Abstract
Introduction
In developed countries, the physician‐patient relationship is moving from a paternalistic model to new decision‐making models that take patient preferences into account.
Objectives
Our aim was to develop a Decision Board (DB) and to test its acceptability in a French Regional Cancer Centre regarding the decision on whether or not to use chemotherapy after surgery in postmenopausal women with breast cancer. This paper presents the development process for this instrument and reports the pretesting phase, as well as the corresponding results.
Methods
A working group was created with oncologists, psychologists and economists. Following the first phase, i.e. the development process, a first version of the instrument was presented to health professionals. Their feedback led to important modifications of the instrument. The DB was then presented to experienced patients, which resulted in slight changes. The second phase consisted of pretesting the comprehension, internal and across‐time consistency of the DB on healthy volunteers.
Results
The DB was pretested in a group of 40 healthy volunteers. Eighteen respondents chose chemotherapy and 22 chose not to have chemotherapy. Comprehension rates were very high (≥87.5%). Internal consistency was assessed considering option attitudes based on outcomes and option attitudes based on process. Women shifted their choices in a predictable way. Across‐time consistency was appraised using the test‐retest method with Visual Analog Scales. The Intraclass Correlation Coefficient was 0.97.
Discussion‐conclusion
Due to cultural differences, the DB developed in our French Cancer Centre is quite different from the DBs previously developed elsewhere. Our instrument showed good comprehension and consistency properties, which are corroborated by the DB literature. Whether our DB is acceptable for patients with breast cancer must still be tested. Patients’ reactions will tell us which type of decision‐making model is at work. Further research is needed in order to explore the shared decision‐making process and clarify the concept.
Keywords: choice of treatment, Decision Board, oncology, decision‐making models, patient preferences
Introduction
Broad social changes have occurred in North America since the 1960s, (though a little later in Europe), in relation to two linked notions: individual autonomy and consumer rights. 1 , 2 Applied to general medical practices, these two notions have stimulated active patient participation in terms of establishing goals and making clinical decisions. Indeed, patients indicate a desire for more information concerning their disease and the need to be more involved in their health care decisions. 3 , 4 In the past, physicians tended to make treatment decisions for their patients, thus endorsing a paternalistic behaviour 1 , 5 and placing patients in a passive and dependent role. 5 , 6, –7 But now, particularly in North America, physicians often see the process of clinical decision‐making as a joint venture between the patients and themselves. 8
In France, important changes have also taken place in clinical decision‐making since the early eighties, inspired by AIDS patient associations. 9 , 10 Physicians are now required by law to ask for patients’ consent before performing any medical investigation or intervention, on the basis of clear, comprehensive and honest information. 11 , 12, 13, –14 More than 50% of the lawsuits brought against physicians are due to a lack of information given to the patients. The Supreme Court of Appeal recently stated that physicians must prove that good information has been provided to their patients. 15 Moreover, in a report to the Minister of Health, the National Advisory Committee of Ethics claimed that ‘shared decision‐making is the best way to proceed’. 16 In this report, shared decision‐making is defined as the ‘full association of the physician’s expertise and responsibility with the patient’s complete understanding of the different options’. However, the report outlines a gap between legal obligation and real practice because shared decision‐making, as defined in the report, is far from being implemented consistently.
The concept of shared decision‐making seems to be well‐suited to different clinical contexts, but especially in situations where, as Coulter 17 underlined, ‘there are several treatment options with different possible outcomes, and particularly those that are likely to have differential effects on the patient’s quality of life’. This is the case, for example, in cardiology, 18 gynaecology, 19 neonatalogy, 20 genetics 21 and oncology. In oncology, decisions often involve a trade‐off between the toxicity of the treatment (i.e. the quality of the patient’s life) and potential survival (i.e. quantity of life). Moreover, the uncertainty of the outcome at the individual level further complicates the problem. According to Sebban et al., 22 the process of shared decision‐making can be restated as follows: in order to make clinical decisions which involve trade‐offs and uncertainty, two components are required: knowledge of the risks and the benefits of each course of action (i.e. the knowledge component), and individual preferences regarding potential outcomes (i.e. the preference component). Yet as knowledge exists in one body, the physician, and preferences in another, the patient, shared‐decision making may take on a variety of different forms.
In their typology of treatment decision‐making models, Charles et al. 23 point out three criteria related to: (1) information exchange, (2) the deliberation process and (3) treatment decision. The ‘shared decision‐making’ model is defined according to these three criteria, as follows. Firstly, the information exchange is two‐way; the physician transfers knowledge to the patient and the patient provides information concerning his/her preferences to the physician. The second stage is deliberation. This refers to the process of expressing and discussing treatment options and is characterized by its interactional nature. As Charles et al. 23 state ‘both parties through the deliberation process work towards reaching an agreement.’ Thirdly, as a result, the treatment decision involves at least two decision‐makers: the physician and the patient.
Thus defined, the shared decision‐making model can be viewed as an intermediary between two other models, namely what can be referred to as the ‘patient as decision‐maker’ model and the ‘physician as decision‐maker’ model. In the former, which is similar to the ‘informed treatment decision‐making’ model described by Gafni et al., 24 ‘information exchange is one‐way, from physician to patient’. 23 There is therefore no deliberation process between patient and physician, as the decision is made by the patient according to his/her preferences, on the basis of the information provided by the physician. The patient is thus the sole decision‐maker and the physician is seen as a technical adviser whose preferences, per se, have no place. 25 , 26
Conversely, in the latter, the decision is made by the physician. But this encompasses two very different situations, namely ‘the paternalistic model’ 23 and the ‘physician as a perfect agent for the patient’ model. 24 As Charles et al. 23 report, ‘in the paternalistic model, the information exchange is largely one‐way and the direction is from physician to patient. At a minimum, the physician must provide the patient with legally required information on treatment options and obtain informed consent on the treatment recommended… In general, this model assumes that the physician knows best, and will make the best treatment decision for the patient’. Therefore, there is no deliberation between patient and physician, and the latter is the sole decision‐maker.
