Abstract
Purpose
This study addresses the hypothesis that age-related stresses upregulate Thy-1 in choroidal endothelial cells (CECs) and contribute to CEC activation and migration, processes important in choroidal neovascularization (CNV).
Methods
Measurements were made of Thy-1 protein (Western blot) in CECs and Thy-1 mRNA (real time quantitative PCR) in CECs treated with VEGF, CCL11, or PBS or in RPE/choroids from young or old donors or lasered or nonlasered mice. Immunolabeled Thy-1 in ocular sections was compared from young versus old human donor eyes or those with or without neovascular AMD or from lasered versus nonlasered mice. Choroidal endothelial cells transfected with Thy-1 or control siRNA or pretreated with Thy-1 blocking peptide or control were stimulated with VEGF or 7-ketocholesterol (7-KC). Choroidal endothelial cell migration, proliferation, cytoskeletal stress fibers, Rac1 activation, and phosphorylated VEGF receptor 2 (VEGFR2), integrin β3, and Src were measured. Statistics were performed using ANOVA.
Results
Thy-1 was expressed in retinal ganglion cells and in vascular endothelial-cadherin–labeled choroid and localized to human or mouse laser-induced CNV lesions. Thy-1 protein and mRNA were significantly increased in CECs treated with VEGF or CCL11 and in RPE/choroids from aged versus young donor eyes or from lasered mice versus nonlasered controls. Knockdown or inhibition of Thy-1 in CECs significantly reduced VEGF-induced CEC migration and proliferation, stress fiber formation and VEGFR2, Src, integrin β3 and Rac1 activation, and 7-KC–induced Rac1 and Src activation.
Conclusions
Thy-1 in CECs regulates VEGF-induced CEC activation and migration and links extracellular 7-KC to intracellular signaling. Future studies elucidating Thy-1 mechanisms in neovascular AMD are warranted.
Keywords: Thy-1, chorodial endothelial cells, VEGF, choroidal neovacularization, cell migration
Neovascular age-related macular degeneration (nvAMD) is a leading cause of blindness worldwide. Besides the role of genetic predisposition, external stresses related to inflammation, oxidative stress, hypoxia-related signaling, and age-related changes in the composition of the extracellular matrix play roles in the development of nvAMD.1–4 Currently, anti-VEGF agents are the standard of care but effective in only approximately 40% of cases.5,6 In addition, successful treatment of nvAMD with anti-VEGF agents does not slow progression of geographic atrophy, the advanced dry form of AMD.7 Also, there are concerns about potential harm from repeated long-term administration of anti-VEGF agents on retinal cells.8,9 One way to reduce the potential risk from repeated anti-VEGF treatments is to limit treatment to nvAMD lesions that result in vision loss.
Clinical studies have shown that vision may be maintained when choroidal neovascularization (CNV) remains beneath the retinal pigment epithelium (RPE), that is, type 1 CNV.10–12 It then follows that one way to stop CNV from entering the sensory retina would be to inhibit choroidal endothelial cells (CECs) from attaching to extracellular matrix and being activated to migrate across the RPE monolayer.
We previously found that activation of the rhoGTPase, Rac1, was necessary for CEC activation and transmigration of the RPE.13,14 Furthermore, CCL11 and VEGF, ligands that activate signaling events important in nvAMD pathophysiology, also induced Rac1-mediated CEC migration.15,16 In this study, we explored the role of Thy-1 on CEC activation and migration. Thy-1 is a highly conserved glycosylphosphatidylinositol (GPI)-anchored protein, known for its expression on thymocytes and ganglion cells. In addition, Thy-1 regulates cell–cell interactions by binding to receptors, like integrins.17 Integrins trigger intracellular signaling and bind proteins, not only on other cells and cell types, but also within the extracellular matrix. In this way, Thy-1 links the cell membrane to the extracellular matrix and other cells. The role of Thy-1 has not been explored in CECs or in nvAMD. However, Thy-1 facilitates neuron–astrocyte interactions and signaling.17 We found Thy-1 was highly expressed in older versus younger human donor choroid and CECs and in CECs exposed to VEGF or CCL11. Furthermore, we found that Thy-1 was necessary for VEGF or 7-ketocholesterol (7-KC)–induced Rac1-mediated CEC migration. Thy-1, which is not a transmembrane protein, triggered intracellular signaling by activating Src kinase, VEGF receptor 2 (VEGFR2), or integrin β3.
Methods
Animal and Laser-Induced CNV Model
C57Bl/6 wild-type mice (Jackson Laboratory, Bar Harbor, ME, USA) between 6 and 8 weeks old and of both sexes were used in the laser-induced CNV model. The care and use of animals in our studies adhered to the University of Utah guidelines (Guide for the Care and Use of Laboratory Animals) and treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
The laser-induced CNV model was carried out as previously described.14,17 Five to 7 days after laser, mice were euthanized, and eyes were collected for RNA analysis or for immunostaining of retinal cryosections.
Human Donor Eye Specimens
Institutional review board review was not required for these studies as tissue and cells are from deceased donors and are not considered human studies. Donor eyes were deidentified, and only age, sex, and whether AMD was present were known. For mRNA, samples were taken from three different donor eyes from young (female, 22 years old; female, 36 years old; and male, 37 years old) and old donors (female, 79 years old; male, 88 years old; and female, 79 years old). Paraffin-embedded eye sections from three different donor eyes with AMD (female, 75 years old; male, 79 years old; and female, 88 years old) or three age-matched controls. Young donor eyes were taken from donors of ages 25 years (male) and 37 years (female).
