Abstract
Although guidelines for treating stage IV non‐small cell lung cancer suggest that the patient’s values should be considered in decision‐making, there are no practical tools available to assist them with their decision‐making.
Objective
To develop and evaluate a decision aid that incorporates patient values.
Design and sample
(1) Before/after evaluation with patients referred to a regional cancer centre. (2) Mailed survey of thoracic surgeons and respirologists in Ontario.
Intervention
An audio‐tape guided individuals to review a booklet describing stage IV non‐small cell lung cancer, its impact and possible coping strategies, treatment options, benefits and risks, and examples of the decision‐making of others. Patients then used a worksheet to consider and communicate personal issues involved in the choice, including: personal values using a ‘weigh‐scale’; questions; preferred role in decision‐making; and predisposition.
Measures
(1) Patient questionnaires eliciting knowledge, the decision, decisional conflict and acceptability of the decision aid. (2) Physician questionnaires eliciting attitudes toward the decision aid.
Results
(1) Twenty of 30 patients used the aid in decision‐making. Users thought that the aid was acceptable and significantly improved their knowledge about options and outcomes (P < 0.001), and reduced their decisional conflict (P < 0.001). (2) The majority of the 29 physicians who reviewed the decision aid found it acceptable, were comfortable providing it to patients and said that they were likely to use it.
Conclusion
The decision aid is a useful and acceptable adjunct to personal counselling.
Keywords: decision aid, lung cancer, patient education, shared decision‐making
Introduction
Lung cancer is the leading cause of cancer deaths for North American men and women. 1 Owing to its insidious onset, 25–30% of patients presenting with non‐small cell lung cancer have stage IV disease, which has a 3% 5‐year survival rate. 2 According to the Cancer Care Ontario Practice Guidelines Initiative, treatment options include supportive care with palliative radiation therapy as needed, with the possible addition of chemotherapy. 3 The guideline suggests that chemotherapy may provide some relief of symptoms, and may increase the 1‐year survival rate from 10% to 20%. However, the benefits of chemotherapy are short‐lived. By 2 years there is a non‐significant increase in median survival of only 6 weeks. Therefore the decision to take chemotherapy involves weighing the improvements in symptom relief and short‐term survival against the side‐effects and inconvenience of treatment.
There is considerable variation in opinion as to whether the benefits of chemotherapy outweigh the risks. Practice guidelines suggest that patients’ values be considered in deliberating about options. 3 This is a challenging task for clinicians with limited time to counsel patients, and for patients who may be sick and anxious, and have limited education. Currently, there are no practical tools to assist patients to consider the personal importance that they attach to the benefits and risks of chemotherapy for advanced lung cancer.
Decision aids have been used with other types of patients as adjuncts to counselling. They prepare patients and families for decision‐making by describing choices and their probable outcomes based on research evidence. They also assist in clarifying values or desirability of each of the expected outcomes. 4 Evaluation studies have shown that decision aids help the uncertain to make decisions, and increase the likelihood that decisions are based on better knowledge, realistic expectations of outcomes, and personal values. 9
Our study objective was to develop and evaluate a take‐home, self‐administered decision aid incorporating patient values as an adjunct to counselling.
Methods
Ethical approval for this study was obtained from the research ethics committee of the hospitals affiliated with the regional cancer centre.
Decision aid development
We developed a decision aid entitled ‘Making Choices: Treatment of Stage IV Non‐Small Cell Lung Cancer’. Development was guided by constructs in the Ottawa Decision Support Framework 4 and was based on the evidence and recommendations of the Cancer Care Ontario practice guideline on treatment for stage IV non‐small cell lung cancer. 3
The decision aid used three basic strategies from the Ottawa Decision Support Framework: (1) providing information on options and outcomes to improve knowledge and create realistic expectations; (2) providing guidance and examples in the steps in decision‐making to improve decision‐making skills; and (3) including a ‘weigh‐scale’ exercise to clarify and communicate personal values. 4
The decision aid is a self‐administered, self‐paced booklet, audio‐tape and worksheet. The audio‐tape guides patients for 35 min to review information in a booklet and to complete a personal worksheet. The information focuses on: lung cancer, staging, the functional impact of disease and coping strategies; the treatment options of supportive care, radiation therapy and chemotherapy; the benefits, risks and side‐effects of chemotherapy; and steps in decision‐making.