In the ‘physician as a perfect agent for the patient’ model, the physician uses their ‘extensive knowledge to make the treatment decision for the patient, taking the patient’s point of view’. 24 Here the information flow of patient preferences is one‐way (from patient to physician), because the physician ‘needs to know each patient’s utility function’ in order to make a decision. 19 In this case, there is no deliberation between patient and physician, as the latter is the sole decision‐maker. Interestingly, as Gafni et al. 24 argue, in theory the ‘physician as a perfect agent’ model and the ‘informed treatment decision‐making’ model result in the same outcomes.
Of course, these decision‐making models relate to theoretical paradigms, whereas situations in the real world most often conform to in‐between approaches. All of the models, except the paternalistic one, include patient preferences, and eliciting them is becoming a major issue. In France, it is now time to develop and test strategies aimed at eliciting patient preferences at the physician‐patient level. We are not aware of any systematic attempts at this except by Sebban et al., 22 who developed a Decision Board for allogenic bone‐marrow transplant which was pretested on healthy volunteers in the Lyon area, but never tested with patients at the decision point. Thus, the acceptability of this new kind of decision‐making process in the French sociological and cultural context still needs to be tested. 27
As stated before, such a decision‐making process seems to be well suited to different clinical contexts, particularly in oncology. Up until now, the use of postoperative adjuvant chemotherapy for postmenopausal women with node‐positive breast cancer (N < 8) and positive hormonal receptors (ER ≥ 10) has been controversial. According to randomized clinical trials on adjuvant therapy for postmenopausal breast cancer patients, 28 , 29 chemotherapy decreases relapse rates with no significant impact on overall survival. Therefore, in clinical practice guidelines developed by the French Federation of Cancer Centre, chemotherapy is not considered as a therapeutic standard but only as an option, as opposed to hormonotherapy and radiotherapy, which are both part of standard treatment in this situation. 30 In such cases, the patient and the oncologist are both faced with the question of whether the short‐term reduction in the patient’s quality of life due to chemotherapy is worth the long‐term reduction in the risk of recurrence.
Various strategies taking patient preferences into account could have been explored in this therapeutic situation, such as video techniques, 31 , 32 booklets and audio cassette tapes, leaflets, 18 , 19 , 33 and the Decision Board (DB) method which uses visual aid and written material. 22 , 34 , 35, 36, –37 We chose the latter because it comes closest to everyday interactions between patients and physicians in France. To the best of our knowledge, this approach was first used in the Hamilton Regional Cancer Centre (Ontario, Canada), for women with node‐negative breast cancer. 34 They were faced with the decision of whether or not to receive adjuvant chemotherapy. Because the DB was found acceptable and helpful for both patients and physicians, it is now used routinely in this Canadian Cancer Centre.
Objectives
Our general objective was to develop and test a DB that would allow doctors to transfer clear and comprehensive information to patients, so that the patient could then make a decision or discuss it with another doctor. Accordingly, our approach is consistent with two of the decision‐making models defined above, the ‘patient as decision‐maker’ model and the ‘shared decision‐making’ model. This paper presents the instrument development process in our Regional Cancer Centre located in Lyon, France, and reports the pretesting phase in terms of comprehension, internal consistency and across‐time consistency, as well as the corresponding outcomes.
Methods
As mentioned above, we developed a decision‐making instrument for postmenopausal women with node‐positive breast cancer (N < 8) and positive hormonal receptors (ER ≥ 10). The aim was to present the different postoperative treatments available to these women, thus allowing them to elicit a preference for one of the two options (one which included chemotherapy and the other which did not). A working group was created with five oncologists, one psychologist and two 2 economists, who met once a month from the beginning of 1997. Two phases can be clearly identified: the instrument development phase and the pretest phase.
Instrument development phase
An initial version of the DB was first elaborated by the working group, then presented for testing with health professionals and experienced patients.
DB – initial version
The two treatment options are described with their risks and benefits in an unbiased manner by means of written material and visual aids. The written material includes all the information to be transferred and can be seen as the physician’s guide for using the visual aids. The visual aid is empty at the beginning of the interview and, each time a piece of information is orally provided to the patient, a corresponding card with written information is attached to the board with Velcro. By the end of the interview, all the information cards are on the board and all the information included in the written material has been transferred.
As regards the type and amount of information to be conveyed, the first version of the instrument was close to the one developed by Levine et al. 1992. 34 Information concerning relapse, free survival and overall survival was based on available results from randomized trials. 28 , 29
Psychologists and economists have shown that the preferences elicited may depend on the way information is presented to respondents. 38 , 39 Even if other authors did not observe this phenomenon in the field of shared health care decision‐making, 40 we attempted to minimize these potential presentation effects when designing the visual aids. Firstly, the order of presentation of the two therapeutic options could cause sequencing effects. We therefore decided to present the two options simultaneously in eight successive points: (1) results of cancer surgery, (2) presentation of mandatory treatments (tamoxifen and radiation treatment), (3) formulation of therapeutic choice, (4) description of chemotherapy treatment, (5) presentation of the risks of relapse induced by the two options, (6) information about overall survival, (7) presentation of chemotherapy side‐effects, and (8) treatment schedules. Secondly, in order to avoid format effects, the two treatment options were both presented in the visual aid by colours that would have as little influence as possible on the patient’s choice: pale yellow for chemotherapy and pale orange for no chemotherapy. For the same reason, information concerning relapse rates was provided using bar charts instead of pie charts. This choice is rather unusual, but it was guided by the results of a survey conducted by Siminoff et al., 41 where respondents found bar charts easier to understand, although less pleasant, than pie charts.