Cell Culture and siRNA Transfection
Choroidal endothelial cells were isolated from young donor eyes obtained from the Utah Zions Eye Bank (Salt Lake City, UT, USA), as previously reported.18 Endothelial cell (EC) phenotype was confirmed by labeling of EC markers (CD31, von Willebrand factor [vwF], and vascular endothelial [VE]-cadherin), and CECs of passages 2 through 5 were used in experiments. Choroidal endothelial cells were maintained in endothelial growth medium-2 (EGM-2; Lonza, Walkersville, MD, USA) with 5% fetal bovine serum (FBS). Human RPE cells were obtained from Lonza and grown in medium (RtEGM Retinal Pigment Epithelial Cell Growth Medium; Lonza) plus 2% FBS and penicillin-streptomycin. Cells below passage 5 were used for experiments.
To knockdown Thy-1, CECs were transfected using reagent (Lipofectamine 2000; Life Technologies, Grand Island, NY, USA) per the commercial protocol, with siRNA targeting the human Thy-1 gene or silencer selective negative control siRNA (both from Life Technologies). Forty-eight hours after transfection, cells were analyzed.
CEC Migration Assay
Human recombinant VEGF165 (20 ng/mL) (catalog no. 2933-VE; R&D Systems, Minneapolis, MN, USA) was mixed with growth factor–reduced matrix material (Matrigel; BD Biosciences, Billerica, MA, USA), diluted 1:1 with serum-free endothelial basal media (EBM-2; Lonza). Five hundred microliters EBM-2 was added to each well of a 24-well plate, and 6.5-mm diameter inserts (Transwell, 8-μm pores; Corning, NY, USA) were placed into the wells. Human CECs in EBM-2 were prestained (Vybrant DiO; Invitrogen, Carlsbad, CA, USA) for 30 minutes at 37°C and seeded into the inserts at 50,000 cells per 200 μL of serum-free EBM-2 media. To block Thy-1–mediated signaling, Thy-1 blocking peptide (250 ng/mL) (GeneTex, Inc., Irvine, CA, USA) or PBS, as respective controls, was added to the medium. The plates were allowed to incubate for 24 hours at 37°C, 5% CO2. The migrated CECs were imaged with a fluorescence microscope (TB190; Olympus, Tokyo, Japan).
Cell Proliferation Assay
Choroidal endothelial cells were plated into 96-well plates at a density of 5000 cells per well. Twenty-four hours after transfection with siRNA, cells were starved in serum-free media EBM2 for 24 hours and incubated with VEGF (20 ng/mL) or control PBS for another 24 hours. To inhibit Thy-1, CECs were pretreated with the Thy-1 blocking peptide (250 ng/mL) for 30 minutes before addition of VEGF. In the control situation, cells were pretreated with DMSO vehicle or PBS controls before the addition of VEGF. Cell number was measured with a cell proliferation assay kit (Vybrant MTT; Invitrogen).
CEC Stress Fiber Staining
Choroidal endothelial cells were plated onto growth factor–reduced Matrigel-coated (BD Biosciences) coverslips. Forty-eight hours after transfection, CECs were pretreated with Thy-1 blocking peptide (250 ng/mL) or control PBS for 30 minutes and then with VEGF or PBS control for 30 minutes. Choroidal endothelial cells were then fixed in 3.7% formaldehyde for 30 minutes, permeabilized in 0.2% TX-100/PBS for 5 minutes, and then incubated with rhodamine phalloidin (1:500, Invitrogen), an F-actin probe conjugated to the red-orange fluorescent dye, tetramethylrhodamine, for an hour at room temperature. After washing, coverslips with CECs were mounted onto glass slides with mounting medium (DAPI-Fluoromount-G; SouthernBiotech, Birmingham, AL, USA) and imaged using confocal microscopy (OLYMPUS 1X81; Olympus).
Rac-1 Activity Assay
Human CECs were cultured in EGM-2 containing 5% FBS. Prior to starting the assay, cells were serum starved with basal medium EBM2 (Lonza) overnight. Cells were then stimulated with VEGF (20 ng/mL) or 7-keto-cholesterol (7-KC) (5 or 12.5 μM) for 30 minutes after pretreatment with Thy-1 blocking peptide (250 ng/mL) or PBS vehicle control for 30 minutes. Equal amounts of lysates (500 μg) were incubated with (glutathione S-transferase -p21-activated kinase 1 p21 binding domain agarose beads (Millipore, Temecula, CA, USA) to pull-down active guanosine triphosphate (GTP)–bound Rac1 at 4°C for 30 minutes, with rotation. The samples were subsequently analyzed for GTP-bound Rac1 by Western blot analysis using an anti–Rac-1 antibody (BD Transduction Laboratories, San Jose, CA, USA). Rac1 activity was determined by GTP-Rac1 normalized to total Rac1 in the cell lysate.
Immunolabeling of Thy-1 in Human or Mouse Retinal/RPE/Choroidal Sections
Human retinal paraffin sections of eyes from a deidentified 75-year-old female donor obtained from the Georgia Eye Bank (Atlanta, GA, USA) were deparaffinized and an antigen retrieval step performed with antigen retrieval solution from Dako (Carpinteria, CA, USA) prior to immunostaining. Human retinal cryosections were processed from an eye of a 25-year-old a deidentified male donor, which was obtained from the Utah Lions Eye Bank (Salt Lake City, UT, USA). Mouse retinal cryosections were prepared from mice 5 or 7 days after treatment with laser. Retinal sections were incubated in 5% normal goat serum in PBS/0.1% Triton X-100 for 1 hour to block nonspecific binding of the primary antibody. Sections were incubated with rabbit anti-Thy-1 (1:100 dilution; ABCAM, Cambridge, MA, USA) overnight at 4°C. After three washes in PBS, sections were incubated for 1 hour with a 1:200 dilution of Alexa 488–conjugated goat anti-rabbit secondary antibody (Invitrogen) for Thy-1. The sections were rinsed in PBS and mounted in medium (DAPI-Fluoromount-G; SouthernBiotech). Images were captured with an inverted microscope (OLYMPUS 1X81; Olympus). Some sections were incubated with dye (TO-PRO-3 iodide; Invitrogen) to stain nuclei and mounted in medium without DAPI (Fluoromount-G; SouthernBiotech).