The survival benefits are presented both as an absolute improvement in 1‐year survival (10 more people out of 100 are alive after 1 year because of chemotherapy) and as a median improvement in survival (the typical patient lives 1.5 months longer because of chemotherapy).
The steps in decision‐making show patients how to consider the personal issues involved in the decision using a worksheet. The use of the personal worksheet is illustrated first by showing how three other patients completed it. The different cases also reinforce the notion that decision‐making is variable and should be individualized according to a person’s health history, values and preference for decision participation.
Patients are guided in the personal worksheet to: (1) clarify personal values regarding the pros and cons of chemotherapy using a ‘weigh‐scale’ exercise; (2) identify questions for their practitioners; (3) identify their preference for participation in decision‐making; and (4) indicate their predisposition toward treatment options. The ‘weigh‐scale’ exercise ( Fig. 1) asks patients to write in the pros and cons of chemotherapy that have meaning for them, and to shade in their personal importance.
Figure 1.
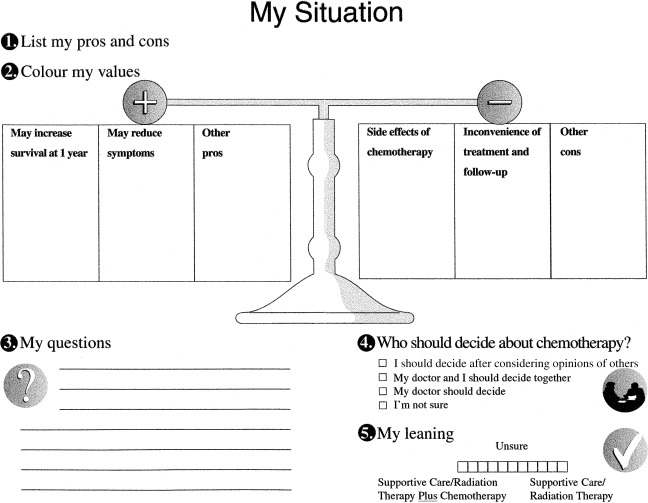
The patient’s personal worksheet.
The decision aid has illustrative icons to represent each concept. The text was adjusted to a grade seven reading level, but was comprehensible to those with less formal education because of the accompanying audio‐tape and illustrations. Patients used the aid in a self‐paced fashion, alone or with family members.
Development and review panels
Three panels were involved in development: a development panel; a practitioner panel; and a patient panel.
The development panel (comprising two medical oncologists, including the chair of the Cancer Care Ontario Guideline Committee on Lung Cancer, two decision‐making researchers and two oncology nurses) reviewed each draft to ascertain face and content validity.
Then the practitioner panel (all medical and radiation oncologists in the region who treat lung cancer) evaluated the face and content validity, and ensured that the information in the decision aid reflected actual practice. The aid was revised until they were comfortable using it with their patients.
Finally, the content validity and acceptability of the decision aid were evaluated with a panel of six patients who had already made the decision about chemotherapy for lung cancer (four had accepted and two had declined chemotherapy). The panel found the decision aid acceptable in terms of amount of information, length, clarity, and appropriateness and usefulness for patients faced with this decision. However, three participants (all taking chemotherapy) found it slightly or clearly slanted toward chemotherapy, in part because more time was spent describing this option. Three (also all taking chemotherapy) found the absolute survival benefit information upsetting. They had not realized that the survival benefit was so low, and two of them thought that it should be removed because it would be very upsetting for patients who had not heard it first from their physicians.
The decision aid was modified prior to the next phase of evaluation. The description of the benefits of chemotherapy was rewritten to emphasize that the range of survival benefit from chemotherapy was wide. Previously, this had only been stated in the audio‐tape. It was decided to leave in the information on absolute survival benefit so that patients would have this information to make an informed decision. However, it was agreed that the decision aid would only be given to patients after the oncologist had briefly introduced the options to them, so that it would not be so surprising. The order of information on the side‐effects of chemotherapy was also changed to ensure better flow and comprehension of the information.