Test with health professionals
In order to test the content and presentation of the information conveyed, the first version of the instrument was presented to two groups of health professionals (one group of four nurses and one group of eight physicians), from the staff of the Cancer Centre. In both groups, the information was presented in the same way as it would to a patient. Reactions were approximately the same in the two groups and can be summarized as follows: (1) giving detailed information to the patient about the effects of chemotherapy and metastatic relapse may be viewed as too violent, (2) speaking of overall survival may be very frightening for the patient and can generate strong adverse reactions, (3) telling her that overall survival is not affected by chemotherapy, whereas the relapse rate is, can be difficult to understand and misleading, and (4) asking the patient to decide can result in an opinion that the physician may not know what the best option is, and therefore may decrease her confidence in her doctor. This last point throws into question the very principle of using DBs for health care decision‐making. However, the working group decided to continue, knowing that it would always be possible for a patient to refuse either to be informed and/or to decide. Such patient reactions, if they occurred, would provide very useful information concerning the acceptability of the instrument. Regarding the second and third points on overall survival, the choice was between giving the corresponding information to the patient or not. While recognizing that the points made by the health professionals were relevant, the working group decided to maintain the information. It was considered that this information was essential, and omitting it would be paradoxical since it would not allow the patient to make an ‘informed‐decision’. 5 , 34 , 36 , 42 Taking into account the first point regarding the amount of information provided, the working group decided to modify the initial version. Certain developments were shortened, especially those concerning metastatic relapse. Moreover, as many authors recommended, the language was simplified and made as unspecialized as possible. 43 , 44, –45
Test with experienced patients
After the written material and visual aid had been tested with health professionals and modified accordingly, another test was conducted with women who had already completed chemotherapy. The aim was to assess the clarity of language and presentation of the instrument, and to determine whether it reflected their experience. Women were selected according to two criteria: (1) they had received both chemotherapy and radiotherapy and (2) they had been operated on for breast cancer more than five years ago. The reason for the second point was that we did not want them to feel concerned retrospectively by the information provided on the five‐year relapse rates. The psychologist for the working group presented the instrument to six women. All six women found that the instrument was clear, comprehensible and reflected their experience, except for tiredness induced by chemotherapy which was not specifically mentioned. Consequently, we added the possibility of feeling tired after each course as one of the side‐effects of chemotherapy.
In the final version of the instrument, the two major consequences of chemotherapy as compared to no chemotherapy (decrease in relapse risk and side‐effects), were depicted as follows: the five‐year relapse rates were estimated at 30% with chemotherapy vs. 40% without chemotherapy. 28 , 29 Four major potential side‐effects were finally presented: hair loss, nausea and vomiting, tiredness and infection requiring hospitalization.
Pre‐test phase
Comprehension, internal consistency and across‐time consistency of the instrument were tested on a group of healthy volunteers. We felt that it would be unethical to manipulate information concerning therapies and outcomes with patients at the decision point without first having tested these properties on healthy volunteers.
Pre‐test subjects
The instrument was tested on a group of healthy volunteers who were more than 50 years old and who had never experienced cancer. The sample was chosen as close as possible to the patient population in terms of the characteristics which, according to the literature, could influence patients’ attitudes towards oncology treatments. 46 , 47, 48, –49 We selected four criteria: (1) age, (2) family situation, (3) parenthood and (4) professional situation. Healthy volunteers were recruited so that distribution in these four categories was the same as the distribution observed in the patient population. Once again, the same skilled interviewer used the instrument to interview all the volunteers, followed by a two‐week retest on their decision to accept or reject chemotherapy treatment.
Intervention materials
Based on the outcomes of the development phase, we created a visual aid made of light cardboard, 35 cm wide and 50 cm high – large enough for the respondent to be able to read the cards without effort, but small enough to be carried around easily. In line with the DB approach, a paper take‐home version was created as well and given to the patient at the end of the interview (Fig. 1). This would allow her to have further discussions with different people, such as relatives and family doctor, before making a decision. [Link]
Figure 1.
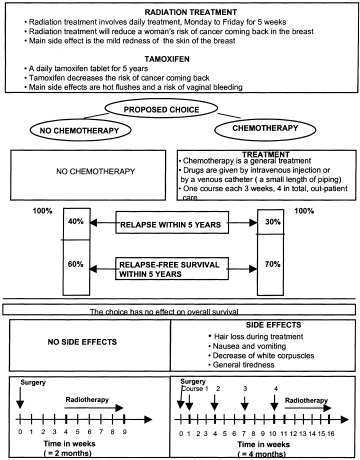
The decision board (take‐home version).
Comprehension
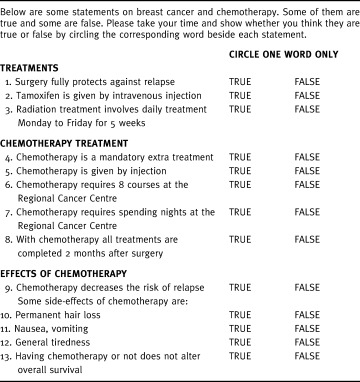
We decided to test comprehension in the same way as Whelan et al. when they developed a DB for breast irradiation postlumpectomy. 35 During the first test with the DB, the interviewer provides the healthy volunteer with information, then asks her to answer 13 true or false statements regarding basic information items, which included: three statements related to general postoperative information, five regarding chemotherapy treatment and five concerning chemotherapy side‐effects (Table 1). The items selected were those considered as key information to be understood by patients. It seemed important to assess comprehension rather than mere recall, because information must be fully understood by patients in order to justify claims to informed consent.
Table 1.
Comprehension questionnaire
Internal consistency
As there is no gold standard to determine whether the expressed choice is the patient’s true preference, only the internal consistency of the preference structure surrounding the treatment options can be evaluated. 50 In order to assess whether the choice of treatment changed in a predictable way according to changes in relapse rates and chemotherapy characteristics, two different approaches were used. One related to option attitudes based on outcomes, and the other to option attitudes based on process. Corresponding questions were asked after the comprehension questionnaire had been administered.
