Abstract
Purpose
To investigate changes in volumes of extraocular muscle (EOM) compartments in unilateral superior oblique (SO) palsy using magnetic resonance imaging (MRI).
Methods
High-resolution, surface-coil MRI was obtained in 19 patients with unilateral SO palsy and 19 age-matched orthotropic control subjects. Rectus EOMs and the SO were divided into two anatomic compartments for volume analysis in patients with unilateral SO palsy, allowing comparison of total compartmental volumes versus controls. Medial and lateral compartmental volumes of the SO muscle were compared in patients with isotropic (round shape) versus anisotropic (elongated shape) SO atrophy.
Results
The medial and lateral compartments of the ipsilesional SO muscles were equally atrophic in isotropic SO palsy, whereas the lateral compartment was significantly smaller than the medial in anisotropic SO palsy (P = 0.01). In contrast to the SO, there were no differential compartmental volume changes in rectus EOMs; however, there was significant total muscle hypertrophy in the ipsilesional inferior rectus (IR) and lateral rectus (LR) muscles and contralesional superior rectus (SR) muscles. Medial rectus (MR) volume was normal both ipsi- and contralesionally.
Conclusions
A subset of patients with SO palsy exhibit selective atrophy of the lateral, predominantly vertically acting SO compartment. Superior oblique atrophy is associated with whole-muscle volume changes in the ipsilesional IR, ipsilesional LR, and contralesional SR; however, SO muscle atrophy is not associated with compartmentally selective volume changes in the rectus EOMs. Selective compartmental SO pathology may provide an anatomic mechanism that explains some of the variability in clinical presentations of SO palsy.
Keywords: superior oblique palsy, extraocular muscle, magnetic resonance imaging, strabismus, hypertropia
It has long been recognized that many individual skeletal muscles are composed of two or more distinct neuromuscular compartments capable of selective motor functions.1–5 These include trapezius,2,5 tibialis anterior,3 and peroneus longus4 in humans. Such compartmentalization expands the functional repertoires of each of these muscles. Compartmentalization has recently become recognized in the extraocular muscles (EOMs), as demonstrated by histologic6,7 and magnetic resonance imaging (MRI) studies.8–12 The medial rectus (MR) and lateral rectus (LR) muscles are each supplied by separate superior and inferior branches of motor nerves that have nonoverlapping distributions.6,7 The inferior rectus (IR) muscle, although mostly innervated by one motor nerve branch, has an additional separate nerve branch that co-innervates only the lateral third of the IR.7 The motor nerves to both oblique EOMs, the trochlear nerve,11 and the distal branch of the oculomotor nerve12, bifurcate before entering into the EOM bellies, and innervate two nonoverlapping compartments. The superior rectus (SR) muscle is the only EOM for which selective intramuscular innervation has not been identified.7 Functional MRI studies have demonstrated differential compartmental functions of specific EOMs during ocular counter-rolling, asymmetrical convergence, vertical fusional vergence, and vertical duction.8–10,13
The two compartments of the superior oblique (SO) muscle have different mechanical advantages for vertical versus torsional actions.11 Tendon fibers of the medial SO compartment insert near the globe equator and thus mainly implement torsion. Fibers of the lateral SO compartment insert posterior to the equator and have mainly vertical action. This anatomic difference has been supported by an MRI study of the association of morphologies of SO atrophy with clinical features in SO palsy.14 Denervation of the SO rapidly produces neurogenic atrophy of the EOM belly.15 Patients with isotropic atrophy (symmetrically rounded SO muscle cross section) showed greater excyclotorsion and hypertropia in infraversion than patients with anisotropic atrophy (asymmetric atrophy with preservation of the long axis of the SO muscle cross section). Isotropic SO atrophy is consistent with atrophy of both SO compartments, whereas anisotropic SO atrophy is consistent with atrophy of only one of the two SO compartments. However, anatomic examination of anisotropic SO atrophy by MRI has as yet not been able to indicate whether the atrophy is in the medial or lateral compartment.
Compartmental mechanisms provide surprising contributions to normal ocular motor physiology, and may complicate clinical presentation of strabismus in ways not intuitive to clinicians. For example, recent functional MRI studies have demonstrated differential compartmental function even in horizontal rectus muscles during vertical duction.10,13 In this study, we sought to investigate the compartmental volumes of all four rectus EOMs in unilateral SO palsy to determine if SO muscle atrophy is associated with secondary changes in other EOM compartments that might influence the clinical presentation of SO palsy. Furthermore, we asked if the medial or lateral SO might be selectively vulnerable to pathology in compartmentally selective SO palsy.