Before/after patient evaluation
A before/after study was conducted to evaluate the decision aid with patients at the point of decision‐making. It was hypothesized that the decision aid would: (1) increase knowledge of treatment alternatives, benefits and risks; (2) create realistic perceptions of the likelihood of survival benefit; and (3) reduce decisional conflict or uncertainty about which treatment to take.
A convenience sample of patients at the point of decision‐making was recruited from the Ottawa Regional Cancer Center in Ontario, Canada, to evaluate the decision aid. Inclusion criteria were that patients should be (1) newly diagnosed with stage IV non‐small cell lung cancer, (2) being followed at the Cancer Center, (3) making the decision regarding chemotherapy for stage IV non‐small cell lung cancer, (4) able to read and speak English, and (5) consenting to participate. Excluded were patients (1) having metastases to the brain causing significant cognitive impairment, and/or (2) being judged unsuitable to participate by Cancer Center staff owing to physical or mental impairment or emotional distress.
All medical oncologists in the region who treated patients with lung cancer agreed to refer patients to the study. During the first referral visit, a medical oncologist confirmed the patient’s eligibility, introduced the treatment options, and asked if the patient would be interested in participating in the study. A researcher then proceeded to (1) obtain informed consent, (2) administer a baseline questionnaire, and (3) give the decision aid to the patient to review at home. On return to the Cancer Center, the patient completed the post‐test questionnaire and then met with their oncologist to ask any further questions that they may have identified, and to make a decision regarding treatment.
The baseline questionnaire elicited the following data: clinical and demographic information; knowledge of options and outcomes; predisposition toward options; and decisional conflict. The post‐test questionnaire assessed the same variables plus acceptability of the decision aid.
Knowledge was assessed by asking patients to respond to items derived from information in the decision aid using a true/false/unsure response format. The focus was on information essential for decision‐making, including the nature of metastatic disease, the options, and the benefits, risks and side‐effects. This approach has been used previously in other evaluations of decision aids. 4
Quantitative perceptions of survival benefit were elicited by asking patients to estimate how much chemotherapy increases a patient’s chance of survival at 1 year (e.g. 10 out of 100 more people will be alive at 1 year; 20 out of 100; 30 out of 100, etc.). The perception was judged to be ‘realistic’ if a person responded that 10 out of 100 more people will be alive at 1 year, in accordance with the published evidence, which was presented in the decision aid.
The decisions patients made were quantified using an 11‐point rating scale anchored by ‘supportive care/radiation therapy plus chemotherapy’ and ‘supportive care/radiation therapy’, with ‘unsure’ situated in the centre of the scale. The test–retest reliability coefficient for this type of scale in a hormone replacement therapy study was 0.91. 5
Decisional conflict was assessed using two subscales of the decisional conflict scale: uncertainty in making a choice; and modifiable factors contributing to uncertainty such as feeling uninformed, unclear about personal values and unsupported in decision‐making. The scale has been evaluated in over 1000 individuals making a variety of health care decisions, and is reliable, valid and responsive to change. 5 , 6
Acceptability was assessed using several of the standardized questions used in evaluations of shared decision‐making programmes. 7 Patients were asked about the amount and clarity of information, and the balance, helpfulness and usefulness of the decision aid. A question was added regarding how upsetting the information was.
Physician survey
A cross‐sectional mail survey of Ontario thoracic surgeons and respirologists who currently treat lung cancer patients was undertaken to elicit their opinions of the decision aid. The sampling frame for the survey consisted of all physicians listed by the Ontario Medical Association as thoracic surgeons and respirologists. As part of the Cancer Care Ontario Practice Guidelines Initiative, all physicians on the list were mailed copies of three Cancer Care Ontario practice guidelines on the treatment of lung cancer, along with a copy of the decision aid and a three‐page evaluation questionnaire. The survey consisted of two mailings (no reminders were sent) and occurred between August and October 1997.
The evaluation questionnaire asked about the respondent’s practice characteristics (specialty, number of years practising their specialty, number of patients with lung cancer seen each year), whether they agreed with Cancer Care Ontario’s practice guideline related to chemotherapy for stage IV non‐small cell lung cancer, and whether they had reviewed the decision aid. Respondents who had reviewed the decision aid were then asked the extent to which they agreed with 18 Likert‐type questions intended to elicit their attitudes about the acceptability and usefulness of the aid. Response categories ranged from 1 (strongly agree) to 5 (strongly disagree). Respondents were also asked how comfortable they would be providing the decision aid to their patients, and how likely they were to share it with future patients. Response categories for these two questions were 1 (very uncomfortable) to 5 (very comfortable), and 1 (very unlikely) to 5 (very likely), respectively.