(1) Option attitudes based on outcomes
The hypothesis was that systematic manipulation of the five‐year relapse rates would lead to predictable shifts in expressed choice 51 , 52 (Table 2). For women who chose chemotherapy, the relapse rate without chemotherapy was held constant, while the relapse rate with chemotherapy was progressively increased. It was predicted that for a small or no gain in relapse rate, a woman would choose not to receive chemotherapy. For women who chose no chemotherapy, the relapse rate with chemotherapy was held constant and the relapse rate without chemotherapy was systematically increased until relapse was certain within 5 years. The prediction here was that a high enough increase in the relapse rate without chemotherapy would lead a woman to choose to receive chemotherapy.
Table 2.
Internal consistency: option attitudes based on outcomes (5‐year relapse rates)

(2) Option attitudes based on process
The hypothesis tested here was that modifying certain characteristics of the chemotherapy treatment (conditions of administration and side‐effects) would lead to predictable shifts in expressed choices (Table 3). The characteristics of the chemotherapy treatment were changed one by one, the others being held constant. Because we wanted them to be realistic, only slight changes were made. For a woman who chose chemotherapy, side‐effects such as hair loss and risk of infection were increased. Conditions of administration were worsened: the catheter became mandatory, eight courses of chemotherapy instead of four were required, or hospitalization was implied. It was predicted that these changes would lead the woman to give up chemotherapy. Conversely, for a woman who chose no chemotherapy, the side‐effects previously mentioned were suppressed, and conditions of administration improved: chemotherapy could be received at home, or could be orally administered. The prediction here was that these changes would lead the woman to choose chemotherapy.
Table 3.
Internal consistency: option attitudes based on process (characteristics of chemotherapy)

It was not possible to use a parallel structure for the two subgroups. In fact, side‐effects, such as tiredness, could be clearly suppressed in the no chemotherapy group. Conversely, worsening them in the chemotherapy group was not easy to express in a precise way. Moreover, some changes in the conditions of chemotherapy administration only pertained to one subgroup. This was the case for home chemotherapy, which was considered as an improvement for the no chemotherapy group.
Across‐time consistency
The across‐time consistency of the preference structure elicited by the DB intervention was assessed using the test‐retest method, which means that the instrument was used on the same respondent on two separate occasions. We chose a two‐week time interval, which is appropriate in this sort of situation. 50 At the end of the first test with the instrument, each healthy volunteer was asked not only to choose whether or not to be treated, but also to indicate her strength of preference on a Visual Analog Scale (VAS) by moving a cursor along the scale. It was graduated from 0, representing indifference between the two options, to 10, corresponding to an absolute preference for the chosen option. The gradation was only visible to the interviewer, who could score the stated preference between 0 and 10.
Two weeks later, the instrument was administered again to the same respondent by the same skilled interviewer. Each woman was asked again to state her decision and specify a strength of preference on a VAS. Reliability was assessed using the Intraclass Correlation Coefficient based on variance analysis (ANOVA) for repeated measures. 53
Results
Characteristics of pretest participants
Comprehension, internal consistency and across‐time consistency of the instrument were tested on a group of 40 healthy volunteers. In the published literature on DBs, sample sizes range from 25 to 42. 22 , 34 , 36 , 37 Healthy volunteers were selected so that the distribution of the sample in terms of the four selected criteria (see Methods) would be the same as the distribution of the patient population. This distribution was obtained from the patient database at the Cancer Centre concerning 348 postmenopausal breast cancer women admitted in 1996 and 1997. Mean age was 61 years: 16 women between 50 and 59, 14 between 60 and 69, and 8 over 70. Seventeen (42%) lived alone, and 7 (17%) had no children. Fifteen were retired, 15 were employed, and 10 were unemployed.
The first interview lasted 1 hour on average, and the second one 30 min.
Initial choice, strength of preference and comprehension
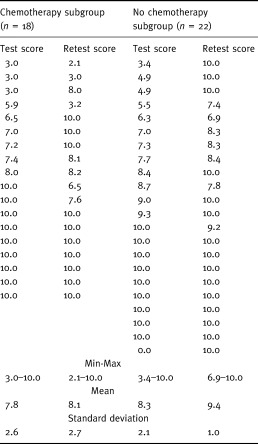
Eighteen respondents chose chemotherapy (45%), nine of whom definitely preferred this option with a VAS score of 10 (Table 4, initial test). Twenty‐two respondents chose not to have chemotherapy (55%), 10 of whom definitely preferred this option. Scores presented mean values of 7.8 (σ=2.64) and 8.3 (σ=2.1), respectively, in the two subgroups. A moderate preference being defined by a score less than 5 on the VAS (graduated from 0 to 10), there were 5 moderate preferences out of 40, none of them being less than 3.
Table 4.
Preference scores resulting from a VAS
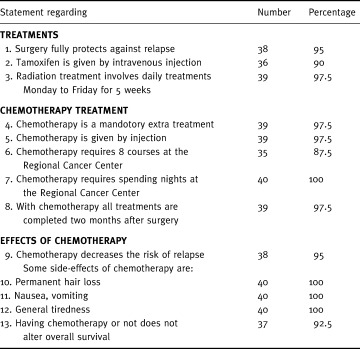
Comprehension rates, defined as the proportion of right answers for each of the 13 questions, were never lower than 87.5% (Table 5). Therefore, none of the questions were randomly answered (P < 5·10−6, one‐tailed χ2 test).
Table 5.