Methods
Subjects
Nineteen patients (14 male and 5 female) with unilateral SO palsy and 19 age-matched orthotropic control volunteers (10 male and 9 female) were included in this study. These patients comprised all subjects recruited for an ongoing, prospective study of strabismus between 2002 and 2015 who had unilateral SO atrophy in quasicoronal MRI planes obtained in central gaze that was of adequate image quality for analysis. Control subjects were volunteer participants in the same study who were matched as closely as possible in age to the patients. The mean (±SD) age of the patients was 43 ± 22 (range, 14–78) years, similar to controls at 37 ± 21 (range, 18–74) years (P = 0.43). Before participation, subjects gave written, informed consent according to a protocol approved by the University of California, Los Angeles Institutional Review Board that conformed to the tenets of the Declaration of Helsinki. The diagnosis of unilateral SO palsy was based on evidence of significant ipsilesional SO muscle atrophy on quasicoronal MRI. Patients were excluded if they had other causes of hypertropia, such as thyroid ophthalmopathy, orbital wall fracture, and sagging eye syndrome,16 or had undergone previous strabismus surgery. Congenital SO palsy was presumed when there was a history of first recognition of abnormal head posture or vertical strabismus in early childhood, supported by childhood photographs demonstrating head tilt when available; all other cases were considered acquired. Control subjects underwent comprehensive eye examinations to verify normal corrected visual acuity, normal binocular alignment at both distance and near, and normal stereoacuity of at least 40 arcsec measured by Titmus test. Patients with SO palsy underwent complete ophthalmic evaluation including binocular alignment measured by alternating prism cover testing in cardinal gazes and head tilt positions, ocular versions, Hess screen test, and stereoacuity measured by Titmus test. Subjective torsion was measured using double Maddox rods.
Magnetic Resonance Imaging
With a 1.5-T General Electric Signa scanner (Milwaukee, WI, USA), high-resolution T1 or T2 fast spin-echo MRI was performed using a dual-phased surface-coil array (Medical Advances, Milwaukee, WI, USA) and fiberoptic fixation target, as described in detail elsewhere.17–19 Each eye was scanned in central gaze fixating an afocal, monocularly viewed target that does not induce convergence. Contiguous 2-mm-thick quasicoronal images were obtained perpendicular to the long axis of each orbit, using a 256 × 256 matrix over an 8-cm field of view, giving 312 μm in-plane resolution.
Analysis
Digital MRI images were processed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA), and were quantified using the program ImageJ (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) and custom software written in MATLAB (MathWorks, Natick, MA, USA). Images of left orbits were reflected to the orientation of right orbits. Image planes were numbered negatively posterior and positively anterior to the reference plane containing the globe-optic nerve junction, designated as plane 0. Cross sections of rectus and SO EOMs were manually outlined in contiguous images and quantified using the “area” function of ImageJ (Fig. 1). Cross-sectional areas of EOMs in contiguous image planes from −7 to 0 were summed and multiplied by 2-mm slice thickness to obtain the extended posterior partial volume (ePPV) of each EOM.
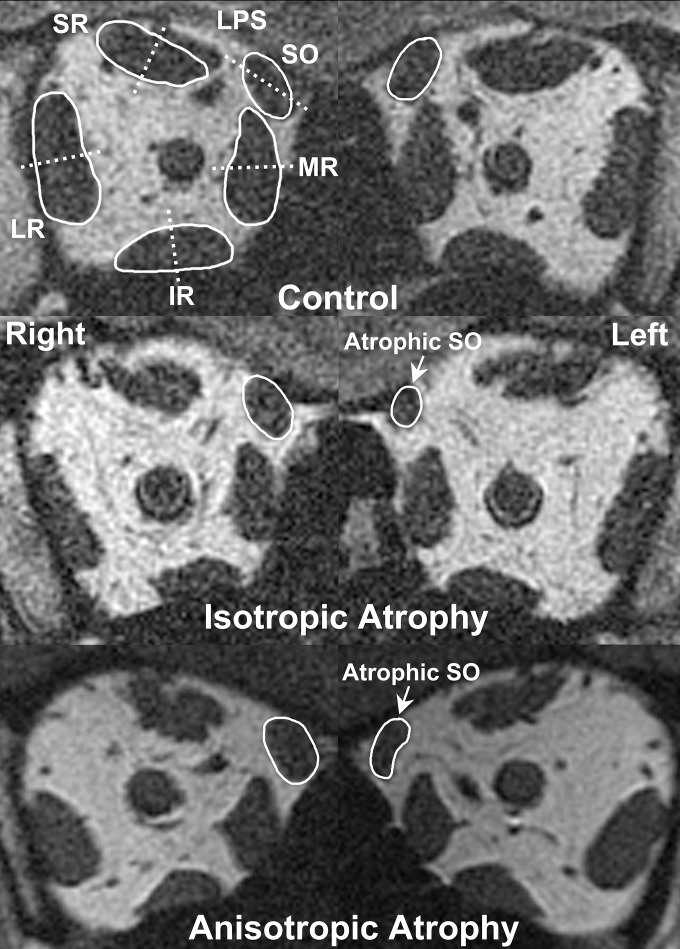
Figure 1.
