Abstract
Aging is associated with reduced abilities to selectively allocate attention across multiple domains. This may be particularly problematic during everyday multitasking situations when cognitively demanding tasks are performed while walking. Due to previous limitations in neuroimaging technology, much remains unknown about the cortical mechanisms underlying resource allocation during locomotion. Here, we utilized an EEG-based Mobile Brain/Body Imaging (MoBI) technique that integrates high-density event-related potential (ERP) recordings with simultaneously acquired foot-force sensor data to monitor gait patterns and brain activity concurrently. To assess effects of motor load on cognition we evaluated young (N=17; mean age=27.2) and older adults (N=16; mean age=63.9) and compared behavioral and ERP measures associated with performing a Go/No-Go response inhibition task as participants sat stationary or walked on a treadmill. Stride time and variability were also measured during task performance and compared to stride parameters obtained without task performance, thereby assessing effects of cognitive load on gait. Results showed that older, but not young adults’ accuracy dropped significantly when performing the inhibitory task while walking. Young adults revealed ERP modulations at relatively early (N2 amplitude reduction) and later (earlier P3 latency) stages within the processing stream as motor load increased while walking. In contrast, older adults’ ERP modulations were limited to later processing stages (increased P3 amplitude) of the inhibitory network. The relative delay and attenuation of ERP modulations accompanied by behavioral costs in older participants might indicate an age-associated loss in flexible resource allocation across multiple tasks. Better understanding of the neural underpinnings of these age-related changes may lead to improved strategies to reduce fall risk and enhance mobility in aging.
Keywords: cognitive aging, dual-task design, event-related potentials, gait, inhibitory control, EEG, Mobile Brain/Body Imaging
Graphical abstract
INTRODUCTION
Walking, traditionally assumed to be a largely automatic motor function regulated primarily by subcortical processes, is now considered a behavior with significant cognitive involvement (Hausdorff, Yogev, Springer, Simon, & Giladi, 2005; Woollacott & Shumway-Cook, 2002). Walking in the real world depends on the ability to effectively pursue internally generated goals and negotiate competing demands from the environment while simultaneously maintaining gait stability. When individuals are engaged in an attention-demanding task while walking (e.g., talking or texting) cortical resources required for safe locomotion can become overburdened, resulting in deficits to either the cognitive task, gait stability, or both. The issue of limited resources is of particular concern in the elderly population since cognitive impairment has been linked to reduced mobility and increased risk of falling (Beurskens & Bock, 2012; Mirelman et al., 2012).
The relationship between cognition, gait and aging has been explored in the laboratory by way of dual-task walking paradigms (Woollacott & Shumway-Cook, 2002; Yogev-Seligmann, Hausdorff, & Giladi, 2008). Resulting behavioral costs to either the cognitive or motor task have been cited as evidence of cognitive-motor interference (CMI), suggesting at least a partial reliance upon common cortical processes (Holtzer, Verghese, Xue, & Lipton, 2006; Killane et al., 2014), albeit indirectly. Yet, the neurophysiological mechanisms associated with dual-task walking remain largely unexplored. Only recently have technological advancements (Gramann, Ferris, Gwin, & Makeig, 2014; Reis, Hebenstreit, Gabsteiger, von Tscharner, & Lochmann, 2014) enabled the acquisition of high-density electro-cortical activity during locomotion, termed Mobile Brain/Body Imaging (MoBI) (Gramann et al., 2011; Gramann, Jung, Ferris, Lin, & Makeig, 2014; Makeig, Gramann, Jung, Sejnowski, & Poizner, 2009). Previously, we employed this method with a group of young adults and found that although our participants demonstrated a lack of behavioral costs while performing an inhibitory control task when walking (dual-task load) compared to sitting (single-task load), they exhibited substantial task load modulations in the electrophysiological components associated with inhibitory network activity (De Sanctis, Butler, Malcolm, & Foxe, 2014). Thus, MoBI offers significant potential in attempting to characterize the neural correlates of dual-task walking. Furthermore, the deployment of this technique in an older population will allow for the assessment of age-related differences in the cortical underpinnings of CMI. The classification of such age-linked modulations may serve to identify possible biomarkers of increased fall risk (Verghese et al., 2014; Verghese, Wang, Lipton, & Holtzer, 2013).
Normal aging is associated with functional declines in gait cycle stability, including reduced speed and stride length, and an increased double support phase – i.e. longer periods where both feet are in contact with the ground (Winter, Patla, Frank, & Walt, 1990). Previous studies have also observed increased variability across several spatiotemporal gait parameters including swing and double-support times, step length and width (Callisaya, Blizzard, Schmidt, McGinley, & Srikanth, 2010; Hausdorff, 2007). However, these impairments may be further exacerbated under dual-task load (Hausdorff et al., 2005; Lindenberger, Marsiske, & Baltes, 2000; Plummer-D’Amato, Altmann, & Reilly, 2011), indicating an increased susceptibility to CMI with aging (Beurskens & Bock, 2012). It should be noted though that whether or not older adults exhibit additional motor costs, or any costs at all in comparison to young adults, may largely depend upon the relative difficulty of current task demands (Bock, 2008; Springer et al., 2006).
In the cognitive domain, age-related declines have been well-documented for executive function (EF) processes, particularly involving the ability to selectively attend to relevant information and monitor responses (Kramer, Humphrey, Larish, Logan, & Strayer, 1994; Prakash et al., 2009; Royall, Palmer, Chiodo, & Polk, 2005). Consequently, common findings from dual-task walking paradigms have shown older adults to exhibit greater performance deficits on secondary tasks recruiting EF compared to young adults, while walking compared to sitting (Al-Yahya et al., 2011; Lovden, Schaefer, Pohlmeyer, & Lindenberger, 2008; Srygley, Mirelman, Herman, Giladi, & Hausdorff, 2009). Secondary tasks requiring other cognitive processes such as memory, verbal IQ or visuospatial skills appear to be less susceptible to motor interference (Hausdorff et al., 2005; Herman, Mirelman, Giladi, Schweiger, & Hausdorff, 2010; Holtzer et al., 2006), although see (Theill, Martin, Schumacher, Bridenbaugh, & Kressig, 2011).