Western Blots
Retinal pigment epithelium cells and CECs were lysed in radio immunoprecipitation assay (RIPA) buffer with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA) and orthovanadate (Sigma-Aldrich Corp., St. Louis, MO, USA). Lysates were clarified by centrifugation at 16.1 × 1000g for 5 minutes at 4°C. Protein concentration in the supernatant was quantified by bicinchoninic acid assay (BCA) (Pierce, Rockford, IL, USA). Twenty micrograms of protein from each treatment was loaded into 4% to 12% BIS-TRIS gels (NuPAGE; Invitrogen), transferred to a polyvinylidene fluoride membrane, and incubated with antibodies to phosphorylated VEGFR2 (p-VEGFR2, Y951) (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), phosphorylated integrin β3 (p-integrin β3, Y773), phosphorylated Src (p-Src, Y529), or Thy-1 (all from ABCAM) at 4°C overnight. After incubating with primary antibodies, membranes were then probed with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:3000; Santa Cruz Biotechnology) at room temperature for 1 hour. All the membranes were reprobed with HRP-conjugated β-actin (1:3000; Santa Cruz Biotechnology) as loading controls. All experimental conditions were plated in triplicate.
RNA Isolation and Quantitative Real Time PCR (qPCR) Analysis
Total RNA of RPE/choroidal tissues was extracted by reagent (TRI Reagent; Sigma-Aldrich Corp.). RNA was quantified using a spectrophotometer (NanoDrop; Thermo Fisher Scientific, Waltham, MA, USA). cDNA was generated with the use of a high-capacity cDNA archive kit (Life Technologies, Grand Island, NY, USA). Quantitative PCR was performed (Mastercycler ep realplex; Eppendorf, Hauppauge, NY, USA) with the use of reagents and primers (TaqMan Universal PCR Master Mix; Life Technologies). Expression levels for Thy-1 were normalized to the mean value of the internal control GAPDH.
Statistical Analysis
Significant differences between groups were determined by ANOVA with post hoc protected corrections using the Bonferroni multiple comparison test. Results were mean ± SEM. A minimum P value of <0.05 was considered statistically significant. Western blot or PCR analyses included three to six samples per condition. For in vitro studies, each experimental condition included an n = 6–9, and each experiment was performed two to three times.
Results
Thy-1 Is Expressed in CECs and Upregulated by AMD-Related Ligands and Stresses
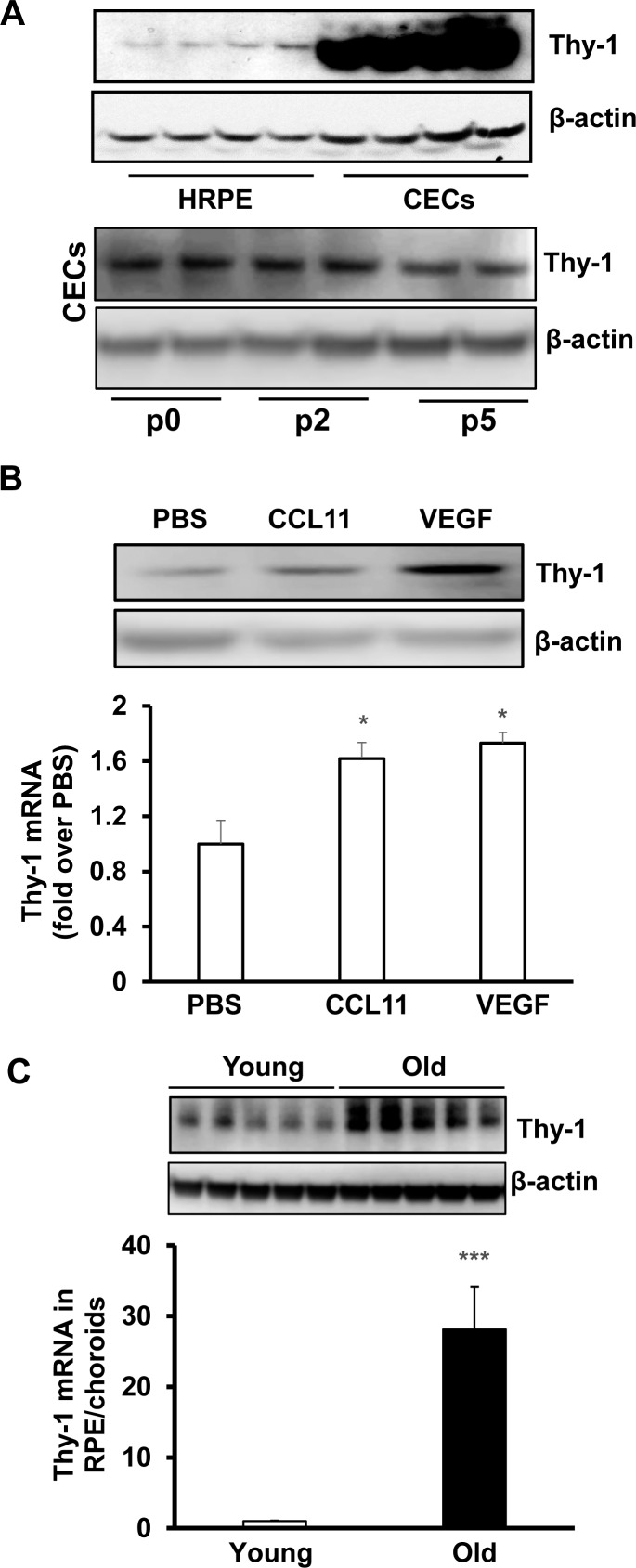
Choroidal endothelial cell activation and migration precede the development of CNV in nvAMD. We were interested in identifying factors involved in the activation and migration of CECs. We detected Thy-1 protein, a cell surface protein implicated in cell adhesive and migratory events,19 in both human RPE (HRPE) and CECs, but the protein level was substantially higher in CECs compared to HRPE. Thy-1 protein expression in CECs did not appear affected by passage in culture in that there was no difference in expression between primary CECs harvested without passaging and that in CECs used in experimentation, taken from passages 2 and 5 (Fig. 1A, Supplementary Fig. S1). In CECs, Thy-1 protein and mRNA were both significantly increased by overnight treatment with VEGF or CCL11 (Fig. 1B), factors implicated in the pathophysiology of nvAMD, and greater in RPE/choroids from aged human donor eyes compared to young donor eyes (Fig. 1C).