Healthy volunteers comprehension: Correct responses by statement (n = 40)
Internal and across‐time consistency
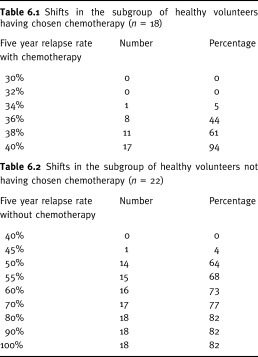
As regards internal consistency, the first approach (related to option attitudes based on outcomes) tested whether choices changed in a predictable way according to changes in relapse rates. For the 18 women who chose chemotherapy, the relapse rate without chemotherapy was held at 40%, whereas the relapse rate with chemotherapy was progressively increased by increments of 2%, from 32% to 40% (Table 6). In 17 women out of 18 (95%), preferences changed in the predicted way: women shifted to no chemotherapy when the two relapse rates were both equal to 40%. In other words, one woman out of 40 (2.5%, 95% Confidence Interval (CI): 0.06–13.16) still chose the chemotherapy option even when its benefits were suppressed. For the 22 women who chose no chemotherapy, the relapse rate with chemotherapy was held at 30%, whereas the relapse rate without chemotherapy progressively increased by increments of 5% until 60%, then by 10% until 100% (Table 6). When it rose to 50%, 14 women (65%) shifted to chemotherapy. When the five‐year relapse rate reached 100%, 18 women (80%) shifted to chemotherapy. However, this means that four women out of 40 (10%) still chose the no chemotherapy option even when relapse was certain within five years (95% CI: 2.79–23.66).
Table 6.
Preference shifts associated to changes in five‐year relapse rates
The second approach tested whether the treatment choice changed in a predictable way according to changes in chemotherapy characteristics (side‐effects and conditions of administration). For the 18 women who chose chemotherapy, certain conditions of administration were worsened, and side‐effects increased one by one (Table 3). Changes in the choice of treatment were rather slight: 5% when either eight courses of chemotherapy were required instead of four or when each course needed a two day hospitalization, 11% when hair loss was worsened, 17% when catheter was mandatory, and 28% in case of systematic hospitalization due to infection (Table 7). This last change led 5 women out of 40 (12.5%) to shift to no chemotherapy (95% CI: 4.19–26.8). Overall, the choice for chemotherapy was not very sensitive to increasing side‐effects or worsening conditions of the administration of the treatment, as defined in Table 3.
Table 7.
Preferences shifts associated to changes in the characteristics of chemotherapy
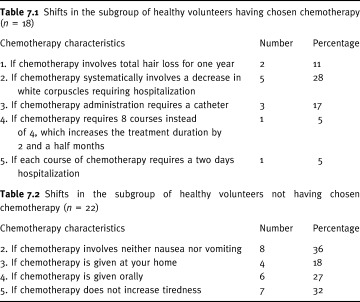
For the 22 women who chose no chemotherapy, certain conditions of administration were improved, and side‐effects suppressed one by one (Table 3). 18% shifted their choice to chemotherapy when chemotherapy was administered at home, 27% when chemotherapy was administered orally, 32% when the treatment was not tiring, 36% when vomiting and nausea disappeared, and 50% when hair loss was eliminated (Table 7). According to this result, eliminating hair loss led 11 women out of 40 (27.5%) to shift to chemotherapy (95% CI: 14.6–43.89). Overall, the no chemotherapy choice was rather sensitive to changes in the conditions of administration and side‐effects of chemotherapy.
As regards across‐time consistency, none of the women shifted their choice between the two interviews (Table 4), and the Intraclass Correlation Coefficient was 0.97.
Discussion and conclusion
The development of a Decision Board in our French Cancer Centre showed that this instrument is highly influenced by sociological and cultural contexts. For this reason, and not surprisingly, our DB is rather different from the one developed by Levine et al. in Ontario, Canada. 34 The latter addressed the role of adjuvant chemotherapy postoperatively in women with axillary node‐negative breast cancer, which is close to our situation. But, to make the instrument acceptable for the health professionals in our Cancer Centre, we had to omit certain information essentially related to metastatic relapse. This most likely reflects fundamental differences between the two sociological and cultural contexts. After the DB had been tested with health professionals, another test was conducted with experienced patients, where its clarity of language and presentation were confirmed. Comprehension and internal and across‐time consistency of the instrument were then tested on a group of 40 healthy volunteers, who chose either chemotherapy (45%) or no chemotherapy (55%). These two percentages are close to those reported by Levine et al. 34 in a group of 30 healthy women (57% and 43%, respectively). Comprehension rates were very high, none being less than 87.5% in each of the 13 true or false statements. This finding is close to the result obtained by Elit et al. in advanced epithelial ovarian cancer. 36 According to the authors, ‘the comprehension questionnaire was correctly answered by 96% of healthy volunteers’.
With regard to internal consistency, we first tested whether choice changed in a predictable way according to changes in relapse rates. In the two subgroups of women who chose either chemotherapy or no chemotherapy, they shifted their choice in the predicted way. This finding is consistent with Levine et al., 34 Sebban et al., 22 Elit et al., 36 and Whelan et al. 37 It must be emphasised that four out of 40 women chose the no chemotherapy option even when the five‐year relapse was assumed to be certain, which reflects a strong aversion to chemotherapy.
Secondly, we tested whether choice changed in a predictable way according to changes in chemotherapy characteristics. For the women who chose chemotherapy, their decision was not very sensitive to increasing side‐effects or worsening conditions of treatment administration. Our results are not as affirmative as those reported by Levine et al., which is that: 94% of the women ‘switched their choice when the toxicity of treatment was increased such that the chance of a fatal side‐effect was 50%’. 34 But this change is much more extreme than those we tested. With regard to the no chemotherapy choice, it was rather sensitive to changes in the conditions of administration and side‐effects of chemotherapy, especially hair loss: 50% shifted to chemotherapy when hair loss was eliminated, all other characteristics being held constant. Our findings are supported by what Levine et al. reported: ‘100% shifted when toxicity was eliminated’. 34 However, it is important to remember that all these results are highly dependent on presumed changes in chemotherapy characteristics (nature and magnitude).
