GSD and ANT (adjacent normal tissues).
Fig. 3a.
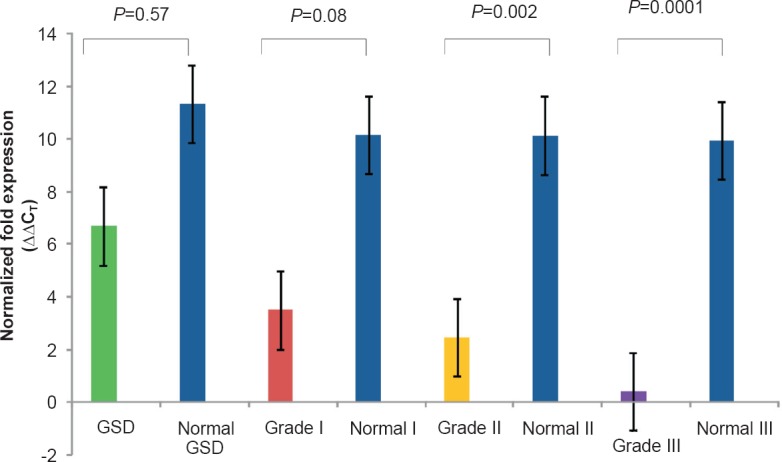
Real-time PCR analysis showing normalized relative fold expression, ΔΔCT of APC exon 1 in GSD and different GBC grades as compared to their respective adjacent normal tissues.
Fig. 3b.
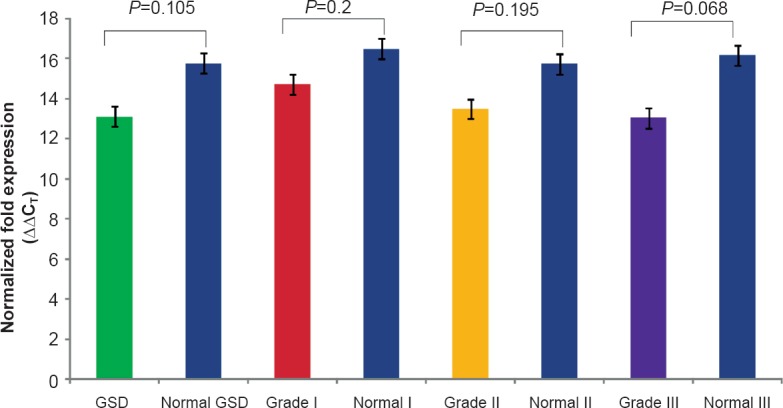
Real-time PCR analysis showing normalized relative fold expression, ΔΔCT of APC exon 2 in tissues of GSD and different GBC grades as compared to their respective adjacent normal tissues.
The approximate quantities of exon 2 in different GBC tissue samples were 36.06 U in grade I (62.7 U in adjacent normal tissues from grade I), 23.9 U in grade II (61 U in adjacent normal tissues from grade II) and 65.56 U in grade III (67 U in adjacent normal tissues from grade III) (Fig. 2b). However, the real time analysis of exon 2 was not significant.
Fig. 2b.
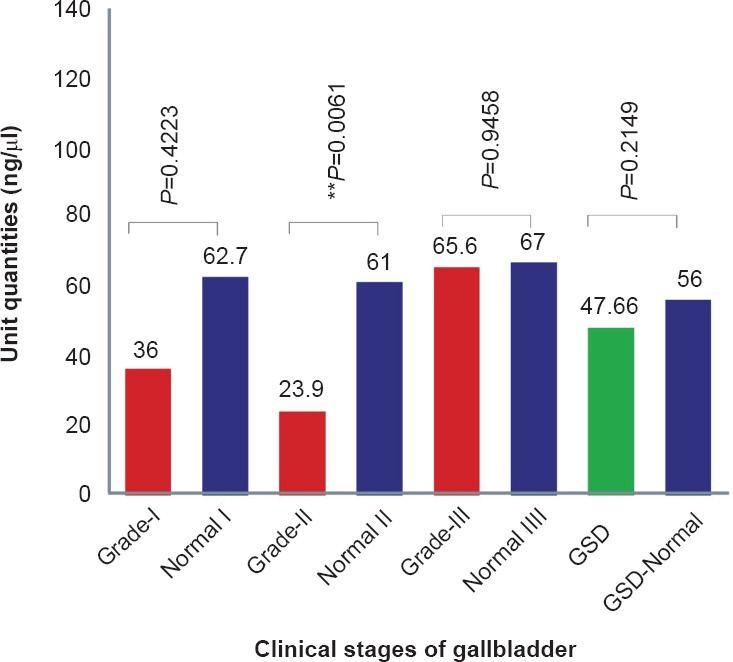
Semi-quantitative PCR analysis showing the estimated concentration or expression level (U or ng/μl) of APC exon 2 in GBC, GSD and ANT (adjacent normal tissues).