Figure 1.
Thy-1 is expressed in CECs and upregulated by AMD-related stresses. (A) Western blot of Thy-1 protein in cultured HRPE (top panel) and in CECs before passaging (p0) and after in vitro culturing (passages 2 [p2] and p5) (bottom panel); Western blot of Thy-1 protein (top panel) and qPCR of Thy-1 mRNA (bottom panel) (B) in CECs treated with CCL11 (10 ng/mL) or VEGF (20 ng/mL) overnight (*P < 0.05 vs. PBS, n = 6) and (C) in RPE/choroids isolated from young (20- to 40-year-old) and old (>60-year-old) donor eyes (***P < 0.001 vs. Young, n = 3).
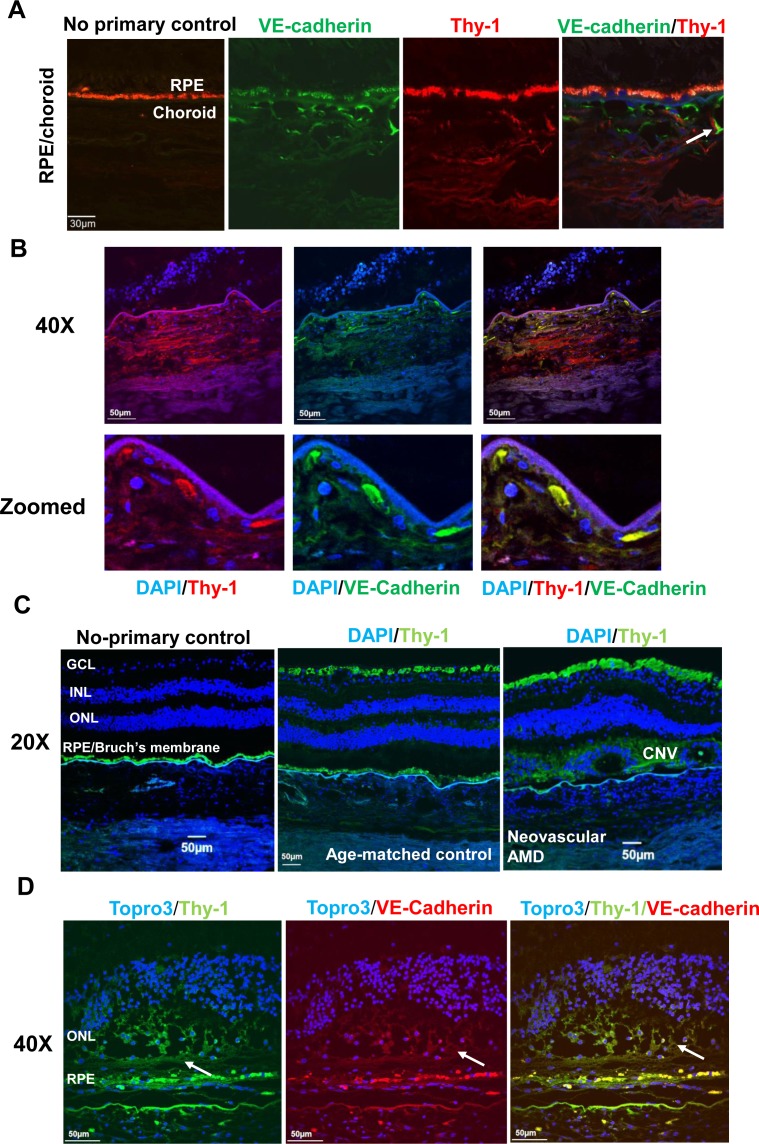
Thy-1 Is Expressed in Human Choroidal Vessels and Human CNV
To further determine if Thy-1 was associated with CNV in human nvAMD, immunolabeling of Thy-1 was performed in human retina/RPE/choroid sections. As shown in Figure 2, Thy-1 not only localized to the retinal ganglion cell layer but also colocalized with VE-cadherin in CECs in choroid (Fig. 2A), and in VE-cadherin–labeled migrating CECs (Fig. 2B), in paraffin sections from donors with neovascular AMD (Fig. 2C), and in VE-cadherin–labeled CNV lesions (Fig. 2D). Taken together, the data in Figures 1 and 2 suggest that Thy-1 was expressed in choroidal endothelium and its expression was associated with CNV.
Figure 2.
Thy-1 is expressed at human choroids and CNV lesions. Immunostaining of Thy-1 (A) in retinal cryosections from a 25-year-old donor (red, Thy-1; green, VE-cadherin; autofluorescence of RPE shown in Alexa Fluor 568 and no primary control); (B) in paraffin-embedded sections from a 79-year-old donor with neovascular AMD (blue, 4′,6-diamidino-2-phenylindole [DAPI]; green, VE-cadherin; red, Thy-1); and (C, D) in paraffin-embedded sections from 75-year-old donor with neovascular AMD and an age-matched control donor without AMD (C) (blue, DAPI; green, Thy-1; magnification 20×) and at VE-cadherin–labeled CNV lesions (D) (green, Thy-1; red, VE-cadherin; blue, TO-PRO3, magnification, 40×; arrows point to CNV lesion).