Quasicoronal plane MRI in a healthy control and two subjects with unilateral SO palsy. Extraocular muscles are outlined in white solid lines and compartmental bisectors indicated with white dashed lines (top row). Note the rounded left SO in isotropic atrophy (middle row) compared with the elongated left SO in anisotropic atrophy (bottom row).
Further image analysis was based on statistical criteria and automated to avoid subjective bias. The diagnosis of unilateral SO palsy was made by MRI criterion when the ratio of ipsilesional to contralesional maximum SO cross-sectional area was below the 95% confidence intervals for the ratios between both SO muscles in control subjects. The shape of the palsied SO muscle was determined using the “ellipse” function of ImageJ, which automatically determines the major and minor axes of an ellipse best fitting the SO cross section as manually outlined. When measured in the quasicoronal plane of the globe-optic junction, best-fit SO ellipses with major axis length less than 3.9 mm were regarded as exhibiting isotropic SO atrophy, whereas those with major axis length of at least 3.9 mm were regarded as exhibiting anisotropic atrophy. This criterion has been validated by post hoc discriminant analysis published elsewhere.14
Compartmental analysis was automated using custom software in MATLAB that segmented each EOM's cross section into two compartments.8–10,13 In brief, the maximum transverse dimension of each rectus EOM was aligned with scanner vertical for horizontal rectus EOMs and scanner horizontal for vertical rectus EOMs.8 Superior and inferior putative compartments of horizontal rectus muscles were calculated as cross sections above and below the perpendicular bisector of the vertical best-fit line, and cross-sectional areas of medial and lateral putative compartments of vertical rectus muscles were calculated relative to the perpendicular bisector of the horizontal best-fit line (Fig. 1).8 The central 20% of each rectus EOM flanking the demarcating line was not analyzed due to anatomic variation in compartmental border and possibility of mixed innervation crossing any arbitrary linear border.8 The presumed intercompartmental border of the SO was determined by a bootstrap technique, which analyzed the orientation of the border in 15° in increments between 15° and 180° from the major axis of the SO cross sections, as described in detail elsewhere.10,13 Medial and lateral putative compartmental areas of the SO muscle were then calculated, again excluding the central 20% to account for possible irregularities in the intercompartmental border (Fig. 1). To obtain the ePPV for each compartment, cross-sectional areas in contiguous images planes from −7 to 0 were summed and multiplied by the 2-mm slice thickness.
Main outcome measures were ePPV of the total EOM and each of the EOM compartments. Nonparametric statistical analysis was used to avoid assumptions about data distributions, and included the Mann-Whitney and Wilcoxon signed-rank tests performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
Results
Clinical Findings
Seventeen patients with SO palsy had vertical binocular diplopia of 5 years' median duration (range, 2 months–28 years). Two others with SO palsy had hypertropia without diplopia. Three of the 19 patients were diagnosed with congenital SO palsy, 1 patient had a history of surgical manipulation causing SO palsy, and all other acquired cases were regarded as idiopathic. Median ipsilesional hypertropia was 12Δ (range, 2–27Δ) in central gaze, 6Δ (range, 0–25Δ) in supraversion, and 10Δ (range, 0–25Δ) in infraversion. Median hypertropia was greater in contralesional gaze at 17Δ (range, 1–42Δ) than in ipsilesional lateral gaze at 6Δ (range, 0–30Δ). Median hypertropia in ipsilesional head tilt was 10Δ (range, 2–45Δ) and 0Δ (range, 0–15Δ) in contralesional head tilt. On a scale of zero to ±4, median overelevation in adduction of the palsied eye was 1.5 (range, 0–4), and median underdepression was −1 (range, −2.5 to 0). Median excyclotorsion in upright position was 5° (range, 0–25°). Three subjects with SO palsy had no measurable strereoacuity, 2 subjects only perceived the Titmus stereo fly, and median stereoacuity in the remaining 14 subjects was 60 arcsec (range, 40–200 arcsec). None of the clinical features differ significantly between patients with isotropic versus anisotropic atrophy.