While the above-mentioned studies provide indirect evidence of shared cognitive-motor resources and CMI, it has only recently become possible to directly assess cortical involvement in walking. Experiments employing functional near-infrared spectroscopy (fNIRS) and electrophysiological measures have been conducted with (Beurskens, Helmich, Rein, & Bock, 2014; Doi et al., 2012; Holtzer et al., 2011; Uehara, Higashi, Tanabe, & Sugawara, 2011) and without (Gramann et al., 2011; Gwin, Gramann, Makeig, & Ferris, 2011; Harada, Miyai, Suzuki, & Kubota, 2009; Kurz, Wilson, & Arpin, 2012; Miyai et al., 2001; Suzuki, Miyai, Ono, & Kubota, 2008) engagement in a secondary task. Results from fNIRS studies have shown reduced oxygenation levels in older adults over prefrontal cortex while walking alone and under increased load (while talking) (Holtzer et al., 2011). Similar fNIRS findings were described by Beurskens et al. (2014), who revealed decreased prefrontal activation associated with dual-task load in old but not in young participants (Beurskens et al., 2014).
However, in contrast to hemodynamic measures, electroencephalography (EEG) affords considerably more precise temporal resolution in order to evaluate neurophysiological mechanisms of cortical involvement during dual-task walking (Makeig et al., 2009). Our group (De Sanctis, Butler, Green, Snyder, & Foxe, 2012; De Sanctis et al., 2014; Nolan et al., 2012; Nolan, Whelan, Reilly, Bulthoff, & Butler, 2009) and others (Castermans & Duvinage, 2013; Castermans, Duvinage, Cheron, & Dutoit, 2014; De Vos, Gandras, & Debener, 2014; Debener, Minow, Emkes, Gandras, & de Vos, 2012; Duvinage et al., 2012; Gramann, Ferris, et al., 2014; Gramann, Gwin, Bigdely-Shamlo, Ferris, & Makeig, 2010; Hoellinger et al., 2013; Reis et al., 2014) have shown that it is entirely feasible to record robust event-related potentials (ERPs) from a cognitive task while participants are in motion, without a significant difference in signal-to-noise ratio compared to stationary conditions. The MoBI approach integrates high-density electro-cortical activity with simultaneously acquired body tracking data to investigate brain activity and gait pattern as participants walk on a treadmill while also performing a cognitive task. For our previous study, we employed the MoBI system in young adult participants and assessed the neural correlates of an attentionally-demanding visual Go/No-Go task under different motor load conditions, ranging from sitting (single-task) to walking (both deliberately and briskly) (De Sanctis et al., 2014). Temporal parameters of the gait cycle were recorded from foot force sensors to assess the effect of increased cognitive load on stride time and stride time variability. We found that participants took longer strides under dual-task load, a result that has been reported previously and interpreted as an adaptation to lessen inter-task interference (Li, Abbud, Fraser, & Demont, 2012; Lovden et al., 2008). Furthermore, the young adults exhibited no dual-task behavioral costs performing the Go/No-Go task while walking compared to sitting (i.e., no differences in reaction time or accuracy). However, under increased task load, we observed a substantial reduction in the amplitude of the N2 component, a negative-going ERP component time-locked to the No-Go-stimulus presentation, representing automatic inhibitory (Eimer, 1993; O’Connell, Dockree, Bellgrove, et al., 2009) and conflict detection processes (Dockree, Kelly, Robertson, Reilly, & Foxe, 2005; Morie et al., 2014). Additionally, we reported that the P3, a later positivity also evoked by successful response inhibition, occurred earlier and exhibited a more frontal distribution when participants changed from single-task to dual-task performance mode. We have interpreted the reduction of the N2 and earlier initiation of the P3 as an adaptive processing strategy, permitting the redeployment of motor-cognitive processes to optimize performance under increased task load.
To our knowledge, previous MoBI studies have so far only been conducted in young adult populations. Therefore, the objective of the present study was to investigate age-related differences in the recruitment of cortical mechanisms underlying CMI during a dual-task walking scenario. We utilized the same Go/No-Go task to measure inhibitory response control (De Sanctis et al., 2014) and recorded high-density EEG while young and old participants walked on a treadmill. Foot force sensors were again employed to evaluate age and task load effects on temporal indices of the gait cycle. Based on previous work demonstrating increased susceptibility to CMI, we predicted that older adults would exhibit a less flexible performance strategy during dual-task load compared to younger adults. Specifically, we hypothesized that older participants would show increased behavioral costs in both cognitive and motor domains while dual-tasking, i.e., slower responses, decreased inhibitory response control and increased stride-to-stride variability. Moreover, we speculated that along with these behavioral costs, older participants would show a differential pattern of inhibitory network activity associated with the Go/No-Go task, reflecting a diminished flexibility in the re-deployment of processing resources as task load increased.
METHODS
Participants
18 young adults and 18 older adults were recruited from the community and from the lab’s existing database. All volunteers underwent an initial phone screening to assess general health and mobility. Study inclusion was limited to individuals with normal or corrected-to-normal vision, free from any neurological or psychiatric deficits or disorders likely to affect gait (e.g., vestibular, orthopedic or neurological diseases) and able to walk comfortably on a treadmill for approximately one hour of total recording time. In addition, older individuals were screened with the Mini-Mental State Examination (MMSE) using a cutoff score of 24 to exclude participants with signs of cognitive impairment (Folstein, Folstein, & McHugh, 1975). Data from one older participant were excluded due to the presence of walking-related artifacts. Additionally, we chose to exclude the data from two other participants (one young adult and one older adult) because their behavioral performance was more than two standard deviations from their respective group means. Thus, results reported here consist of data from 17 young adults (8 female) and 16 older adults (9 female). The age range was 21.8 to 36.1 years for the young group (mean = 27.2; SD = 4.6) and 57.7 to 71.0 years for the old group (mean = 63.9; SD = 4.0). The Institutional Review Board of the Albert Einstein College of Medicine approved the experimental procedures and all participants provided their written informed consent. Participants were modestly compensated at a rate of $12 per hour. All procedures were compliant with the principles laid out in the Declaration of Helsinki for the responsible conduct of research.
Stimuli and task
Participants performed a speeded visual Go/No-Go paradigm consisting of 168 images from the International Affective Picture System (IAPS), a database of photographs with normative ratings of emotional status (Lang, Bradley, & Cuthbert, 2008). Only those photographs that were classified as affectively neutral or positive were included. Images were presented centrally for 600ms with a random stimulus-onset-asynchrony (SOA) ranging from 800 to 1000ms. Stimuli were presented using Presentation software version 14.4 (Neurobehavioral Systems, Albany, CA, USA) and projected (InFocus XS1 DLP, 1024 × 768 pxl) onto a black wall. On average, images subtended 28° horizontally by 28° vertically. Participants were instructed to quickly and accurately perform the response inhibition task by clicking a wireless computer mouse button in response to the presentation of each image, while withholding button presses to the second instance of any picture repeated twice in a row. The probability of Go and No-Go trials was 0.85 and 0.15, respectively. The task was presented in blocks, each lasting approximately 4 minutes. All participants took part in a practice block before undertaking the main experiment. Walking blocks were performed on a treadmill (LifeFitness TR-9000) positioned approximately 1.5m from the wall onto which stimulus images were displayed. No specific task prioritization instructions (i.e., walking versus cognitive task) were given. To guard against falls, a custom-designed safety harness was worn while walking and all participants rested for a minimum of two minutes between blocks to prevent fatigue.