Thy-1 Is Upregulated in RPE/Choroids and Within CNV Lesions in a Laser-Induced CNV Model
To study the role of Thy-1 in the development of CNV, the laser-induced CNV model was performed in mice. Five days following laser injury, RPE/choroids were collected from lasered mice, and age-matched nonlasered mice were used as controls. Compared to nonlasered mice, Thy-1 mRNA in RPE/choroids was significantly increased in lasered mice (Fig. 3A). In ocular cryosections, Thy-1 staining was not only found in the retinal ganglion cell layer but also in choroid and in migrating cells within CNV lesions 5 days after laser (Fig. 3B). At 7 days after laser injury, immunostaining of Thy-1 was found within CNV lesions, colabeled with lectin-stained choroidal ECs, but not with retinal vascular ECs (Fig. 3C).
Figure 3.
Thy-1 is upregulated in laser-treated RPE/choroids and expressed at CNV lesions. (A) Quantitative real time PCR of Thy-1 mRNA in RPE/choroids from nonlasered control eyes or laser-treated eyes at day 5 after laser (*P < 0.05 vs. nonlasered, n = 5); immunostaining of Thy-1 in retinal cryosections of nonlasered eyes or lasered eyes (B) 5 days after laser (blue, DAPI; green, Thy-1) or (C) 7 days after laser (green, Thy-1; red, lectin; gray, TO-PRO3) (magnification, 20×; n = 3).
Thy-1 Regulates VEGF-Mediated CEC Activation and Migration Through VEGFR2-Src Signaling
Vascular endothelial growth factor plays important roles in the development of CNV,20 and anti-VEGF treatment is a standard of care for neovascular AMD.5,6 We previously found that activation of the rhoGTPase, Rac1, was necessary for VEGF-induced CEC migration.13 Thy-1 is a GPI-anchored glycoprotein that can interact with integrins within other cell membranes that link cells to the extracellular matrix.17,21 We therefore determined if Thy-1 was involved in VEGF-mediated CEC activation and migration. Vascular endothelial growth factor–induced Rac1-mediated CEC migration, proliferation, and stress fiber formation were measured in CECs knocked down for Thy-1 by siRNA (Fig. 4). As shown in Figure 4, following 30-minutes incubation with VEGF and compared to CECs transfected with control siRNA, knockdown of Thy-1 in CECs significantly reduced VEGF-induced Rac1 activation (Fig. 4A), CEC migration (Fig. 4B), proliferation (Fig. 4C), and cytoskeletal stress fibers (Figs. 4D, 4E).
Figure 4.
Knockdown of Thy-1 inhibits VEGF-mediated Rac1 activation and angiogenic effects in CECs. (A) Rac1 activity assay, (B) CEC migration, (C) CEC proliferation, and (D, E) cytoskeleton stress fibers stained by rhodamine phalloidin (D, representative images; E, quantification of fluorescent density of stress fiber staining [normalized to control siRNA/PBS]) in CECs transfected with Thy-1 siRNA and treated with VEGF (20 ng/mL) for 30 minutes. Note intracellular fibers induced by VEGF and reduction in Thy-1 siRNA-transfected CECs compared to control siRNA (*P < 0.05, **P < 0.01, ***P < 0.001 vs. PBS of control siRNA; ††P < 0.01, †††P < 0.001 vs. VEGF of control siRNA; ‡P < 0.05 vs. PBS of Thy-1 siRNA; n = 6–9).
As another method to inhibit Thy-1, we used a Thy-1 blocking peptide that blocks binding of Thy-1 to other cell membrane proteins at its extracellular tail. Choroidal endothelial cells were pretreated with Thy-1 blocking peptide for 30 minutes prior to incubation with VEGF for another 30 minutes. Inhibition of Thy-1 binding significantly inhibited VEGF-induced Rac1 activation (Fig. 5A), migration toward VEGF (Fig. 5B) and VEGF-induced proliferation (Fig. 5C), and cytoskeletal stress fiber formation in CECs (Figs. 5D, 5E). Altogether, these data provide evidence that Thy-1 regulates VEGF-mediated angiogenesis in CECs.
Figure 5.
Inhibition of Thy-1 inhibits VEGF-mediated Rac1 activation and angiogenic effects in CECs. (A) Rac1 activity assay (left panel, representative images; right panel, quantification of densitometry of GTP-Rac1 to total Rac1 [normalized to PBS of control]), (B) CEC migration, (C) CEC proliferation, and (D, E) cytoskeleton stress fibers stained by rhodamine phalloidin (D, representative images; E, quantification of fluorescent density of stress fiber staining [normalized to control/PBS]) were measured in CECs pretreated with Thy-1 blocking peptide (Thy-1Inh) for 30 minutes prior to incubation with VEGF (20 ng/mL) for additional 30 minutes. Note intracellular fibers induced by VEGF and reduction in CECs pretreated with Thy-1 blocking peptide compared to control (*P < 0.05, **P < 0.01, ***P < 0.001 vs. PBS of control; †P < 0.05, ††P < 0.01, †††P < 0.001 vs. VEGF of control; n = 6–9).
As a GPI-anchored protein, Thy-1 is located in lipid raft microdomains within the cell membrane.21,22 Thy-1 is not a transmembrane protein. However, neuronal Thy-1 has been shown to trigger intracellular signaling through PKCα by engaging β3 integrin on astrocytes, and β3 has been linked to VEGF-mediated signaling.17,23 Thy-1, as part of lipid rafts, is also in proximity to signaling molecules, such as cytoplasmic tyrosine kinases like Src. To determine potential signaling mechanisms involved in Thy-1–regulated VEGF-mediated angiogenesis, VEGF-induced phosphorylation of VEGFR2 (p-VEGFR2), integrin β3 (p-integrin β3), and Src (p-Src) were measured in CECs transfected with Thy-1 siRNA or pretreated with Thy-1 blocking peptide. Compared to respective controls, VEGF-induced p-VEGFR2 (Figs. 6A, 6B), p-integrin β3 (Figs. 6C, 6D), and p-Src (Figs. 6E, 6F) were all significantly decreased in CECs when Thy-1 was knocked down by siRNA transfection or inhibited by a blocking peptide.