Data management and analysis
Data entry, quality assurance and analysis were performed at the Clinical Epidemiology Unit of the Loeb Health Research Institute, Ottawa Hospital–Civic Campus, using spss for Windows V.6.1.2 (SPSS, Chicago, IL, USA). Descriptive statistics were generated for all variables. Hypotheses in the patient study were analysed using paired t‐tests or McNemar tests for change. The estimated sample size in the patient study was based on the paired t‐test for comparing the means of the outcome ‘decisional conflict’ before and after using the decision aid. For a level of significance α=0.05, a power (1 − β)=0.80, a standard deviation of 0.6 4 and a correlation between before/after scores of 0.70, the sample size was estimated at 20 participants to detect a before/after difference of 0.3 in the decisional conflict score, out of a possible score of 1–5. The proposed effect size was 0.50, which Cohen defines as a medium effect. 8 It is clinically important in that effect sizes among those who make or delay decisions range from 0.43 to 0.82.
Results
Before/after patient evaluation
Participants were recruited for the before/after phase of the study from 20 May 1997 to 30 April 1998. The accrual and completion rates are shown in Fig. 2. Of 33 patients approached by the oncologist to participate in the study, 30 agreed, and 20 used the decision aid and completed both the baseline and post‐test questionnaires.
Figure 2.
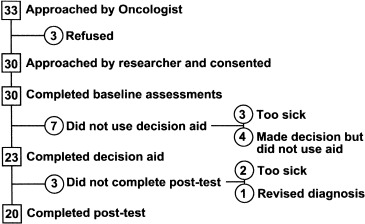
Accrual and completion rates.
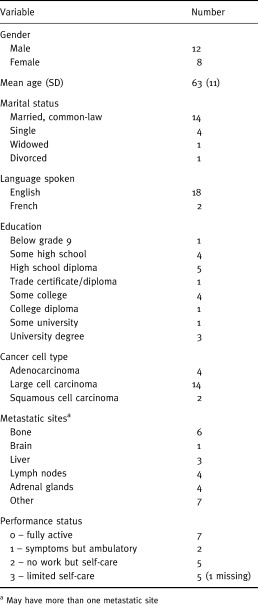
The demographic and clinical status of the participants is shown in Table 1. The ages of the participants ranged from 38 to 83 years old. The typical patient was 63 years old, male, English speaking and married. Participants varied considerably in terms of level of education and clinical/performance status.
Table 1.
Demographic and clinical characteristics (n = 20)
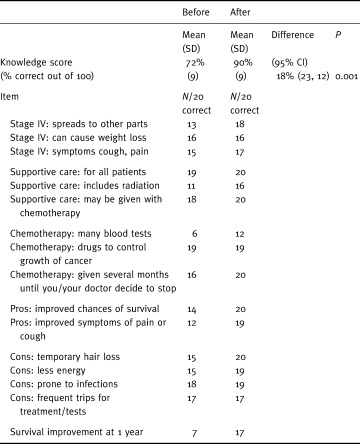
Patients’ knowledge of options, benefits and risks is presented in Table 2. As hypothesized, knowledge improved significantly (P < 0.001) from a mean of 72% correct responses at baseline to 90% correct responses after using the decision aid. When individual items were examined, there were improvements in 14 of 16 items. Larger improvements were noted in participants’ understanding of the number of blood tests per week, and the benefits of chemotherapy in terms of improved survival and improved symptoms.
Table 2.
Changes in knowledge test results (n = 20)
Patients’ quantitative perceptions of the degree to which chemotherapy improved chances of survival also improved in the hypothesized direction, from seven out 20 patients being realistic before using the decision aid to 17 out of 20 being realistic after using the decision aid (P=0.013, McNemar’s test for change).