Rectus EOM Volumes
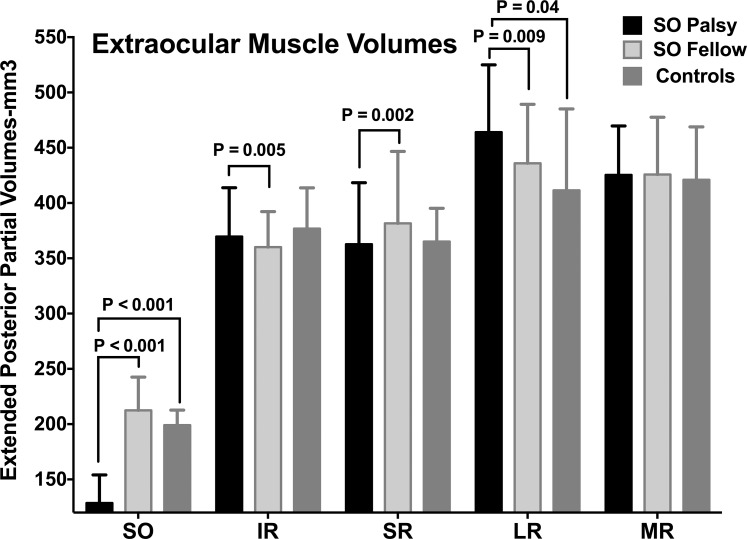
Muscle volumes of all subjects are shown in Figure 2. Median ePPV of the palsied SO muscle was 129 mm3 (range, 48–229 mm3), which was significantly smaller than that of the fellow eye at 212 mm3 (range, 164–295 mm3) and controls at 199 mm3 (range, 148–252 mm3, P < 0.001 for both). Median ipsilesional IR ePPV at 370 mm3 (range, 278–521 mm3) significantly exceeded that of the contralesional eye at 360 mm3 (range, 264–514 mm3, P = 0.005). Neither significantly differed from controls at 377 mm3 (range, 288–486 mm3, P > 0.5 for both). Median ipsilesional SR ePPV at 363 mm3 (range, 298–517 mm3) was significantly smaller than that of the contralesional eye at 382 mm3 (range, 311–512 mm3, P = 0.002). Neither significantly differed from controls at 365 mm3 (range, 246–544 mm3, P = 0.65 and P = 0.11, respectively). Median ipsilesional LR ePPV at 464 mm3 (range, 364–570 mm3) significantly exceeded both that of the contralesional eye at 436 mm3 (range, 325–540 mm3) and controls at 411 mm3 (range, 345–556 mm3, P = 0.009 and P = 0.04, respectively). Median ipsilesional MR muscle ePPV at 425 mm3 (range, 327–504 mm3) was similar to that of the contralesional eye, 426 mm3 (range, 331–559 mm3), and controls, 421 mm3 (range, 309–531 mm3) (P > 0.25 for both).
Figure 2.
Extended posterior partial volumes (ePPVs) of extraocular muscles in unilateral SO palsy. Bars indicate median values and error bars interquartile ranges.
Superior Oblique Compartmental Volumes
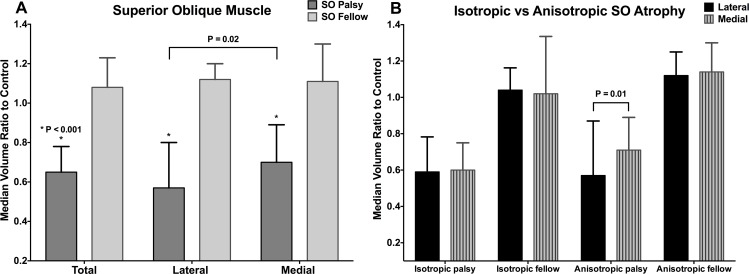
The ePPV ratio to controls was compared for each eye in total muscle, lateral (SOl), and medial (SOm) compartments (Fig. 3A). In the palsied eye, the median ePPV ratio for total muscle was 0.65 (range, 0.24–1.16). The median ePPV ratio for SOl was 0.57 (range, 0.19–1.26), and for SOm was 0.70 (range, 0.21–1.20). The ePPV of the palsied SOl was significantly smaller than that of the SOm (P = 0.02). All these ratios were significantly smaller than those for the fellow eye, in which the median ePPV ratio was 1.08 (range, 0.83–1.50) for total muscle, 1.12 (range, 0.79–1.67) for SOl, and 1.10 (range, 0.75–1.48) for SOm (P < 0.0001 for all).