Expression status (transcription) of exon 1 (1A) and exon 1 (1B) in gallstone diseases: Semi-quantitative PCR showed downregulation of 1A in GSD, as also observed in GBC. Exon 1 was amplified at an average value of 17.67 U and 25 U in GSD and adjacent normal tissues, respectively. Of the GSD samples, two showed visibly faint band. Exon 2 was transcribed normally in 19 GSD samples with an average value of 47.66 U, as compared to 56 U in adjacent normal tissues. Only one sample showed weak amplification. Real-time PCR analysis also revealed normalized 6.5 unit fold expression of exon 1 in comparison to their respective adjacent normal tissues with normalized 12 unit fold expression (Fig. 3a, 3b). Real time analysis of exon 2 was not significant in GSD.
Expression level of APC protein in gallbladder cancer and gallstone diseases: In TMA analysis for APC expression, we observed complete silencing or negative scores of APC in grades II and III of GBC, as well as in various subtypes of GBC, including poorly differentiated adenocarcinoma, colloid carcinoma and mucinus carcinoma. Both adjacent normal tissues and GSD were indiscriminately scored. In all GBC cores, less than 6 per cent of tumour cells showed weak expression of APC (Fig. 4), about 34.48 per cent of GBC cases scored negative (P=0.057), about 24.14 per cent of GBC cases showed very low intensity of stain, i.e. 1+ score (P=0.005), 25.85 per cent of early stage or GSD showed normal expression of APC, i.e. scores 2+ (P=0.091), and the remaining 15.52 per cent showed exceptionally intense stain of APC antibody (P=0.078).
Fig. 4.
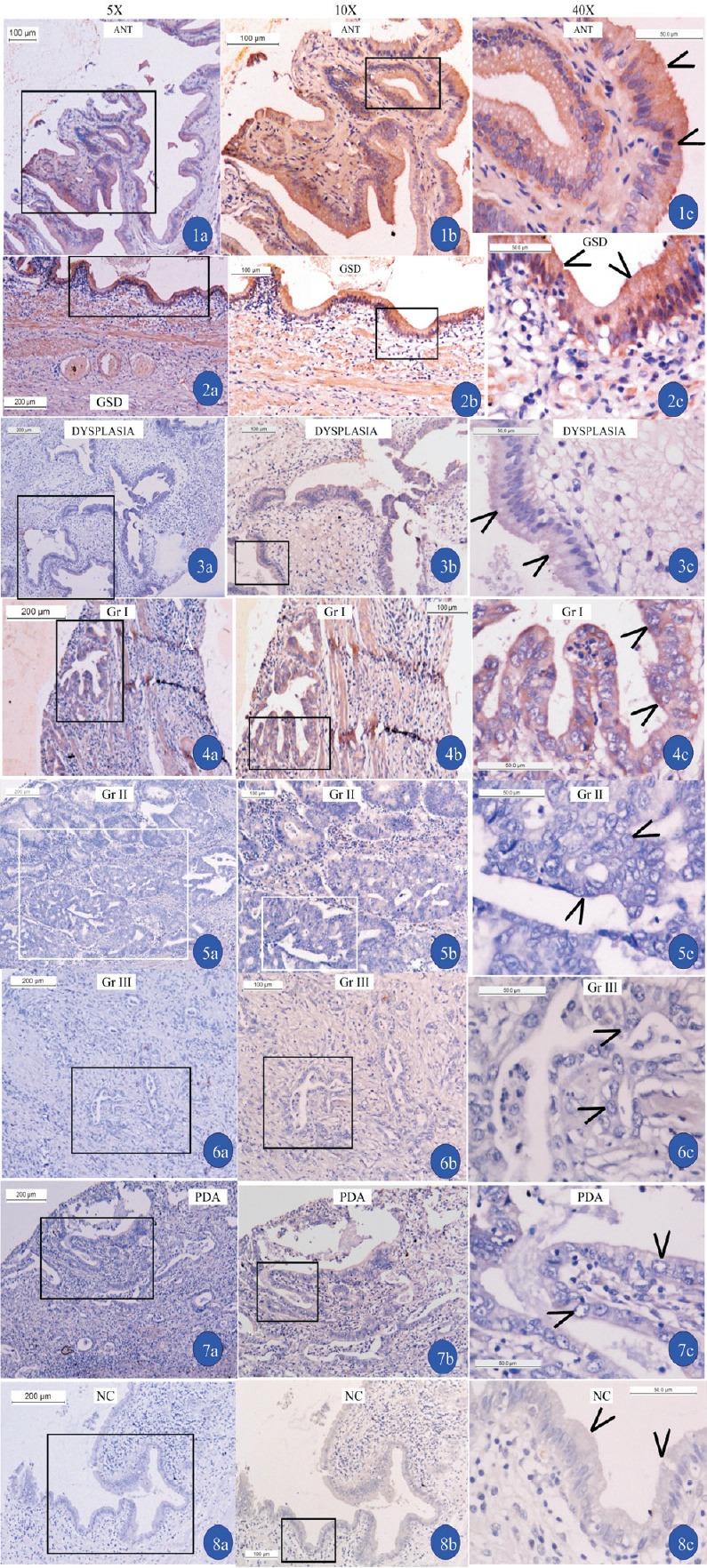
Immunohistochemistry of APC in ANT (adjacent normal tissues) gallbladder tissues (1), GSD (2), dysplasia (3) grades I (4), II (5), III GBC (6), and poorly differentiated adenocarcinoma (PDA). Negative control (NC) slide (8) is without primary antibody to check the non-specific stain in gallbladder tissue and positive controls were taken from eight different tissues. Arrow heads show the cells where cytoplasmic staining of antibody APC is observed (a=5X, b=10X and c=40X magnifications). Inset shown in (a) is magnified in (b), and inset shown in (b) is magnified in (c).
Discussion