Our previous study was designed to assess the effects of progressively increased walking demands on cognitive performance (De Sanctis et al., 2014). Consequently, young adult participants performed the walking blocks (including dual-task and walking only blocks) at two different speeds (2.4 km/h and 5 km/h). However, the majority of age-related dual-task investigations have utilized individual self-selected walking speeds during both over-ground (Lindenberger et al., 2000; Springer et al., 2006) and treadmill walking (Li et al., 2012; Lovden et al., 2008). Therefore, to be consistent with previous literature and to provide a relatively demanding walking task for each group, the older adult participants chose a comfortable walking speed at the beginning of the experimental session and maintained this preferred speed for its duration. Average walking speed for this group was 3.5 km/h (range: 2.4 to 4.8 km/h). For the current investigation, we chose to compare the older adults’ walking performance with that of the young adults walking at 5 km/h. This decision was based on findings from several large-scale field studies (n > 3000) indicating that 5 km/h is a close approximation to the average walking speed (5.3 km/h) of young adults (Knoblauch RL, 1996; Silva, da Cunha, & da Silva, 2014). Consequently, the complete experimental protocol involved several different task conditions presented to participants in a pseudorandom order. Each older adult performed five blocks of the response inhibition task while sitting, 9 or 10 blocks while walking and an additional two blocks only walking (i.e., without performing the task). Young participants completed either three or four blocks sitting, a minimum of four blocks walking slowly (range: 4 – 8 blocks), at least four blocks walking quickly (range: 4 – 8 blocks) and two blocks of each speed walking without the task.
Gait cycle recording and analysis
Foot force sensors recorded temporal parameters of the gait cycle while participants walked on the treadmill during either uninterrupted walking or while concurrently engaged in the Go/No-Go task. Three sensors (Tekscan FlexiForce A201 transducers) were positioned on the sole of each foot: at the center of the back of the heel, the big toe ball and midway along the outer longitudinal arch. These positions enabled the detection of changes in plantar pressure during various stance phases including initial contact, loading response, mid-stance, terminal stance and pre-swing. Force signals were sampled at 512 Hz using an Analog Input Box (BioSemi) connected and integrated via optical fiber with the Biosemi ActiveTwo EEG system. Continuous data were butterworth low-pass filtered at 7Hz, epoched into 10 sec intervals, and normalized against the standard deviation. To assess stride time we measured peak-to-peak intervals using the force signal derived from a heel sensor (e.g., time of a complete gait cycle is heel contact to next heel contact of that same foot). Automatic peak detection software (MATLAB custom scripts) with one standard deviation as threshold was used to determine if each peak was significantly larger than the data around it. Peak-to-peak intervals were included for further analysis only if the duration to complete a cycle was > 500ms and < 1500ms. Foot sensors were not recorded from one young participant; therefore the effects of age and cognitive load on the gait cycle are reported from 16 young and 16 older participants.
Event related potential recording and analysis
Scalp recordings were conducted with a 72-channel EEG system (BioSemi ActiveTwo, Amsterdam, The Netherlands), digitized at 512 Hz and bandpass filtered from 0.05 to 100 Hz (24 dB/octave). Offline, data were processed using custom MATLAB scripts (MathWorks, Natick, MA), EEGLAB (Delorme & Makeig, 2004) and the FieldTrip toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011). EEG was bandpass filtered from 1 to 30 Hz to remove low frequency drift and high frequency noise. An artifact rejection criterion of ±75 µV was applied to all electrode sites to reject trials with excessive eye movements, EMG or other noise. Trials with more than 6 bad channels were excluded from further analysis. Electrode data were interpolated using a nearest neighbor spline correction for trials in which there were 6 or fewer bad channels (Perrin, Pernier, Bertrand, Giard, & Echallier, 1987). Data were then re-referenced to an average reference. Epochs time-locked to stimulus presentation with a 800ms post-stimulus period and a 50ms pre-stimulus baseline were computed for Go trials during which the participant successfully responded (Hit trials) and No-Go trials during which the participant successfully withheld a response (Correct Rejection trials [CRs]). Incorrect trials were excluded from the analysis. The average number of accepted trials for young participants was 485 (Go) and 71 (No-Go) while sitting and 749 (Go) and 103 (No-Go) during walking. The average number accepted for the older group was 748 (Go) and 103 (No-Go) while sitting and 1,326 (Go) and 180 (No-Go) during walking. A comprehensive description and analysis of rejection rates across conditions is provided in the Supplementary Materials section for the interested reader, since the performance of these systems during mobility sessions will be of interest to those researchers considering the use of MoBI technology. Please see the Supplementary Materials document and Supplementary Figures 1 and 2, for information on trial acceptance rates as well as the number and location of interpolated electrode channels, respectively.
Signal-to-noise statistics
To assess the signal-to-noise ratio (SNR) for each group for both experimental conditions (sitting vs. walking), we computed the global field power (GFP) for hits and CR trials. The background noise was estimated from the pre-stimulus period (-100 to −50ms) and the signal was estimated from the first post-stimulus positive peak (150 to 210ms). The squared signal was divided by the squared noise and converted to decibels in order to be scale-invariant. The resulting SNRs were subjected to a 2 (Condition: sitting, walking) x 2 (Trial type: hits, CRs) x 2 (Group: young, old) mixed repeated measures ANOVA. The reason for using a relatively narrow early time window is based on the assumption that early evoked potentials (150 to 210ms, e.g., N1) are to a lesser degree modulated by endogenous higher-order cognitive processes compared to later ones (e.g., N2/P3). ERP modulations driven by endogenous processes such as the reallocation of cognitive resources under increased task load could mimic differences in SNR. This would raise the ambiguity and hinder the interpretation of SNR as a measure to compare EEG signal quality between the sitting and walking conditions. In addition, to test for muscle and eye movement-related contamination of the broadband evoked response, artifacts most prominent in frequencies of 8Hz or higher, we performed a Fast Fourier Transform on the epoched Go trials for each participant and computed the correlation coefficient matrix between conditions (Nolan et al., 2009).