Figure 3.
Median ratio to control of SO muscle extended posterior partial volume in unilateral SO palsy. The lateral compartment is more atrophied than the medial compartment (A), but this difference is significant only for anisotropic SO palsy (B). Error bars indicate interquartile ranges.
Based on the shape of the palsied SO cross section, 4 patients were considered to have isotropic atrophy and 15 patients to have anisotropic atrophy. Median SO muscle ePPV ratios to controls were compared for SOl and SOm compartments in patients with isotropic and anisotropic SO atrophy (Fig. 3B). In isotropic SO atrophy, the median ePPV ratio of the palsied SOl was 0.59 (range, 0.19–0.80), similar to 0.60 (range, 0.21–0.79) for the SOm (P = 0.87). However, in anisotropic SO atrophy, the median ePPV ratio of the palsied SOl was 0.57 (range, 0.31–1.26), significantly less than that of SOm at 0.71 (range, 0.36–1.20, P = 0.01). Median ePPV ratios of the fellow SOl and SOm did not differ significantly between isotropic and anisotropic atrophy (P > 0.45).
Rectus EOM Compartmental Volumes
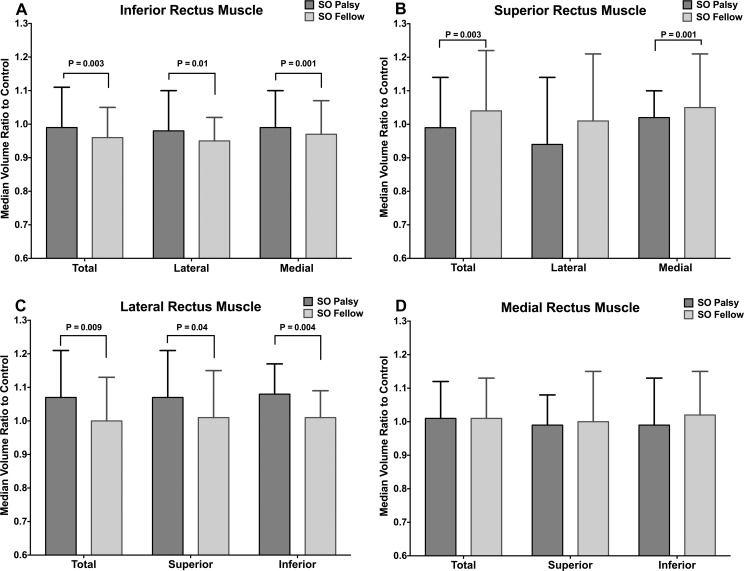
The median ePPV ratios to controls also were compared between the eyes ipsilesional and contralesional to SO palsy (Fig. 4). The ipsilesional IR was significantly larger than the contralesional IR in both the lateral (IRl) and medial (IRm) compartments (P = 0.01 and P= 0.001, respectively). The contralesional SR muscle was significantly larger than the ipsilesional SR only in the medial (SRm, P = 0.001) but not lateral (SRl) compartment (P = 0.13). Similar to IR, the ipsilesional LR's superior (LRs) and inferior (LRi) compartments were both significantly larger than those of the contralesional LR (P = 0.04 and P = 0.004, respectively). Ipsilesional and contralesional MR muscle volumes showed no significant differences between the superior (MRs) and inferior (MRi) compartments (P > 0.1).
Figure 4.
Median ratio to control of rectus extraocular muscle extended posterior partial volumes in unilateral SO palsy. Error bars indicate interquartile ranges.
Discussion
The present MRI study demonstrates the novel finding in a subgroup of patients with unilateral SO palsy of selective atrophy of the lateral SO compartment, the region of the SO that has selective mechanical advantage for infraduction, with preservation of the medial compartment known to have selective advantage for incycloduction. This MRI study also demonstrated that unilateral SO palsy is not associated with selective compartmental changes in other EOM volumes either in ipsi- or contralesional orbit.