The present study showed epigenetic alteration of APC in the molecular pathogenesis of gallbladder cancer and gallstone diseases as reported in tumorigenesis of stomach7, melanoma8, prostate20, breast21 and colorectum22. Lower frequency of methylation in 27 and 30 per cent of GBC13,14 in Chilean population has encouraged us to further investigate the expression of APC in our sample. We observed both methylated and unmethylated APC 1A alleles in 12 GBC, two GSD and five ANT which could be due to the presence of mono-alleles or heterogenous hypermethylation. High frequency of APC 1A promoter methylation in GBC and GSD indicates importance of differential role of exon 1A (not exon 1B) in gallbladder carcinogenesis. About 80 per cent of GBC possesses stones irrespective of size and number23 suggesting the mechanistic influence of stones to the epithelial cells to display hypermethylation. Single nucleotide polymorphisms (SNPs) and mutations are the common mechanisms to inactivate APC in familial adenomatous polyposis, colorectal, breast, pancreatic and Lung cancers2,10,11,24,25,26,27,28. Such a mechanism is also possible in GBC.
Functional inactivation of APC in GBC and not in GSD: High frequency of methylation of APC was further processed to evaluate the expression pattern in GBC and GSD. A differential expression pattern of APC exon 1 and 2 was observed in GBC. A slight downregulation of APC exon 1 in GSD indicates an early onset of epigenetic regulation in GSD. Exon 2 was not of significance. Promoter methylation of APC is responsible for loss of expression in stomach7, breast and lung9 cancers. Mutation in APC is one of the mechanisms responsible for tumorigenic transformation of normal cells of colon24, pancreas25, lung26 and stomach27,28. Two cases of GBC and ANT showed similar pattern of expression of APC exon 1A suggesting normal transcription mechanism. Moreover, two GSD amplified very weak signal of exon 1A. This may likely be explained due to the presence of heterozygous methylated alleles which might mask the binding of transcription factors, thereby, blocking the transcription of exon 1A. A low level of insignificant expression of exon 2 in GBC and GSD may have protected the normal cells from transformation. This explain the role of exon 1 (promoter 1A), rather than exon 2 (promoter 1B), in the tumorigenesis of gallbladder.