N2/P3 amplitude and latency
The N2 and P3 ERP components associated with successful response inhibition in a Go/No-Go paradigm have been well characterized in previous studies (Bokura, Yamaguchi, & Kobayashi, 2001; Donkers & van Boxtel, 2004; Eimer, 1993; Falkenstein, Hoormann, & Hohnsbein, 2002; Garavan, Ross, Murphy, Roche, & Stein, 2002; Katz et al., 2010; Morie et al., 2014) and have been shown to produce maximal amplitudes over fronto-central scalp sites. Thus, the three midline sites of FCz, Cz and CPz were chosen to represent the task-evoked N2/P3 components. For each age group and task load condition, we used the average peak amplitude across the three electrode sites of interest to encapsulate a 100ms time window for the N2 and a 200ms time window for the P3 (see Table 1), which were then used to compute mean amplitude and detect peak latency across the respective time periods. ERP amplitude may either be quantified by the mean amplitude across the corresponding time period of interest, or by the amplitude of the highest peak. In this case we chose to use the former, as this method is better able to provide a more comprehensive account of the componentry across the entire time window (Luck, 2004). The latency on Hit\CR trials and difference waves was quantified using an automatic peak-picking procedure (MATLAB custom scripts) which identifies the maximal deflection within the given time window. A peak was identified such that an ascending and descending difference of 0.2 µV had to be reached between consecutive sample points. Results were verified by manual visual inspection. Walking and age-related differences in N2 and P3 mean amplitude and peak latency for Hit, CRs and difference waves (CRs minus Hits) were statistically assessed by three-way repeated measures ANOVA with factors of Group (young, old), Task Load (cognitive task performed while sitting vs. walking) and Electrode site (FCz, Cz, CPz). Greenhouse– Geisser corrections were applied when appropriate.
Table 1.
Time windows used for the statistical analysis of N2 and P3 component latency and amplitude on Correction Rejection (CR) trials, Hit trials and difference waves (CRs minus Hits), computed separately for each age group and task-load condition.
| Young | Old | ||||
|---|---|---|---|---|---|
| N2 Window (ms) | P3 Window (ms) | N2 Window (ms) | P3 Window (ms) | ||
| CRs | Sitting | 221–321 | 371–571 | 250–350 | 394–594 |
| Walking | 213–313 | 300–500 | 249–349 | 391–591 | |
| Hits | Sitting | 207–307 | 371–5712 | 256–356 | 394–594 |
| Walking | 206–306 | 300–500 | 265–365 | 391–591 | |
|
Difference Waves |
Sitting | 238–338 | 347–547 | 253–353 | 363–563 |
| Walking | 221–321 | 315–515 | 233–333 | 377–577 | |
For both age groups, ERPs for Hit trials produced no apparent P3 component (as P3 is often associated with rare events) thus we used the time window encompassing the P3 for CRs to compute the amplitude and latency statistics for Hits.
Topographical voltage maps
Topographic maps display interpolated voltage distributions, derived from 64 scalp electrode measurements. These interpolated potential maps are displayed on a 2D reconstruction of a rendered scalp surface as implemented in FieldTrip analysis software (Oostenveld et al., 2011). Maps were computed over the time periods of 240–340ms and 400–550ms for the N2 and P3 components, respectively, in order to convey maximal ERP differences between task-load conditions for each age group.
RESULTS
Behavioral results
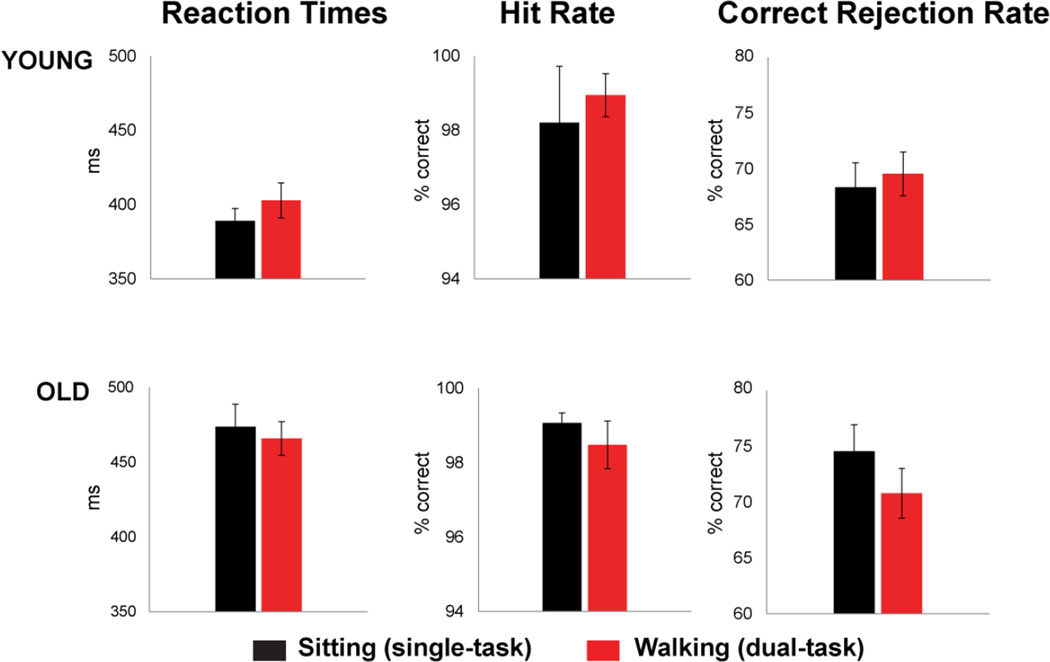
Figure 1 shows mean reaction times (RTs) and accuracy rates on Go trials (Hits) in addition to rates of correct rejections (CRs) on No-Go trials, during both sitting (black) and walking (red) conditions. Two-way repeated measures analyses of variance (ANOVA) were used to statistically assess each dependent measure with within-subjects factor of Task Load (cognitive task performed while sitting vs. walking) and between-subjects factor of Group (young vs. old). RTs yielded a main effect of Group (F1,31 = 22.50, p < .001) indicating that older participants (RTsitting = 474ms; RTwalking = 466ms) were overall slower than young participants (RTsitting = 389ms; RTwalking = 403ms) to respond to image presentation. Hit rates did not differ between groups (p = .86) nor as a function of Task Load (p = .89), demonstrating that both age groups achieved highly accurate response performance (mean hit rates above 98%) to Go trials for both sitting and walking conditions.
Figure 1.
Behavioral performance on the Go/No-Go task for young (top row) and older participants (bottom row). Reaction times on Go trials, percentage of correct responses on Go trials (hit rate) and percentage of correct rejections (CR rate) on No-Go trials are shown in black for sitting (single-task load) and red for walking (dual-task load) conditions.