The demonstration of compartmentally selective SO atrophy confirms and extends this finding from what was previously believed limited only to the LR muscle. Selective atrophy of the superior lateral rectus compartment (LRs) has been identified by MRI in a substantial proportion of patients with LR palsy who demonstrated clinical evidence of greater residual LR function than did patients with atrophy of the entire LR, and thus with presumed denervation of both the LRs and LRi compartments.20 In the present study, selective SOl atrophy was identified in patients with anisotropic residual SO cross sections. The common occurrence of selective SOl weakness, predicted on anatomic grounds to mainly impair infraduction,11 would explain why most of the patients with SO palsy complain of vertical rather than torsional diplopia. Perhaps this biased the patient sample toward selective SOl weakness, because such cases are arguably more likely to be clinically diagnosed as SO palsy on the basis of vertical diplopia and hypertropia. We speculate the SOm weakness might preferentially cause only excyclotropia that might not be symptomatic for diplopia, and at any rate would be more difficult to detect and clinically diagnose than SO palsy.
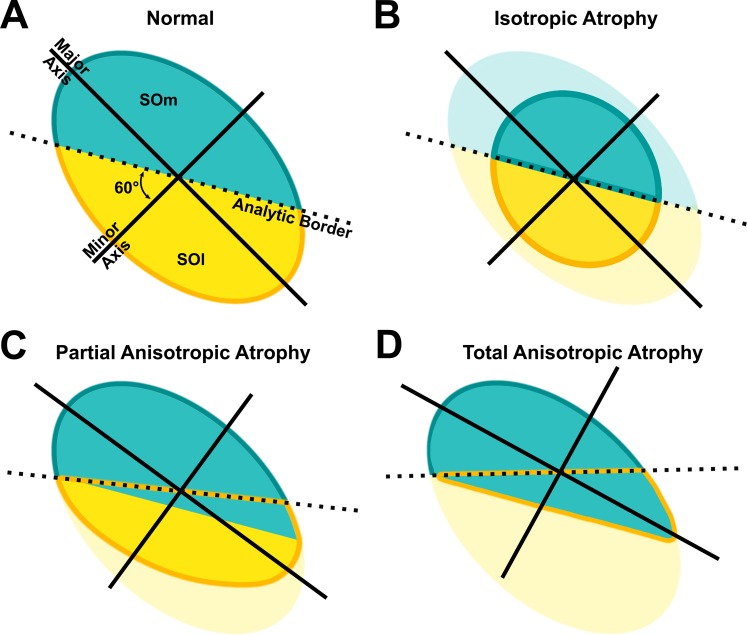
There are several complexities involved in interpretation of compartmental SO atrophy from MRI cross sections. Although the automated analytic algorithm must surely be imperfect, the effects of the errors would always make it more difficult to detect selective compartmental atrophy of the SO and never create the illusory appearance of selective atrophy where none was actually present (Fig. 5). Because differentiation between isotropic versus anisotropic palsy was initially based on SO cross-section morphology, the anisotropic atrophy group might in theory have included both selective SOm and SOl palsy; if equal numbers of each were present in the sample of patients, asymmetries in each group could cancel one another mathematically. Moreover, if there were total atrophy of only one SO compartment, the automated analytic algorithm arbitrarily dividing the cross section into two compartments at an intercompartmental boundary line oriented 60° to the minor axis of the SO would erroneously parse the single remaining compartment into two regions for analysis (Fig. 5D). The analytic method is biased against finding intercompartmental differences in the SO. Thus, the empirical finding of the significant selective atrophy of the SOl in anisotropic palsy almost certainly underestimates the frequency and severity of anisotropic atrophy in individual patients.
Figure 5.
Diagrams of medial (SOm) and lateral (SOl) compartments of normal and atrophied SO muscles indicating how the two different types of atrophy would be conservatively interpreted by the cross-sectional analysis used in this study. Analytic border at 60° to the minor axis of the SO divides the muscle into SOm and SOl (A) and green and yellow lines indicate the new borders of SOm and SOl in atrophied SO (B–D). The atrophied portion of the SO is demonstrated in faded colors. Note in (C) and (D) that the automated algorithm, which has no knowledge of the actual anatomic border between compartments, will tend to misclassify some of the intact compartment as belonging to the atrophic compartment (green area below the dotted line). Such misclassification will tend to reduce, but never erroneously increase, the statistical significance of intercompartmental differences in cases of anisotropic atrophy.