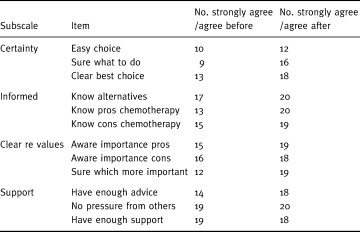
Changes in participants’ decisional conflict scores after using the decision aid are illustrated in Fig. 3. As hypothesized, there was a statistically significant decline in decisional conflict (P < 0.001), with the mean score declining by 0.6 out of 5 (95% confidence interval: 0.4, 0.8). When individual items in the scale were examined (see Table 3), there were improvements in all but one item. Improvements were most pronounced in uncertainty (the patient feeling sure what to do), feeling informed (knowing the pros of chemotherapy), and clarity of values (being clear about what was most important to them). The smallest change related to support items, which were already high at baseline.
Figure 3.
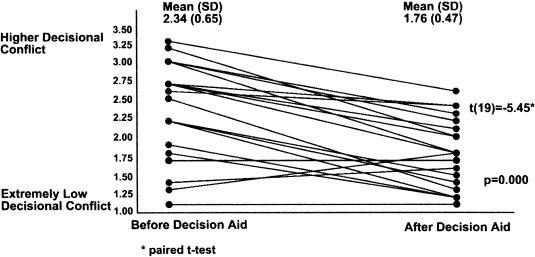
Decisional conflict scores.
Table 3.
Responses to items on decisional conflict scale
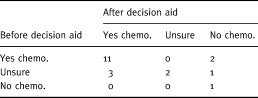
Participants’ decisions regarding chemotherapy both before and after using the decision aid are shown in Table 4. Most change occurred in those who were undecided at baseline, but two participants did change their preference from chemotherapy to no chemotherapy.
Table 4.
Decision before and after decision aid
As indicated on their personal worksheet, most participants indicated that they preferred an active role in decision‐making about treatment: 11 out of 20 preferred to share decision‐making with their physicians; six wanted to decide on their own after considering their physician’s opinion, and the rest were unsure about the role that they wanted in decision‐making.
All 20 participants found the decision aid helpful in aiding decision‐making, and would recommend it to others making the same decision. At least three‐quarters of participants were satisfied with the amount (16/20) and clarity (16/20) of the information, and found that it was balanced (15/20) and that it was not upsetting (16/20). Those who found that it was slightly slanted (4/20) or clearly slanted (1/20) toward chemotherapy had strong baseline predispositions toward taking chemotherapy. Those who found it upsetting (4/20) were bothered about the information regarding survival. They commented that it made them realize the truth, that it made them sad, and that there were many ‘cons’ to choosing chemotherapy.
Eleven participants commented positively on the presentation of the information using an audio‐tape and booklet format. In addition, one participant enjoyed having the decision aid available for use at home, allowing them to review the information and share the information with family members.
Physician survey
Of the 84 thoracic surgeons and 111 respirologists on the list of the Ontario Medical Association, 20 thoracic surgeons and 11 respirologists were no longer at the address provided, or replied indicating that they did not treat adult lung cancer or did not practise the specialty of interest. Thirty‐six per cent of eligible thoracic surgeons (23/64) and 37% of respirologists (37/100) responded to the questionnaire. Of these, 15 thoracic surgeons and 14 respirologists reviewed the decision aid (four listened only to the tape, 12 reviewed only the booklet, 10 reviewed the booklet and listened to the tape, and three did not state what they reviewed).
The majority of respondents (n=35) reported having more than 21 patients diagnosed with lung cancer per year. On average, respondents had been in practice for 12 years (standard deviation=8 years, median=11 years). All but four respondents (all respirologists) agreed with the clinical practice guideline upon which the decision aid was based.