Normal translation of APC requires both 1A and 1B to be active. Our IHC data showed loss of expression of APC in advanced GBC grades II and III, not in GSD. This suggests an altered translation of APC despite normal status of exon 1B. Contradictory findings on APC 1B in cancers of gastric epithelium7, breast9, etc. need to be studied further to unravel the differential role of the two exons in GBC. Negative scoring of grades II and III cases further confirms epigenetic silencing of APC. The similar expression level of APC in GSD and ANT suggests that APC does not have any role in the transformation of GSD to GBC, and downregulation of APC is just initiated during the early stage of GBC, i.e. Grade I. The present study was conducted in limited numbers of human GBC tissue samples (not in blood). But, the final validation needs large number of samples as well as in vitro analysis using GBC cell lines.
In conclusion, 1A promoter plays a cardinal role in the epigenetic silencing of APC in GBC. Epigenetic guided loss of expression of APC may be one of the key steps towards gallbladder carcinogenesis. Thus, detection of methylation pattern followed by expression analysis of APC may be useful in the disease management/prognosis or diagnosis of GBC.
Acknowledgment
Authors acknowledge the financial support from Department of Science and Technology (DST), Government of India, New Delhi. The first author (DST) was a recipient of Senior Research Fellowship from Indian Council of Medical Research (ICMR), New Delhi.
Footnotes
Conflicts of Interest: None.
References
- 1.Barbhuiya MA, Singh TD, Poojary SS, Gupta S, Kakkar M, Shrivastav BR, et al. Gallbladder cancer incidence in Gwalior district of India: Five-year trend based on the registry of a regional cancer center. Indian J Cancer. 2015;52:430–7. doi: 10.4103/0019-509X.176736. [DOI] [PubMed] [Google Scholar]
- 2.Joslyn G, Carlson M, Thliveris A, Albertsen H, Gelbert L, Samowitz W, et al. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66:601–13. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Mef CN, Li-Kuo S, Vogelstein B, Tracy MB, Daniel B, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 4.Horii A, Nakatsuru S, Ichii S, Nagase H, Nakamura Y. Multiple forms of the APC gene transcripts and their tissue-specific expression. Hum Mol Genet. 1993;2:283–7. doi: 10.1093/hmg/2.3.283. [DOI] [PubMed] [Google Scholar]
- 5.Lambertz S, Ballhausen WG. Identification of an alternative 5′ untranslated region of the adenomatous polyposis coli gene. Hum Genet. 1993;90:650–2. doi: 10.1007/BF00202484. [DOI] [PubMed] [Google Scholar]
- 6.Hosoya K, Yamashita S, Ando T, Nakajima T, Itoh F, Ushijima T. Adenomatous polyposis coli 1A is likely to be methylated as a passenger in human gastric carcinogenesis. Cancer Lett. 2009;285:182–9. doi: 10.1016/j.canlet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya T, Gen T, Kiyoshi S, Yasushi E, Ken S, Zhe J, et al. Distinct methylation patterns of two APC gene promoters in normal and cancerous gastric epithelia. Oncogene. 2000;19:3642–6. doi: 10.1038/sj.onc.1203704. [DOI] [PubMed] [Google Scholar]
- 8.Korabiowska M, Thilo S, Nils S, Müller A, Cordon-Cardo C, Fischer G, et al. Analysis of adenomatous polyposis coli gene expression, APC locus-microsatellite instability and APC promoter methylation in the progression of melanocytic tumours. Mod Pathol. 2004;17:1539–44. doi: 10.1038/modpathol.3800238. [DOI] [PubMed] [Google Scholar]
- 9.Virmani AK, Rathi A, Sathyanarayana UG, Padar A, Huang CX, Cunnigham HT, et al. Aberrant methylation of the adenomatous polyposis coli (APC) gene promoter 1A in breast and lung carcinomas. Clin Cancer Res. 2001;7:1998–2004. [PubMed] [Google Scholar]