In the current study, we observed significant changes in extended posterior partial volumes (ePPVs) of other EOMs in SO palsy. Contralesional SR and ipsilesional IR hypertrophy were evident here, and may be compensatory mechanisms and secondary to excess innervation to reduce the hypertropia caused by the weak, atrophic SO in unilateral SO palsy. Although contractility was not evaluated in this study, a previous MRI study demonstrated that contralesional SR contractility is also enhanced in patients with unilateral SO palsy.21 Unlike the SR, the present finding of ipsilesional IR hypertrophy may be different from that of a prior study that demonstrated larger maximum cross-sectional area of the contralesional IR than in ipsilesional IR.22 However, this discrepancy may be due to differences in measuring EOM size. The present study measured muscle volume rather than maximum cross-sectional area, justified by a recent MRI demonstration that muscle PPV correlates better with EOM function than does cross-sectional area.13 The mechanism of ipsilesional LR hypertrophy in unilateral SO palsy deserves further investigation, but may be related to the recently recognized role of the LR during vertical duction.10,13
Differential activity of particular EOM compartments has been demonstrated repeatedly by MRI during specific ocular rotations,8–10,13 and has emerged to be very complex. Horizontal rectus EOMs exhibit differential compartmental behavior during ocular counter-rolling8 and asymmetric convergence.9 Significantly greater contraction occurs in LRi but not in LRs in the excycloducting orbit than in the incycloducting orbit during ocular counter-rolling.8 The MRs exhibits greater contractility than inferior compartment (MRi) during convergence.9 Not only the horizontal rectus EOMs but also the IR and SO demonstrate differential compartmental contractility during vertical eye movements.10,13 During vertical fusional vergence induced in healthy volunteers by monocular base-up prism viewing, IRm primarily contracts ipsilateral and relaxes contralateral to the prism despite absence of significant contractile changes in the lateral compartment (IRl).10 Ipsilateral LRs, SOm, and contralateral SOl are also differentially active during vertical fusional vergence, although neither MR compartment demonstrates differential activation during this behavior.10 During infraduction, IRm contracts more than IRl, and selective contractile changes in MRs and SOl also contribute, although LR exhibits little differential compartmental behavior.13 In the context of the foregoing complexity and ubiquity of differential EOM compartmental function, it is actually surprising that in the present study of unilateral SO palsy, rectus EOMs did not exhibit differences in compartmental volumes. Ipsilesional IR and LR were hypertrophic in both of their individual compartments, whereas MR exhibited no size changes in either compartment. Although contralesional SR hypertrophy seemed to be significant only in the putative medial compartment, the magnitude of ePPV difference between the palsied and fellow eyes appeared greater in the lateral compartment. It is not clear if this apparent compartmental asymmetry in SR is real or an artifact, because it has so far been impossible to confirm by anatomic tracing any evidence of selective intramuscular innervation in this muscle.7 Thus, the only unifying theme that has emerged from functional studies of compartmentalization is that differential EOM compartmental function is complex, may involve nearly any one or several EOMs during most normal ocular motor behaviors, and may occur ipsilateral or contralateral to a palsied SO. The main implication of the foregoing studies is that it would be particularly hazardous to draw inferences about functions of specific individual EOMs based on conventional examination of version or vergence eye movements; multiple combinations of differential function in compartments of multiple different EOMs might well be able to produce the same externally observed eye movements. Such overdetermination of ocular motor behavior by a plethora of EOM compartments might also contribute to the heterogeneity of clinical presentations of clinical forms of strabismus.
The present study demonstrated EOM volume increases in the entirety of the ipsilesional IR, LR, and contralesional SR muscles in unilateral SO palsy; however, there were no differential compartmental volume changes in rectus EOMs. The compartmental nature of the SO has been again supported by the current finding that a subset of patients with SO palsy have SO atrophy apparently limited to the lateral compartment. Further study is needed to investigate if selective medial compartmental SO palsy also can be identified in patients who mainly have torsional rather than vertical diplopia.
Acknowledgments
Supported by National Eye Institute Grants EY008313 and EY0031 from the US Public Health Service (JLD), and an unrestricted grant from Research to Prevent Blindness (JLD). The authors alone are responsible for the writing and the content of the paper.