Across‐time consistency assessed using the test‐retest method was excellent. None of the women shifted their choice between the two interviews, and the ICC was as high as 0.97. This result is consistent with the findings of other authors, although they did not score preferences using a VAS, but rather a Likert scale, either with six points 34 or seven points. 22 In addition to good comprehension and consistency properties, another advantage of the DB instrument is its flexibility. It can be easily and inexpensively modified as new relevant data are reported. This is now the case for our therapeutic situation, since new data report a slight significant increase in overall survival with chemotherapy, 54 whereas previous scientific literature did not. 28 , 29
This feature emphasises another important point. Since the DB method involves giving the patient a take‐home version at the end of the interview, developers of such instruments must use the highest quality information available. This ensures that the patient benefits from the best research evidence available related to the treatment options. 55
As previously stated, up until now, several methods have been used to transfer information from physician to patient. The DB approach is one of them and was chosen here because it is well adapted to every day interactions between patients and physicians in our country. All of these methods raise many unresolved questions, 17 , 56 and further empirical research is needed to assess their effects. 57 , 58 Questions to be addressed include which presentation options people find most useful, and how they affect people’s understanding and decisions. With regard to the general application of the DB method, the different stages (tests with health professionals, people who have experienced the options under consideration, and healthy volunteers) could be used to validate any physician‐to‐patient information transfer. It could be applied to any health care decision situation which involves patient trade‐offs, in an oncology context or in another. And, interestingly enough, it could also be used in informed consent decision situations, where the information transferred is not systematically validated today.
Health care decision situations which involve patient trade‐offs require a decision‐making process in which the physician and patient are both involved. 24 , 59 According to the two reference models, namely the “shared decision‐making” model and the ‘patient as decision‐maker’ model, the physician acknowledges the importance of the patient’s preferences and provides him/her with current information related to the different options. Available literature shows that most patients have a high preference for information concerning their disease. However, their preference for participation in decision‐making is not so clear. 3 , 4 , 60 , 61, 62, 63, –64 Therefore, the decision‐making process must take into account the patient’s desire to be informed on the one hand, and willingness to participate in decision‐making on the other. 45 , 65
In our setting, the DB approach is now being tested with real patients. Their reactions will tell us which type of decision‐making model is at work. It might be the ‘patient as decision‐maker’ model, the ‘shared decision‐making’ model, or even the ‘physician as decision‐maker’ model if the patient does ask the physician to make the decision for her.
More generally, further research is needed to explore the process of shared decision‐making and to clarify what the concept means. 23 , 25 It is important as well that we study the determinant characteristics that influence the degree of patient involvement in treatment choices in different sociological and cultural contexts. For this purpose, international comparisons could be quite appropriate.
Acknowledgements
Financial support for this study was provided by the National Centre for Scientific Research (Centre National de la Recherche Scientifique (CNRS)), the National Institute for Health and Medical Research (Institut National pour la Santé et la Recherche Médicale (INSERM)), the Inter‐Ministry Mission for Research (Mission Interministérielle à la Recherche (MIRE)) and the France Foundation (Fondation de France). Nora Ferdjaoui is a PhD student and a beneficiary of a grant from the Ligue Nationale de Lutte Contre le Cancer.
Footnotes
Written material and information cards can be obtained by writing to the authors.
References
- 1. Brody DS. The patient’s role in clinical decision‐making. Annals of Internal Medicine, 1980; 93 : 718 722. [DOI] [PubMed] [Google Scholar]
- 2. Richards MA, Ramirez AJ, Degner LF, Fallowfield LJ, Maher EJ, Neuberger J. Offering choice of treatment to patients with cancers. A review based on a symposium held at the 10th annual conference of The British Psychosocial Oncology Group. European Journal of Cancer, 1995; 31A : 112 116. [DOI] [PubMed] [Google Scholar]
- 3. Cassileth BR, Zupkis RV, Sutton‐Smith K et al Information and participation preferences among cancer patients. Annals of Internal Medicine, 1980; 92 : 832 836. [DOI] [PubMed] [Google Scholar]
- 4. Sutherland HJ, Llewellyn‐Thomas HA, Lockwood GA et al Cancer patients: their desire for information and participation in treatment decisions. Journal of the Royal Society of Medicine, 1989; 82 : 260 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brock DW & Wartman SA. When competent patients make irrational choices. New England Journal of Medicine, 1990; 322 : 1595 1599. [DOI] [PubMed] [Google Scholar]
- 6. Parsons T. The Social System New York: The Free Press, 1951.
- 7. Rose G. Reflections on the changing times. British Medical Journal, 1990; 301 : 683 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hughes TE & Larson LN. Patient involvement in health care. A procedural justice viewpoint. Medical Care, 1991; 29 : 297 303. [DOI] [PubMed] [Google Scholar]
- 9. Barbot J. Science, marché et compassion. L’intervention des associations de lutte contre le sida dans la circulation des nouvelles molécules. (Science, market and compassion. The role of AIDS Associations). Sciences Sociales et Santé, 1998; 16 : 67 95. [Google Scholar]
- 10. Barbot J. Agir sur les essais thérapeutiques. L’expérience des associations de lutte contre le sida en France. (Influencing clinical trials. The role of AIDS associations in France). Revue d’Epidémiologie et de Santé Publique, 1998; 46 : 305 315. [PubMed] [Google Scholar]
- 11. Anonymous Huriet Law (Loi Huriet) Loi 88–1138 du 20 décembre 1988 sur la protection des personnes qui se prêtent à des recherches biomédicales (Law related to the protection of people involved in biomedical research). Journal Officiel, 1988.
- 12. Anonymous Code de déontologie Médicale (Medical Code of Ethics) Professional Code of Ethics . Article 35, alinéa 1 (), 1995.
- 13. Ministère des Affaires Sociales , de la Santé et de la Ville, Charte du Patient Hospitalisé, (In‐patient Charter). Annexe de la circulaire ministérielle no. 95–22 du 6 mai 1995. relative aux droits des patients hospitalisés. Charte du patient hospitalisé, 1995.