Figure 6.
Knockdown or inhibition of Thy-1 inhibits VEGF-induced activation of VEGFR2, integrin β3, and Src. Western blots of (A, B) phosphorylated VEGFR2 (top panel, representative images; bottom panel, quantification of densitometry), (C, D) phosphorylated integrin β3 (top panel, representative images; bottom panel, quantification of densitometry), and (E, F) p-Src (top panel, representative images; bottom panel, quantification of densitometry) in CECs transfected with Thy-1 siRNA (A, C, E: *P < 0.05, **P < 0.01, ***P < 0.001 vs. PBS of control siRNA; †P < 0.05, ††P < 0.01 vs. VEGF of control siRNA) or pretreated with Thy-1 blocking peptide (Thy-1 Inh) (B, D, F: ‡P < 0.05 vs. PBS of control; #P < 0.05, ##P < 0.01 vs. VEGF of control) with 30 minutes incubation with VEGF (20 ng/mL) (n = 3–6).
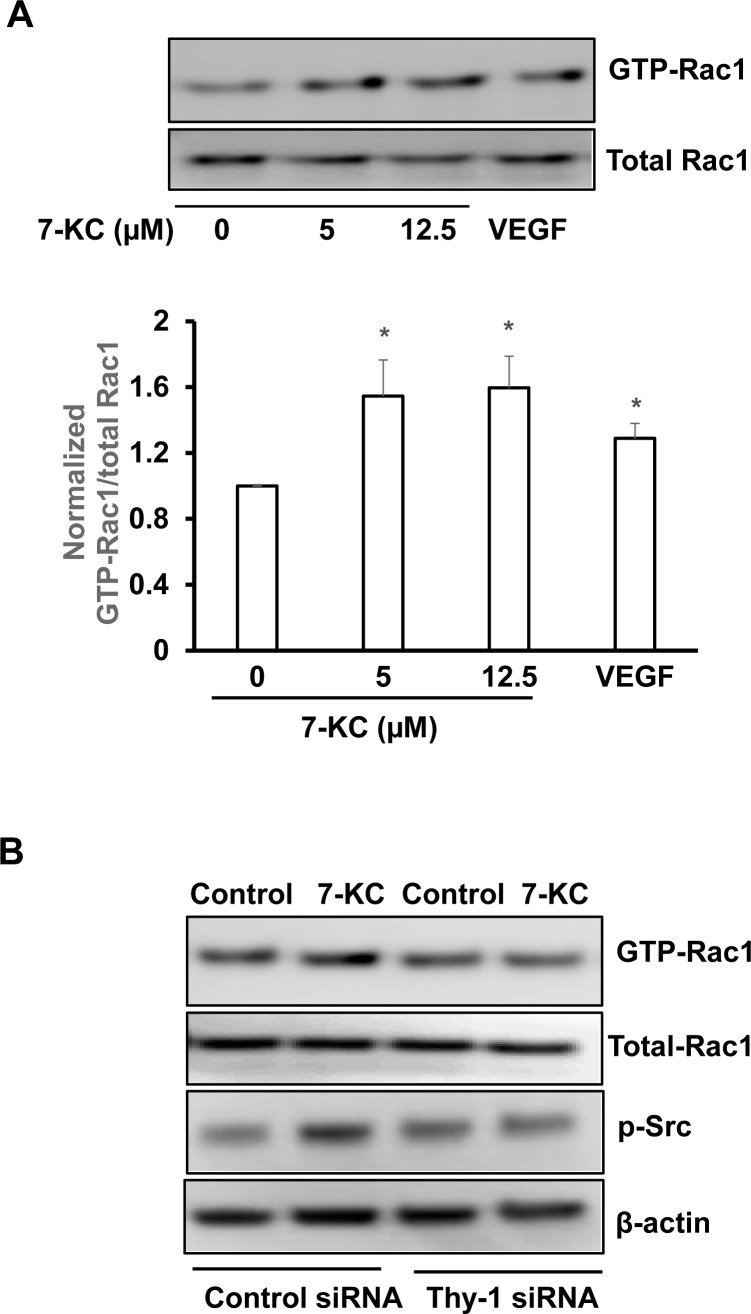
Thy-1 Regulates Extracellular Matrix Protein 7-KC–Induced Rac1 Activation
In nvAMD, the composition of the extracellular matrix and Bruch's membrane changes with accumulation of TIMP3, SerpinA3, vitronectin,24 bisretinoid fluorophores,25 complement,2,26 oxidized lipids, proteins,27,28 and lipoprotein-derived debris,29 like 7-KC.30 Since Thy-1 links proteins within the CEC membrane to those in other cells and the extracellular matrix, we wished to determine if Thy-1 facilitated the regulation of signaling events triggered by age-related changes in the extracellular milieu. We exposed CECs to 7-KC of varying doses for 30 minutes and, compared to vehicle, found that 7-KC caused significant activation of Rac1 in a dose-dependent manner (Fig. 7A). We found that 7-KC did not increase Thy-1 (data not shown). However, knockdown of Thy-1 by siRNA transfection inhibited 7-KC–induced Rac1 and Src activation (Fig. 7B) compared to control.
Figure 7.
Thy-1 regulates extracellular matrix protein 7-KC–induced Rac1 activation. (A) Rac1 activity assay in CECs treated with 7-KC at different doses for 30 minutes (top panel, representative images; bottom panel, quantification of densitometry; *P < 0.05 vs. 7-KC at 0 μM) and (B) Rac1 activity assay and phosphorylation of Src in CECs transfected with Thy-1 siRNA or control siRNA and treated with 7-KC (10 μM) or vehicle for 30 minutes (n = 3–6).