Disclosure: S.Y. Suh, None; R.A. Clark, None; A. Le, None; J.L. Demer, None
References
- 1. English AW,, Wolf SL,, Segal RL. Compartmentalization of muscles and their motor nuclei: the partitioning hypothesis. Phys Ther. 1993; 73: 857–867. [DOI] [PubMed] [Google Scholar]
- 2. Holtermann A,, Roeleveld K,, Mork PJ,, et al. Selective activation of neuromuscular compartments within the human trapezius muscle. J Electromyogr Kinesiol. 2009; 19: 896–902. [DOI] [PubMed] [Google Scholar]
- 3. Bowden JL,, McNulty PA. Mapping the motor point in the human tibialis anterior muscle. Clin Neurophysiol. 2012; 123: 386–392. [DOI] [PubMed] [Google Scholar]
- 4. Mendez GA,, Gatica VF,, Guzman EE,, Soto AE. Evaluation of the neuromuscular compartments in the peroneus longus muscle through electrical stimulation and accelerometry. Braz J Phys Ther. 2013; 17: 427–434. [DOI] [PubMed] [Google Scholar]
- 5. Larsen CM,, Juul-Kristensen B,, Olsen HB,, Holtermann A,, Sogaard K. Selective activation of intra-muscular compartments within the trapezius muscle in subjects with Subacromial Impingement Syndrome. A case-control study. J Electromyogr Kinesiol. 2014; 24: 58–64. [DOI] [PubMed] [Google Scholar]
- 6. Peng M,, Poukens V,, da Silva Costa RM,, Yoo L,, Tychsen L,, Demer JL. Compartmentalized innervation of primate lateral rectus muscle. Invest Ophthalmol Vis Sci. 2010; 51: 4612–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. da Silva Costa RM,, Kung J,, Poukens V,, Yoo L,, Tychsen L,, Demer JL. Intramuscular innervation of primate extraocular muscles: unique compartmentalization in horizontal recti. Invest Ophthalmol Vis Sci. 2011; 52: 2830–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark RA,, Demer JL. Differential lateral rectus compartmental contraction during ocular counter-rolling. Invest Ophthalmol Vis Sci. 2012; 53: 2887–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demer JL,, Clark RA. Magnetic resonance imaging of differential compartmental function of horizontal rectus extraocular muscles during conjugate and converged ocular adduction. J Neurophysiol. 2014; 112: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demer JL,, Clark RA. Magnetic resonance imaging demonstrates compartmental muscle mechanisms of human vertical fusional vergence. J Neurophysiol. 2015; 113: 2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le A,, Poukens V,, Ying H,, Rootman D,, Goldberg RA,, Demer JL. Compartmental innervation of the superior oblique muscle in mammals. Invest Ophthalmol Vis Sci. 2015; 56: 6237–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le A,, Demer JL,, Poukens V. Compartmental innervation scheme for the mammalian superior oblique (SO) and inferior (IO) mucscles (E-Abstract 22). Soc Neurosci. 2014; 62. [Google Scholar]
- 13. Clark RA,, Demer JL. Functional morphometry demonstrates extraocular muscle compartmental contraction during vertical gaze changes. J Neurophysiol. 2016; 115: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shin SY,, Demer JL. Superior oblique extraocular muscle shape in superior oblique palsy. Am J Ophthalmol. 2015; 159: 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demer JL,, Poukens V,, Ying H,, Shan X,, Tian J,, Zee DS. Effects of intracranial trochlear neurectomy on the structure of the primate superior oblique muscle. Invest Ophthalmol Vis Sci. 2010; 51: 3485–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaudhuri Z,, Demer JL. Sagging eye syndrome: connective tissue involution as a cause of horizontal and vertical strabismus in older patients. JAMA Ophthalmol. 2013; 131: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Demer JL,, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005; 94: 3292–3302. [DOI] [PubMed] [Google Scholar]
- 18. Demer JL,, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995; 36: 906–913. [PubMed] [Google Scholar]
- 19. Demer JL,, Dushyanth A. T2-weighted fast spin-echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011; 15: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark RA,, Demer JL. Lateral rectus superior compartment palsy. Am J Ophthalmol. 2014; 157: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clark RA,, Demer JL. Enhanced vertical rectus contractility by magnetic resonance imaging in superior oblique palsy. Arch Ophthalmol. 2011; 129: 904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang L,, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior rectus muscle in superior oblique muscle palsy. Ophthalmology. 2008; 115: 2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]