The ANOVA assessing age and task load-related effects on the rate of correct rejections (CRs) revealed no main effects of Group (p = .21) or Task Load (p = .24) however there was a significant interaction between these factors (F1,31 = 5.33, p < .05). A within-group post-hoc comparison revealed that this interaction was driven by the fact that the older adults showed a drop in their CR rate of about 4% while walking (mean = 70.78%; SD = 8.93) compared to sitting (mean = 74.56%; SD = 9.48), indicating a trend (p = .065) towards increased dual-task costs for only the older group, while young adults demonstrated comparable CR performance between sitting (mean = 68.34%; SD = 9.12) and walking (mean = 69.55%; SD = 8.08). To summarize, young adults performed the cognitive task equally well under single and dual-task load. In contrast, older adults exhibited a general slowing in their response times in addition to a dual-task cost, performing the cognitive task less accurately while walking compared to sitting. Interestingly, older adults performed more accurately overall, but this age-related difference was not found to be significant (p = .21). Note: In order to account for both response speed and accuracy in one measure, we performed a supplementary analysis of the behavioral results in terms of inverse efficiency (IE), computed as RTs divided by the proportion of correct responses (Townsend & Ashby, 1983). IE was calculated for two separate indices of task performance - the proportion of CRs and d’, a measure of response sensitivity (Green & Swets, 1966). For IE based on the proportion of CRs there was a main effect of Group (F 1, 31 = 5.61, p = .024) which may be attributed to the much slowed RTs of the older participants, while no significant effects resulted from the IE calculated from d’ (see Supplementary Figure 3).
Gait cycle results
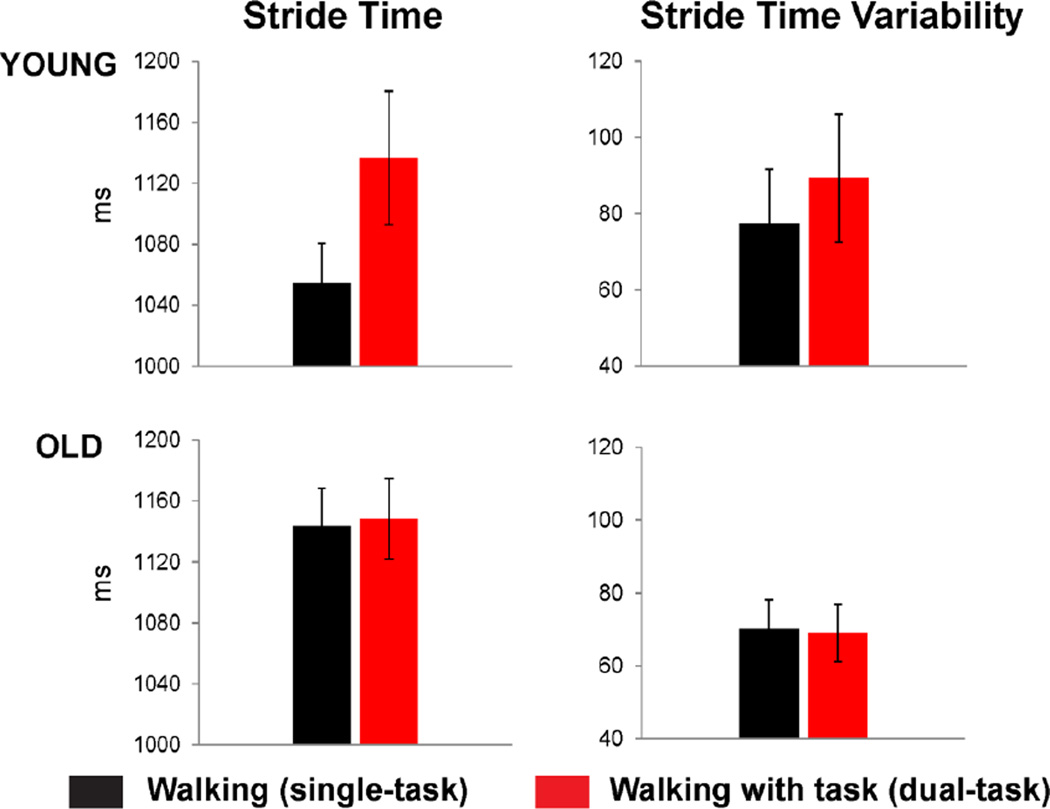
Figure 2 presents the effects of task load on mean stride time and stride time variability for the young adult group (top row) and old adult group (bottom row). The walking-only condition (single-task load) is shown in black and the dual-task walking condition is shown in red.
Figure 2.
Average stride time (left column) and stride time variability (right column) is displayed in milliseconds for young (top row) and older (bottom row) participants, for walking-only blocks (black) and walking while performing the cognitive task (red).
A two-way repeated measures ANOVA with between-subjects factor of Group (young vs. old) and within-subjects factor of Task Load (walking with vs. without cognitive task) revealed a main effect of Task Load (F1,30 = 6.27, p < .02), indicating a relative increase in average stride time under dual-task load. Furthermore, a significant interaction between Task Load and Group (F1,30 = 5.00, p < .05) was found. Post-hoc comparisons revealed that this interaction was driven by a significant dual-task related increase of 82.4ms in average stride duration for the young adult group (t15 = 2.48, p < .05) while the older participants showed a minimal dual-task related increase of less than 5ms (p = .66). There was no main effect of age on average stride time (p = .22). Analysis of stride time variability revealed no significant effects of dual-task load or age. In sum, the young adult group appeared to modify their walking behavior while also performing the inhibitory task by taking longer strides, whereas the older participants maintained an entirely similar walking pattern across task load conditions.