- 14. European Council . Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (Oviedo, April 1997), Strasbourg: European Treaty Series no. 164, 1997.
- 15 Civ. 1, 25 février 1997, Gazette du Palais, 27–29 avril. 1997, 22–28
- 16. National Advisory Committee of Ethics (Comité Consultatif National d’Ethique) Consentement éclairé et information des personnes qui se prêtent à des actes de soin ou de recherche. (Informed consent and patients information). Rapport No. 58, Paris: CCNE, 1998.
- 17. Coulter A. Parternships with patients: the pros and cons of shared clinical decision‐making. Journal of Health Service Research and Policy, 1997; 2 : 112 121. [DOI] [PubMed] [Google Scholar]
- 18. Man Son Hing M, Laupacis A, O’Connor AM, et al A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation. Journal of the American Medical Association, 1999; 282 : 737 743. [DOI] [PubMed] [Google Scholar]
- 19. O’Connor AM, Tugwell P, Wells GA, et al Randomized trial of a portable, self administered decision aid for post‐menopausal women considering long‐term preventive hormone replacement therapy. Medical Decision Making, 1998; 18 : 149 163. [DOI] [PubMed] [Google Scholar]
- 20. Engler‐Todd L, Drake E, O’Connor A, Hunter A. Evaluation of a decision aid for prenatal testing for women of advanced maternal age. Journal of Genetic Counseling, 1997; 6 : 439 439. [DOI] [PubMed] [Google Scholar]
- 21. Shiloh S. Decision‐making in the context of genetic risk. In: Marteau T and Richards M (eds) The troubled helix: social and psychological implications of the new human genetics. Cambridge: Cambridge University Press, 1996: 82–103.
- 22. Sebban C, Browman G, Gafni A et al Design and validation of a bedside decision instrument to elicit a patient’s preference concerning allogenic bone marrow transplantation in chronic myeloid leukemia. American Journal of Hematology, 1995; 48 : 221 227. [DOI] [PubMed] [Google Scholar]
- 23. Charles C, Gafni A, Whelan T. Decision‐making in the physician‐patient encounter: revisiting the shared treatment decision‐making model. Social Science and Medicine, 1999; 49 : 651 661. [DOI] [PubMed] [Google Scholar]
- 24. Gafni A, Charles C, Whelan T. The physician‐patient encounter: the physician as a perfect agent for the patient versus the informed treatment decision‐making model. Social Science and Medicine, 1998; 47 : 347 354. [DOI] [PubMed] [Google Scholar]
- 25. Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean? (or it takes at least two to tango). Social Science and Medicine, 1997; 44 : 681 692. [DOI] [PubMed] [Google Scholar]
- 26. Dowie J. Decision analysis in guideline development and clinical practice: the ‘Clinical Guidance Tree’. In: Selbmann HK (ed) Guidelines in Health Care, Baden‐Baden: Schriftenreihe des Bundersministeriums für Gesundheit, 1997; 162–185.
- 27. Ferdjaoui N, Carrère MO, Charavel M, et al La prise en compte des préférences des patients dans la décision thérapeutique en cancérologie: développement d’un tableau de décision. (Taking patient preferences into account in clinical decision‐making in oncology: development of a decision board). Journal d’Economie Médicale, 1999; 17: 327 342. [Google Scholar]
- 28. Gelber RD, Cole BF, Goldhirsch A et al Adjuvant chemotherapy plus tamoxifen compared with tamoxifen alone for postmenopausal breast cancer: meta‐analysis of quality‐adjusted survival. Lancet, 1996; 347 : 1066 1071. [DOI] [PubMed] [Google Scholar]
- 29. International Breast Cancer Study Group . Effectiveness of adjuvant chemotherapy in combination with tamoxifen for node‐positive postmenopausal breast cancer patients. Journal of Clinical Oncology, 1997; 15 : 1385 1394. [DOI] [PubMed] [Google Scholar]
- 30. Fervers B, Bonichon F, Demard F et al Méthodologie de développement des standards, options et recommandations diagnostiques et thérapeutiques en cancérologie. (Methodology for the development of Standards, Options and Recommendations in oncology). Bulletin Du Cancer, 1995; 82 : 761 767. [PubMed] [Google Scholar]
- 31. Wennberg JE. Outcomes research, patient preference, and the primary care physician. Journal of the American Board of Family Practice, 1991; 4 : 365 367 [PubMed] [Google Scholar]
- 32. Wennberg JE. Outcomes research: What does it really mean? Managed Medicine, 1991; 2 : 7611 7611. [Google Scholar]
- 33. Sawka CA, Goel V, Mahut CA et al Development of a patient decision aid for choice of surgical treatment for breast cancer. Health Expectations, 1998; 1 : 23 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levine M, Gafni A, Markham B, MacFarlane D. A bedside decision instrument to elicit a patient’s preference concerning adjuvant chemotherapy for breast cancer. Annals of Internal Medicine, 1992; 117 : 53 58. [DOI] [PubMed] [Google Scholar]
- 35. Whelan TJ, Levine M, Gafni A, Lukka H, Mohide EA, Patel M et al Breast irradiation postlumpectomy: development and evaluation of a decision instrument. Journal of Clinical Oncology, 1995; 13 : 847 853. [DOI] [PubMed] [Google Scholar]
- 36. Elit LM, Levine MN, Gafni A et al Patients’ preferences for therapy in advanced epithelial ovarian cancer: development, testing, and application of a bedside decision instrument. Gynecological Oncology, 1996; 62 : 329 335. [DOI] [PubMed] [Google Scholar]
- 37. Whelan TJ, Levine M, Gafni A et al Mastectomy or lumpectomy? Helping women make informed choices. Journal of Clinical Oncology, 1999; 17 : 1727 1735. [DOI] [PubMed] [Google Scholar]
- 38. Tversky A & Kahneman D. The framing of decisions and the psychology of choice. Science, 1981; 211 : 453 458. [DOI] [PubMed] [Google Scholar]
- 39. Willinger M. Préférences dépendantes du contexte: les enseignements de l’économie expérimentale. (Context depending preference: learnings from experimental economy). Revue Economique, 1995; 3 : 577 587. [Google Scholar]
- 40. Siminoff LA & Fetting JH. Effects of outcome framing on treatment decisions in the real world: impact of framing on adjuvant breast cancer decisions. Medical Decision Making, 1989; 9 : 262 271. [DOI] [PubMed] [Google Scholar]
- 41. Siminoff LA, Ravdin PM, Gerson N et al Impact of a personal computer based instrument for providing individualized estimates of outcomes for patients with early breast cancer. Congress of the American Society of Clinical Oncology, Abstract No. 404. Los Angeles, CA, 1998.