Discussion
Thy-1 was originally identified in thymocytes,31 and in the eye, Thy-1 has been classically known as a ganglion cell marker.32 Thy-1 also has effects on immune-related cells,33,34 which may be involved in the pathophysiology of nvAMD.35 In an effort to understand how age-related stresses and ligands activate CECs to migrate, we identified Thy-1 as expressed not only on ganglion cells and cells of the inner plexiform layer, but also in aged versus young donor RPE/choroids and on CECs of CNV from specimens of eyes with nvAMD. We also found that the ligands, VEGF and CCL11, which cause CNV and are overexpressed in aged donor eyes and in human nvAMD,15,16 increased the expression of Thy-1 in CECs. Furthermore, VEGF, CCL11, or Thy-1 each was sufficient to activate Rac1 in CECs and cause CEC migration. Knockdown of Thy-1 or use of a peptide to prevent Thy-1 binding to extracellular proteins inhibited VEGF-induced CEC proliferation, Rac1 activation, and CEC migration, providing evidence that Thy-1 was necessary for VEGF-induced CEC angiogenesis and Rac1-mediated CEC migration.
Glycosylphosphatidylinositol-anchored proteins, such as Thy-1, make up lipid rafts, and together with cholesterol, sphingolipids, and Src-family kinases,22 act as platforms for signaling, cell adhesion, migration, and protein trafficking.22 Thy-1 is not a transmembrane protein so it has not been clear how Thy-1 triggers intracellular signaling. In CECs, we found that Thy-1 is necessary for VEGF-induced signaling through VEGFR2 and activation of Rac1, and these events may occur within an individual CEC. We postulate that VEGFR2 signaling triggers Src kinase. Our data also support the hypothesis that Thy-1 facilitates interactions with integrins, thus connecting the CEC to the extracellular matrix, or VEGFR2 on other cells, and provides a link between the extracellular milieu and intracellular signaling. Binding of Thy-1 appeared necessary for the activation of VEGFR2, Src, or integrins from VEGF or 7-KC.
As our data supported the premise that Thy-1 connects the CEC membrane to the extracellular matrix, we determined if compositional changes in Bruch's membrane, similar to what occurs in nvAMD, would affect Thy-1 expression or induced signaling.
In nvAMD 7-KC accumulates in Bruch's membrane30 and also causes microglia to release cytokines including TNF-α,30,36 which upregulates RPE cell–induced VEGF.37 We found that 7-KC did not increase Thy-1 expression but caused a dose-dependent increase in Rac1 activation in CECs. When CECs were knocked down for Thy-1 and treated with 7-KC, Src phosphorylation and Rac1 activation were reduced. Our next steps are to determine signaling mechanisms regarding activation of Src, VEGFR2, or integrin on Rac1 activation mediated by Thy-1. Taken together, our data support the hypothesis that age-related changes in Bruch's membrane, including accumulation of 7-KC, activates microglia to induce RPE-derived VEGF that upregulates Thy-1 in CECs. Vascular endothelial growth factor also induces activation of integrin, Src, or VEGFR2 on CECs. Thy-1 can bind integrins and link the CEC membrane, intracellular signaling, and the extracellular matrix. Inhibiting Thy-1 binding with other signaling partners reduced activation of VEGF-induced Rac1, which is a necessary step in CEC transmigration of the RPE. Inhibition of CEC transmigration of the RPE may reduce the transition of type 1 to type 2 CNV and reduce vision loss.10–12 Information of type 2 CNV may also limit the number of repeated antiangiogenic injections needed in patients with nvAMD.
Supplementary Material
Acknowledgments
The authors thank H. Grossniklaus, Emory Eye Institute (Atlanta, GA, USA) for providing sections of human eyes.
Supported by the National Institutes of Health (Bethesda, MD, USA) Grants EY014800, R01EY015130 (MEH), and R01EY017011 (MEH) and an unrestricted grant from Research to Prevent Blindness, Inc. (New York, NY, USA) to the Department of Ophthalmology & Visual Sciences, University of Utah.