Electrophysiological Results
Feasibility of recording
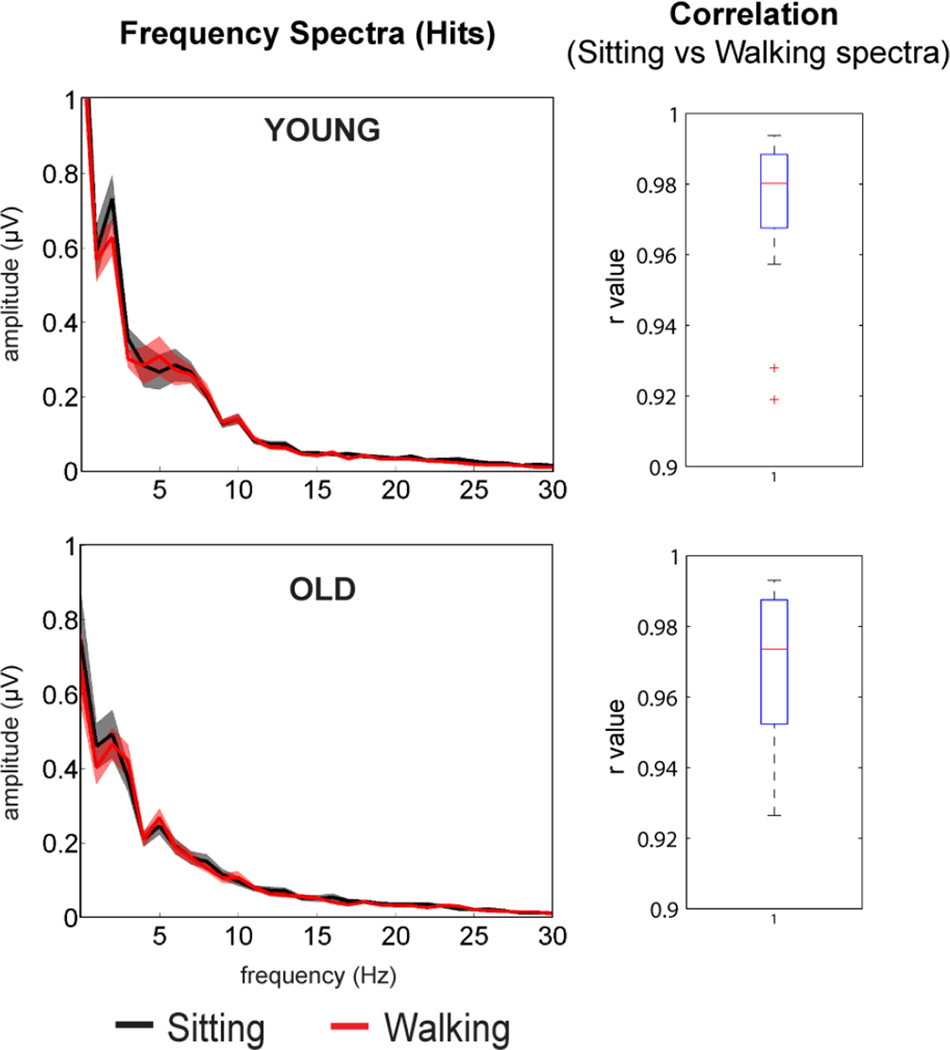
To demonstrate that the signal-to-noise ratio (SNR) of ERPs recorded while participants walked on the treadmill was comparable to ERPs recorded while stationary, we computed the SNR for hits and CR trials for each group for both task load conditions. Three-way repeated measures ANOVA with within-subjects factors of Task Load (sitting vs. walking) and Trial Type (Hits vs. CRs) and between-subjects factor of Group (young vs. old) yielded a main effect of Trial Type (F1,31 = 11.16, p < .005). This effect may be attributed to the difference in probability between Go and No-Go trials. No other effects reached significance. Figure 3 illustrates the grand mean and standard deviation of the frequency spectra of the ERP response on hit trials during the sitting and walking conditions computed using a Fast Fourier Transform. Each group exhibits largely overlapping spectra between conditions, indicating that the ERP frequency spectra for sitting and walking conditions do not significantly differ, and providing further evidence that differences in motor behavior do not compromise the quality of ERP recordings. Correlation coefficient values between conditions for each age group were all found to have an r > 0.95. Finally, to explore the potential for a greater influence of eye movements on ERPs produced during walking blocks, we have included Supplementary Figure 4, showing grand mean ERPs recorded over frontal electrode channel Fp1. Activity closely resembles evoked potentials recorded at FCz, Cz, and CPz with no indication of greater impact of eye movement on the ERP during walking compared to sitting conditions.
Figure 3.
Grand mean and standard deviation (shading) of frequency spectra averaged across hit trials over central scalp regions (left panel). Sitting condition is presented in black, walking red. The right panel depicts box plots of Pearson’s correlation coefficients of the spectra between sitting vs. walking conditions.
ERP results
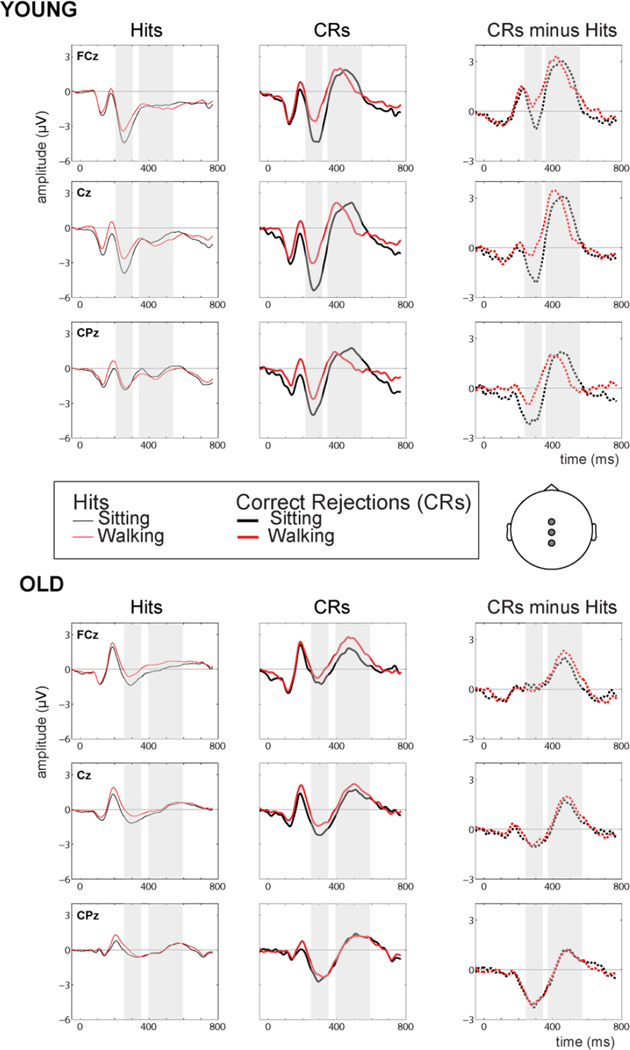
Figure 4 shows the averaged Go/No-Go ERP waveforms plotted over three midline electrode locations (FCz, Cz and CPz) designating hits (thin lines, left column), CRs (thick lines, center column) and difference waves (CRs minus hits, right column). Waveforms are presented for the sitting (black lines) and walking (red lines) conditions, separately for the young (top rows) and old (bottom rows) groups. Highlighted regions represent time periods used for the statistical analysis of N2 and P3 components. As described previously (De Sanctis et al., 2014), young adults showed a robust N2 component (for both CRs and difference waves) over all three electrode sites, with a clear amplitude reduction for dual-task load conditions (walking), compared to performing the inhibitory control task while seated. In contrast, the older group exhibited a substantially reduced N2, particularly over frontal scalp sites, with minimal task-induced amplitude variation for CRs and amplitude of differential activation appearing to be largely independent of task load. Additionally, the young N2 peak showed earlier onset latency compared to the older group (computed within each group’s respective time window) with the greatest difference apparent over the posterior-most recording site. With regards to the later P3 component, a visual inspection of the waveforms confirmed a correspondence with previous results in that, primarily for CRs measured over centro-parietal scalp regions, young adults exhibited clear effects of task load on P3 onset and peak latency. The walking-evoked P3 onset early then quickly declined, peaking approximately 90ms before the more sustained sitting-generated P3. In contrast, the older group showed no modulation in P3 latency as a function of task load, for either CRs or difference waves. However, the older group did exhibit a walking-related enhancement in P3 amplitude, prominent over anterior recording sites. Below, we discuss the results of the statistical evaluation of N2/P3 latencies and amplitudes in detail.