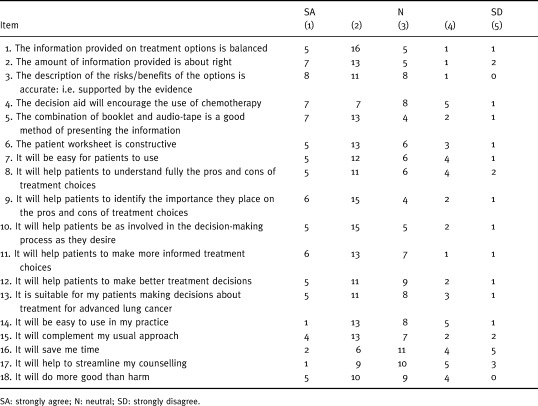
Physicians’ responses to the questions about the acceptability of the decision aid are presented in Table 5. At least two‐thirds thought that the information was sufficient, balanced and accurate. At least two‐thirds liked the delivery method and thought that the decision aid would help the patients to consider their values, to participate in decision‐making as they desired, and to make a more informed treatment choice. The majority thought that the aid would be easy to use, would help patients to make better decisions, would do more good than harm, and would be easy to use and complement their usual approach. Less than half thought that it would save time or streamline their counselling. Half thought that it would encourage the use of chemotherapy.
Table 5.
Physicians’ perceptions of the decision aid (n = 29)
Of the 29 physicians who reviewed the decision aid, 11 stated that they would be very comfortable and eight stated that they would be comfortable providing it to their patients. Six were neutral and four said that they would be uncomfortable (n=3) or very uncomfortable (n=1) providing it to patients. Of 28 responding to a question about future use, eight said that they were very likely, seven were likely, six were somewhat likely, five were unlikely and two were very unlikely to use the decision aid with patients.
Discussion
The decision aid was effective in preparing patients for decision‐making. It was acceptable to patients, improved their knowledge of alternatives, benefits and risks, and reduced decisional conflict about what to choose. Two‐thirds of thoracic surgeons and respirologists who reviewed the decision aid were comfortable with providing it to their patients, and over half indicated that they were likely or very likely to give it to future patients.
Although the results are promising, there are several study limitations that need to be acknowledged. In the patient study, the lack of a randomized controlled design means that the effects of maturation, co‐intervention and testing cannot be ruled out. Decisional conflict may have naturally declined even if patients were not exposed to a decision aid. Uncertainty may have declined once the person had time to consider the choices and discuss the decision with family. Patients may also have improved their comprehension using other sources of information or with subsequent usual care counselling. The repeated use of the knowledge test may also have contributed to improvements. Future evaluations should include a randomized design with usual care controls to rule out these potential confounders.
There are also limitations to the generalization of the study. The sample size was small. Patients may have been biased toward accepting chemotherapy by virtue of being referred to the regional centre for treatment. Future evaluations should be made in thoracic surgery or respirology clinics after patients have received their initial diagnosis, but before they are seen at a cancer centre. Participants may have been influenced by the researcher, who also developed the tool, and these effects may not be replicated if the decision aid is used in another situation.
Despite these limitations, the improvement in knowledge after using the decision aid is consistent with other randomized controlled trials of decision aids. 9 What is particularly noteworthy in this study is the excellent comprehension in such a symptomatic, anxious group, many of whom had limited education. Indeed, knowledge scores of around 75% were usually obtained after using the decision aid. In this study, patients’ scores were that high at baseline, presumably as a result of the explanations given by the oncologists before patient recruitment. What is more impressive is that patients were able to improve their comprehension to 90% after using a decision aid. This result supports other studies showing that reinforcement of verbal information with written material that the patient can review at home may enhance the patient’s comprehension, even if it is high after verbal information has been given. 10 , 11
One of the issues about which patients were not as clear after talking to their oncologists was their perceptions of the probability of benefits with chemotherapy: indeed, only seven out of 20 patients had realistic perceptions. All but three patients’ perceptions were more realistic after using the decision aid, and it is unlikely that they could have obtained appropriate probabilistic information from other sources. The improvement in perceptions of the chances of outcomes is similar to that of other studies of decision aids, and represents one of the clear benefits of decision aids over more general educational material. 9
Decisional conflict also improved as hypothesized. Overall certainty about what to do improved, as well as modifiable factors contributing to certainty, such as feeling informed and clear about values, and having enough advice. This result is supported by other studies. 9 The improvements in feeling informed are also verified by the improvements in the objective tests of comprehension. It is noteworthy that patients also felt clearer about their values, as this is one of the key reasons for using a decision aid. Information about outcomes may help patients to clarify their values. Moreover, the weigh‐scale exercise, in which patients actively consider the personal importance of benefits and risks, also helps to clarify and communicate values.