- 42. Gafni A. When does a competent patient make irrational choices. New England Journal of Medicine, 1990; 323 : 1354 1354. [Google Scholar]
- 43. Bourhis RY, Roth S, McQueen G. Communication in the hospital setting: a survey of medical and everyday language use amongst patients, nurses and doctors. Social Science & Medicine, 1989; 28 : 339 346. [DOI] [PubMed] [Google Scholar]
- 44. Hadlow JAU & Pitts M. The understanding of common health terms by doctors, nurses and patients. Social Science and Medicine, 1991; 32 : 193 196. [DOI] [PubMed] [Google Scholar]
- 45. Ong LML, De Haes JCJM, Hoos AM, Lammes FB. Doctor‐patient communication: a review of the literature. Social Science and Medicine, 1995; 40 : 903 918. [DOI] [PubMed] [Google Scholar]
- 46. Slevin ML, Stubbs L, Plant HJ et al Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. British Medical Journal, 1990; 300 : 1458 1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bremnes RM, Andersen K, Wist EA. Cancer patients, doctors and nurses vary in their willingness to undertake cancer chemotherapy. European Journal of Cancer, 1995; 31A : 1955 1959. [DOI] [PubMed] [Google Scholar]
- 48. Yellen SB & Cella DF. Someone to live for: social well‐being, parenthood status, and decision‐making in oncology. Journal of Clinical Oncology, 1995; 13 : 1255 1264. [DOI] [PubMed] [Google Scholar]
- 49. Albain KS, Green SR, Lichter AS et al Influence of patient characteristics, socioeconomic factors, geography, and systemic risk on the use of breast‐sparing treatment in women enrolled in adjuvant breast cancer studies: an analysis of two intergroup trials. Journal of Clinical Oncology, 1996; 14 : 3009 3017. [DOI] [PubMed] [Google Scholar]
- 50. Streiner DL & Norman GR. Health Measurement Scales: a Practical Guide to Their Development and Use New York: Oxford University Press, 1989.
- 51. Llewellyn‐Thomas HA, Sutherland HJ, Tibshirani R, Ciampi A, Till JE, Boyd NF. Describing health states. Methodologic issues in obtaining values for health states. Medical Care, 1984; 22 : 543 552. [PubMed] [Google Scholar]
- 52. Llewellyn‐Thomas HA, McGreal MJ, Thiel EC, Fine S, Erlichman C. Patients’ willingness to enter clinical trials: measuring the association with perceived benefit and preference for decision participation. Social Science and Medicine, 1991; 32 : 35 42. [DOI] [PubMed] [Google Scholar]
- 53. Norman GR & Streiner DL. PDQ Statistics. Toronto: B.C. Decker, Inc., 1986.
- 54. Early Breast Cancer Trialists’ Collaborative Group . Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet, 1998; 352 : 930 942. [PubMed] [Google Scholar]
- 55. Coulter A. Evidence based patient information. British Medical Journal, 1998; 317 : 225 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Entwistle VA, Sheldon TA, Sowden A, Watt IS. Evidence‐informed patient choice. Practical issues of involving patients in decisions about health care technologies. International Journal of Technology Assessment in Health Care, 1998; 14 : 212 225. [DOI] [PubMed] [Google Scholar]
- 57. Coulter A, Entwistle V, Gilbert D. Informing patients: an assessment of the quality of patient information materials. London: King’s fund, 1998.
- 58. Coulter A, Entwistle V, Gilbert D. Sharing decisions with patients: is the information good enough? British Medical Journal, 1999; 318 : 318 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Emanuel EJ & Emanuel LL. Four models of the physician‐patient relationship. Journal of the American Medical Association, 1992; 267 : 2221 2226. [PubMed] [Google Scholar]
- 60. Strull W, Lo B, Charles G. Do patients want to participate in medical decision‐making? Journal of the American Medical Association, 1984; 252 : 2990 2994. [PubMed] [Google Scholar]
- 61. Blanchard CG, Labrecque MS, Ruckkdeschel JC et al Information and decision‐making preferences of hospitalized adult cancer patients. Social Science and Medicine, 1988; 27 : 1139 1145. [DOI] [PubMed] [Google Scholar]
- 62. Degner L & Sloan JA. Decision‐making during serious illness: What role do patients really want to play? Journal of Clinical Epidemiology, 1992; 45 : 941 950. [DOI] [PubMed] [Google Scholar]
- 63. Beisecker AE & Beisecker T. Patient information‐seeking behaviors when communicating with doctors. Medical Care, 1990; 28 : 19 28. [DOI] [PubMed] [Google Scholar]
- 64. Vick S & Scott. Agency in health care. Examining patients’ preferences for attributes of the doctor‐patient relationship. Journal of Health Economics, 1998; 17 : 587 605. [DOI] [PubMed] [Google Scholar]
- 65. Venin P, Hecquet B, Marcuzzi I, Demaille MC. Cancer du sein: l’information en question (s). Enquête auprès des patientes et des médecins d’un centre de lutte contre le cancer. (Survey with patients and physicians in a Cancer Centre). Bulletin Du Cancer, 1995; 82 : 698 704. [PubMed] [Google Scholar]