Figure 4.
Grand mean ERPs for young (n=17) and older (n=16) participants for hits (left column) and correct rejections (CRs, middle column) to the Go/No-Go task during sitting (black waveforms) and walking (red waveforms) conditions. Difference waves (CRs minus hits) for sitting and walking conditions are plotted in the right column. ERPs (average reference) are displayed at three midline electrode sites over fronto-central, central and centro-parietal scalp regions.
N2 latency for Hits
A three-way repeated measures ANOVA of within-subjects factors of Task Load (sitting vs. walking) and Electrode site (FCz, Cz, CPz) and between-subjects factor of Group (young vs. old) on mean N2 latency revealed a significant main effect of Group (F1,31 = 44.61, p < .001), indicating an earlier onset of the N2 for the young (253ms) compared to the old (278ms) group. Also, a robust effect of Task Load (F1,31 = 6.75, p < .05), and a Task Load x Group interaction (F1,62 = 5.36, p < .05) was found. The interaction appears to be driven mostly by a delay of N2 peak latency in older adults performing under dual-task load.
N2 amplitude for Hits
The three-way ANOVA evaluating the effect of age group, task load and electrode site showed a main effect of Task Load (F1,31 = 17.30, p < .001) and Group (F1,31 = 8.62, p < .05). The N2 modulation by task load reflects an amplitude reduction under increased task load, while the main effect of group indicates an N2 reduction for older adults.
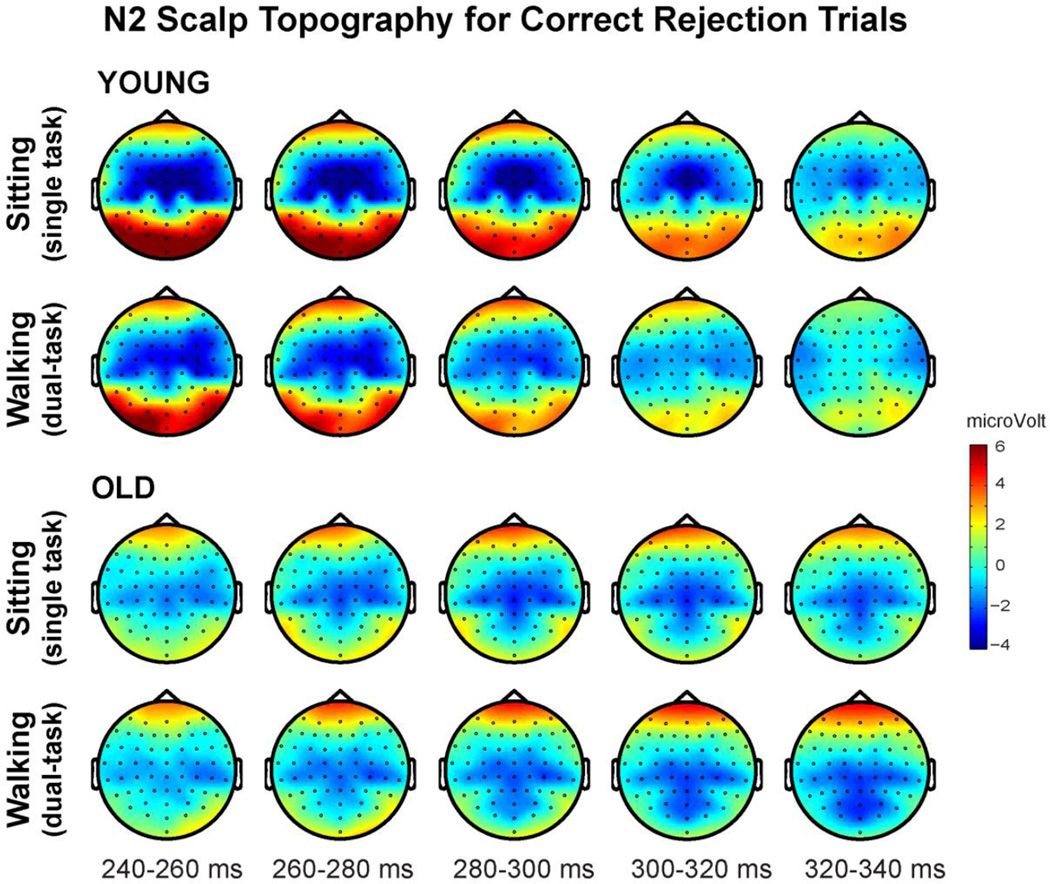
N2 Scalp Topography for Correct Rejections
Group averaged voltage maps for Correct Rejection trials during the N2 time period (240–340ms) are illustrated in Figure 5 for young (top panel) and older participants (bottom panel) as they performed the cognitive task while sitting and while walking. An age-related topographical shift is evident via a fronto-central distribution for young adults while older adults exhibit maximal enhancement over more posterior scalp regions. This shift is supported by our ANOVA findings, revealing a significant Electrode x Group interaction (see below). This effect has been reported previously and considered to reflect age-related decline of frontal-mediated inhibitory processes, which in turn necessitates the recruitment of additional posterior regions (Lucci, Berchicci, Spinelli, Taddei, & Di Russo, 2013; Wascher, Falkenstein, & Wild-Wall, 2011; Willemssen, Falkenstein, Schwarz, Muller, & Beste, 2011). Interestingly, both groups showed largely load-independent topographical distributions. Additionally, the scalp maps clearly illustrate the robust load-dependent N2 amplitude modulation in the young participants.
Figure 5.
The topographical distribution of ERP voltage activity across the scalp for correct response inhibition trials, encompassing the N2 time window (240–340ms) for young adults (top panel) and older adults (bottom panel). Maps are depicted for sitting (single-task) and walking (dual-task) conditions averaged across 20ms time intervals.