Generally, the decision aid was acceptable to the participants. However, the fact that seven participants initially enrolled in the study and then subsequently decided not to use the decision aid suggests that the decision aid is not for everyone facing the decision about chemotherapy for stage IV non‐small cell lung cancer. There are patients who do not want to spend the time using it, are too sick to use it, or feel that they are already quite certain about their decision and so do not need to use it. None the less, all patients do require some form of support. Health care providers have a great role to play in supporting patients and their family members as they make difficult treatment decisions.
To our knowledge, the practitioner survey represents the first time that practitioners who were not purposefully selected have been asked to evaluate a decision aid. Although the majority of physicians responded positively regarding its quality, acceptability and usefulness, the results must interpreted cautiously because of the low response rate and possible selection bias in responders. The physicians were required to spend up to 35 min reviewing the decision aid (this is how long it takes to listen to the audio‐tape) and another 10–15 min completing the questionnaire. Another potential limitation relates to their expressed intention to use the decision aid with patients, which may not translate into actual future use. Further evaluations are warranted, with better incentives for completion and follow‐up of actual use of the aids.
In conclusion, this study has been an important first step in the evaluation of an intervention supporting patients deciding about chemotherapy for stage IV non‐small cell lung cancer. Specifically, the study has shown that the intervention is acceptable to patients and interested physicians, and may improve patient knowledge and reduce decisional conflict. Subsequent evaluations with usual care controls are warranted to confirm if the results can be generalized with larger groups of patients and practitioners.
Footnotes
This project was supported by The Ontario Thoracic Society and the Medical Research Council.
References
- 1. Fry WA, Menck HR, Winchester DP. The national cancer data base report on lung cancer. Cancer, 1996; 77 (9): 1947 1955. [DOI] [PubMed] [Google Scholar]
- 2. Nesbitt JC, Lees JS, Komaki R, Roth JA. Cancer of the lung. In: Holland JF, Bast RC, Morton DL, Frei E, Kufe DW, Weichselbaum RR (eds) Cancer Medicine (4th edn). Philadelphia: Williams and Walkins, 1997: 1723–1803.
- 3. Lopez PG, Stewart DJ, Newman TE, Evans WK, The Provincial Lung Disease Site Group. Chemotherapy in stage IV (metastatic) non‐small cell lung cancer. Cancer Prevention and Control, 1997; 1 (1): 18 27. [PubMed] [Google Scholar]
- 4. O’Connor A, Tugwell P, Wells GA et al A decision aid for women considering hormone replacement therapy after menopause: Decision support framework and evaluation. Patient Education and Counselling, 1998; 33 (3): 267 279. [DOI] [PubMed] [Google Scholar]
- 5. O’Connor AM, Tugwell P, Wells G. Testing a portable, self‐administered, decision aid for post‐menopausal women considering long‐term hormone replacement therapy to prevent osteoporosis and heart disease. Medical Decision Making, 1994; 14 (4): 438 438 (Abstract). [Google Scholar]
- 6. O’Connor AM. Validation of a decisional conflict scale. Medical Decision Making, 1995; 15 (1): 25 30. [DOI] [PubMed] [Google Scholar]
- 7. Barry MJ, Fowler FJ Jr, Mulley AG Jr, Henderson JV Jr, Wennberg JE. Patient reactions to a program to facilitate patient participation in treatment decisions for benign prostatic hyperplasia. Medical Care, 1995; 33 : 771 782. [DOI] [PubMed] [Google Scholar]
- 8. Cohen J. Statistical power analysis for the behavioral sciences Toronto: Academic Press, 1977.
- 9. O’Connor AM, Fiset V, DeGrasse C et al Decision aids for patients considering health care options: Evidence of efficacy and policy implications. Journal of the National Cancer Institute Monographs, 1999; 25 : 67 30. [DOI] [PubMed] [Google Scholar]
- 10. Ley P, Bradshaw P, Eaves D, Walker C. A method for increasing patients’ recall of information presented by doctors. Psychological Medicine, 1973; 3 (2): 217 220. [DOI] [PubMed] [Google Scholar]
- 11. Morrow G, Gootnick J, Schmale A. A simple technique for increasing cancer patients’ knowledge of informed consent to treatment. Cancer, 1978; 42 : 793 799. [DOI] [PubMed] [Google Scholar]