Disclosure: H. Wang, None; X. Han, None; E. Kunz, None; M.E. Hartnett, None
References
- 1. Seddon JM,, Silver RE,, Kwong M,, Rosner B. Risk prediction for progression of macular degeneration: 10 common and rare genetic variants, demographic, environmental, and macular covariates. Invest Ophthalmol Vis Sci. 2015; 56: 2192–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hageman GS,, Luthert PJ,, Victor Chong NH,, Johnson LV,, Anderson DH,, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20: 705–732. [DOI] [PubMed] [Google Scholar]
- 3. Curcio CA,, Presley JB,, Malek G,, Medeiros NE,, Avery DV,, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005; 81: 731–741. [DOI] [PubMed] [Google Scholar]
- 4. Wang H,, Hartnett ME. Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol Vis. 2016; 22: 189–202. [PMC free article] [PubMed] [Google Scholar]
- 5. Gillies MC,, Campain A,, Barthelmes D,, et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015; 122: 1837–1845. [DOI] [PubMed] [Google Scholar]
- 6. Otsuji T,, Nagai Y,, Sho K,, et al. Initial non-responders to ranibizumab in the treatment of age-related macular degeneration (AMD). Clin Ophthalmol. 2013; 7: 1487–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ying GS,, Huang J,, Maguire MG,, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013; 120: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saint-Geniez M,, Kurihara T,, Sekiyama E,, Maldonado AE,, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009; 106: 18751–18756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saint-Geniez M,, Maharaj AS,, Walshe TE,, et al. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008; 3: e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palejwala NV,, Jia Y,, Gao SS,, et al. Detection of nonexudative choroidal neovascularization in age-related macular degeneration with optical coherence tomography angiography. Retina. 2015; 35: 2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jia Y,, Bailey ST,, Wilson DJ,, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014; 121: 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hartnett ME,, Elsner AE. Characteristics of exudative age-related macular degeneration determined in vivo with confocal and indirect infrared imaging. Ophthalmology. 1996; 103: 58–71. [DOI] [PubMed] [Google Scholar]
- 13. Peterson LJ,, Wittchen ES,, Geisen P,, Burridge K,, Hartnett ME. Heterotypic RPE-choroidal endothelial cell contact increases choroidal endothelial cell transmigration via PI 3-kinase and Rac1. Exp Eye Res. 2007; 84: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monaghan-Benson E,, Hartmann J,, Vendrov AE,, et al. The role of vascular endothelial growth factor-induced activation of NADPH oxidase in choroidal endothelial cells and choroidal neovascularization. Am J Pathol. 2010; 177: 2091–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang H,, Wittchen ES,, Jiang Y,, Ambati B,, Grossniklaus HE,, Hartnett ME. Upregulation of CCR3 by age-related stresses promotes choroidal endothelial cell migration via VEGF-dependent and -independent signaling. Invest Ophthalmol Vis Sci. 2011; 52: 8271–8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H,, Geisen P,, Wittchen ES,, et al. The role of RPE cell-associated VEGF189 in choroidal endothelial cell transmigration across the RPE. Invest Ophthalmol Vis Sci. 2011; 52: 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avalos AM,, Valdivia AD,, Muñoz N,, et al. Neuronal Thy-1 induces astrocyte adhesion by engaging syndecan-4 in a cooperative interaction with alphavbeta3 integrin that activates PKCalpha and RhoA. J Cell Sci. 2009; 122: 3462–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geisen P,, McColm JR,, Hartnett ME. Choroidal endothelial cells transmigrate across the retinal pigment epithelium but do not proliferate in response to soluble vascular endothelial growth factor. Exp Eye Res. 2006; 82: 608–619. [DOI] [PubMed] [Google Scholar]
- 19. Leyton L,, Hagood JS. Thy-1 modulates neurological cell-cell and cell-matrix interactions through multiple molecular interactions. Adv Neurobiol. 2014; 8: 3–20. [DOI] [PubMed] [Google Scholar]
- 20. Gragoudas ES,, Adamis AP,, Cunningham ET, Jr,, Feinsod M,, Guyer DR; for the V.I.S.I.O.N. Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. New Engl J Med. 2004; 351: 2805–2816. [DOI] [PubMed] [Google Scholar]
- 21. Rege TA,, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival and cytokine/growth factor responses. Biochim Biophys Acta. 2006; 1763: 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyagawa-Yamaguchi A,, Kotani N,, Honke K. Expressed glycosylphosphatidylinositol-anchored horseradish peroxidase identifies co-clustering molecules in individual lipid raft domains. PLoS One 2014; 9: e93054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravelli C,, Mitola S,, Corsini M,, Presta M. Involvement of alphavbeta3 integrin in gremlin-induced angiogenesis. Angiogenesis. 2013; 16: 235–243. [DOI] [PubMed] [Google Scholar]
- 24. Sohn EH,, Khanna A,, Tucker BA,, Abramoff MD,, Stone EM,, Mullins RF. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci. 2014; 55: 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sparrow JR,, Ueda K,, Zhou J. Complement dysregulation in AMD: RPE-Bruch's membrane-choroid. Mol Aspects Med. 2012; 33: 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hageman GS,, Anderson DH,, Johnson LV,, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005; 102: 7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crabb JW,, Miyagi M,, Gu X,, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Nal Acad Sci U S A. 2002; 99: 14682–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spaide RF,, Armstrong D,, Browne R. Continuing medical education review: choroidal neovascularization in age-related macular degeneration—what is the cause? Retina. 2003; 23: 595–614. [DOI] [PubMed] [Google Scholar]
- 29. Curcio CA,, Millican CL,, Bailey T,, Kruth HS. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001; 42: 265–274. [PubMed] [Google Scholar]
- 30. Rodriguez IR,, Clark ME,, Lee JW,, Curcio CA. 7-ketocholesterol accumulates in ocular tissues as a consequence of aging and is present in high levels in drusen. Exp Eye Res. 2014; 128: 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bradley JE,, Ramirez G,, Hagood JS. Roles and regulation of Thy-1, a context-dependent modulator of cell phenotype. BioFactors. 2009; 35: 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leung CK,, Weinreb RN. Experimental detection of retinal ganglion cell damage in vivo. Exp Eye Res. 2009; 88: 831–836. [DOI] [PubMed] [Google Scholar]
- 33. Malek TR,, Fleming TJ,, Codias EK. Regulation of T lymphocyte function by glycosylphosphatidylinositol (GPI)-anchored proteins. Semin Immunol. 1994; 6: 105–113. [DOI] [PubMed] [Google Scholar]
- 34. Bukovsky A,, Caudle MR,, Carson RJ,, et al. Immune physiology in tissue regeneration and aging, tumor growth, and regenerative medicine. Aging. 2009; 1: 157–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Awasthi A,, Samarakoon A,, Chu H,, et al. Rap1b facilitates NK cell functions via IQGAP1-mediated signalosomes. J Exp Med. 2010; 207: 1923–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Indaram M,, Ma W,, Zhao L,, Fariss RN,, Rodriguez IR,, Wong WT. 7-Ketocholesterol increases retinal microglial migration, activation and angiogenicity: a potential pathogenic mechanism underlying age-related macular degeneration. Sci Rep. 2015; 5: 9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lorenc VE,, Jaldin-Fincati JR,, Luna JD,, Chiabrando GA,, Sanchez MC. IGF-1 regulates the extracellular level of active MMP-2 and promotes muller glial cell motility. Invest Ophthalmol Vis Sci. 2015; 56: 6948–6960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.