- 10.Nishisho I, Yusuke N, Yasuo M, Miki Y, Ando H, Horii A, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–9. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi Y, Hiroshi A, Hiroki N, Nishisho I, Horii A, Miki Y, et al. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA. 1992;89:4452–6. doi: 10.1073/pnas.89.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohlin A, Engwall Y, Fritzell K, Göransson K, Bergsten A, Einbeigi Z, et al. Inactivation of promoter 1B of APC causes partial gene silencing: evidence for a significant role of the promoter in regulation and causative of familial adenomatous polyposis. Oncogene. 2011;30:4977–89. doi: 10.1038/onc.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.House MG, Wistuba II, Argani P, Guo M, Schulick RD, Hruban RH, et al. Progression of gene hypermethylation in gall stone disease leading to gall bladder cancer. Ann Surg Oncol. 2003;10:882–9. doi: 10.1245/aso.2003.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Shivapurkar N, Riquelme E, Shigematsu H, Reddy J, Suzuki M, et al. Aberrant promoter hypermethylation of multiple genes in gall bladder carcinoma and chronic cholecystitis. Clin Cancer Res. 2004;10:6126–33. doi: 10.1158/1078-0432.CCR-04-0579. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Russel DW. Molecular cloning: A laboratory manual. 3rd ed. Vol. 1. New York: Cold Spring Harbor Laboratory; 2001. pp. 64–611. [Google Scholar]
- 16.Herman JG, Jeremy RG, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–6. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinyo Y, Kodama J, Hongo A, Yoshinouchi M, Hiramatsu Y. Heparanase expression is an independent prognostic factor in patients with invasive cervical cancer. Ann Oncol. 2003;14:1505–10. doi: 10.1093/annonc/mdg407. [DOI] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 19.Graph pad prism5. San Diego, California, USA: Available from: www.graphpad.com . [Google Scholar]
- 20.Richiardi L, Fiano V, Vizzini L, De Marco L, Delsedime L, Akre O, et al. Promoter methylation in APC, RUNX3, and GSTP1 and mortality in prostate cancer patients. J Clin Oncol. 2009;27:3161–8. doi: 10.1200/JCO.2008.18.2485. [DOI] [PubMed] [Google Scholar]
- 21.Müller HM, Andreas W, Heidi F, Ivarsson L, Goebel G, Perkmann E, et al. DNA methylation in serum of breast cancer patients an independent prognostic marker. Cancer Res. 2003;63:7641–5. [PubMed] [Google Scholar]
- 22.Lin SY, Yeh KT, Chen WT, Chen HC, Chen ST, Chiou HY, et al. Promoter CpG methylation of tumor suppressor genes in colorectal cancer and its relationship to clinical features. Oncol Rep. 2004;11:341–8. [PubMed] [Google Scholar]
- 23.Singh TD, Barbhuiya MA, Poojary S, Shrivastav BR, Tiwari PK. The liver function test enzymes and higher glucose level are positively correlated in gallbladder cancer from north central India: A cancer registry data analysis from north Central India. Indian J Cancer. 2012;49:125–36. doi: 10.4103/0019-509X.98938. [DOI] [PubMed] [Google Scholar]
- 24.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–4. [PubMed] [Google Scholar]
- 25.Horii A, Shuichi N, Yasuo M, Ichii S, Nagase H, Ando H, et al. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res. 1992;52:6696–8. [PubMed] [Google Scholar]
- 26.D’Amico D, Carbone DP, Johnson BE, Meltzer SJ, Minna JD. Polymorphic sites within the MCC and APC loci reveal very frequent loss of heterozygosity in human small cell lung cancer. Cancer Res. 1992;52:1996–9. [PubMed] [Google Scholar]
- 27.Nakatsuru S, Yanagisawa A, Ichii S, Tahara E, Kato Y, Nakamura Y, et al. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet. 1992;1:559–63. doi: 10.1093/hmg/1.8.559. [DOI] [PubMed] [Google Scholar]
- 28.Horii A, Shuichi N, Yasuo M, Ichii S, Nagase H, Kato Y, et al. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Res. 1992;52:3231–3. [PubMed] [Google Scholar]