N2 latency for Correct Rejections
A Task Load x Electrode x Group ANOVA with within-subjects factors of Task Load (sitting vs. walking) and Electrode (FCz, Cz, CPz) and between-subjects factor of Group (young vs. old) revealed a significant main effect of Group (F1,31 = 15.50, p < .001), indicating an earlier onset of the N2 for the young (253ms) compared to the old (261ms) group. Additionally, an Electrode x Group interaction (F2,62 = 3.77, p < .05) showed that, averaged over task load conditions, the largest difference in peak latency between the groups occurred at electrode CPz.
N2 amplitude for Correct Rejections
The effect of age group, task load and electrode site on mean N2 amplitude revealed significant main effects of Task Load (F1,31 = 28.87, p < .001) and Group (F1,31 = 5.82, p < .05) and a significant Task Load x Group interaction (F1,31 = 7.06, p < .02). The interaction indicates that as task load increased young participants’ N2 response exhibited a prominent reduction over a widespread scalp area, while the N2 in older adults showed a relatively smaller reduction confined to central scalp, with increased task-load. Follow-up t-tests comparing task-load conditions averaged across the three electrode sites confirmed an N2 reduction for the dual-task compared to the single-task condition in young (t16 = 4.62, p < .001) and older adults (t15 = 2.98, p < .005). Furthermore, a significant Electrode x Group interaction (F2,62 = 5.81, p < .005) was found. Follow-up t-tests showed significant age differences between anterior sites (FCz: p = .002, Cz: p = .002), but not over the more posterior channel (CPz: p = .15).
N2 latency for difference waves
The peak latency for N2 difference waves was modulated by Task Load (F1,31 = 19.80, p < .001), indicating an earlier onset of the N2 under high (252ms) compared to low task load (261ms), and by an interaction between Electrode and Group (F2,62 = 6.81, p < .003).
N2 amplitude for Difference Waves
ANOVA showed a main effect of Task Load (F1,31 = 9.63, p < .005) and a significant Task Load x Group interaction (F1,31 = 6.05, p < .05). The interaction appears to be driven mostly by task load differences for only the young group, exhibited by a prominent N2 reduction under increased task load, while the older group displayed minimal modulation across task load conditions. Post-hoc comparisons for data averaged over all three electrode sites confirmed that N2 amplitude was significantly reduced under increased task load in the young (t16 = 3.19, p < .01) but not in the older group (p= .49). Interestingly, the older group demonstrated an almost complete lack of negative-going waveforms during the N2 time window over frontal scalp regions.
P3 latency for Hits
Due to a virtual lack of P3-like activity we refrained from statistically analyzing this time period.
P3 amplitude for Hits
The three-way ANOVA revealed a Task Load x Group interaction (F1,31 = 11.24, p < .005), driven by a somewhat reduced amplitude under high task load in young adults, while the reverse pattern was seen in older adults. Also, a significant main effect of Group (F1,31 = 18.05, p < .001) indicated a relative reduction of P3 in young adults. It should be noted however that hit trials evoked only minimal to no P3-like activation for both age groups.
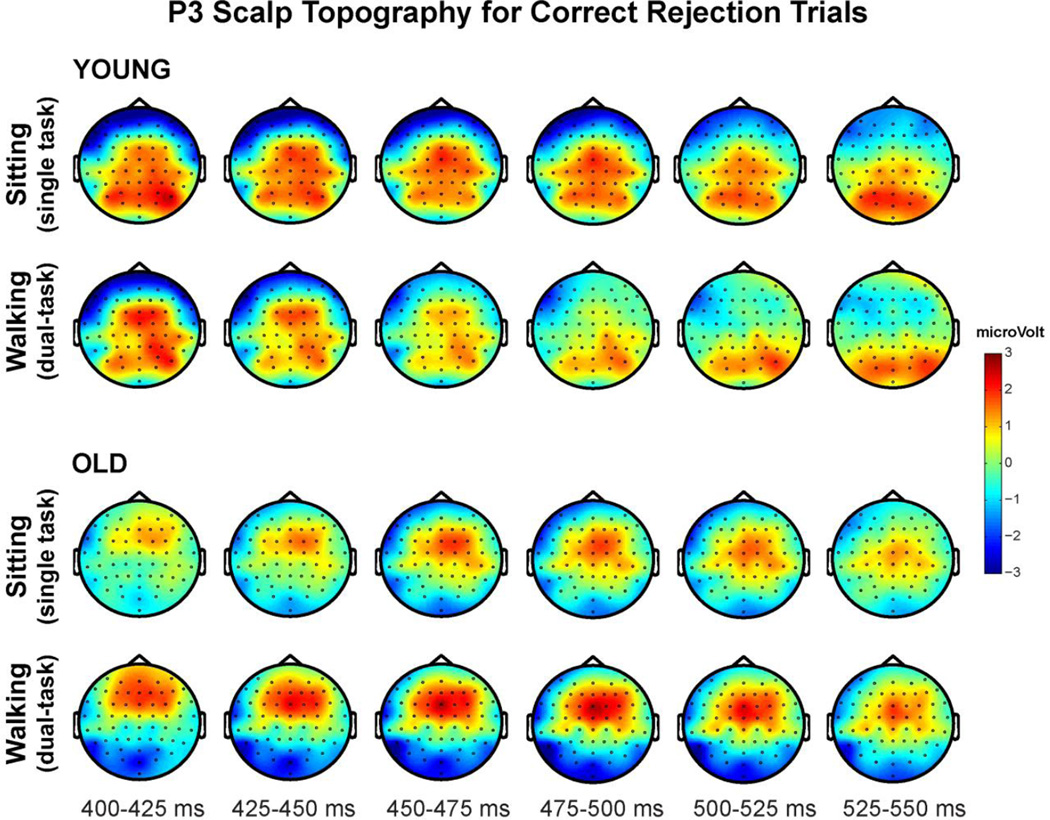
P3 Scalp Topography for Correct Rejections
Figure 6 shows averaged scalp topographies for CR trials during the P3 time period in young (top panel) and older participants (bottom panel) as they performed the task while sitting and walking. The most evident differences appear to be age-related. Young adults reveal a broad distribution of enhanced positivity over centro-parietal scalp while older adults display a more focused distribution over fronto-central regions. This age-related pattern appears to be largely independent of task load with a more frontally-distributed P3 in older adults apparent during both sitting and walking conditions. Topographic maps also reveal, as reported in relation to the waveforms above, that the P3 in young adults is more sustained across the entire 150ms time period for the sitting condition, while the walking P3 attains maximal amplitude at the beginning of the time window (~400ms) and then quickly diminishes.
Figure 6.
Topographical distribution of ERP voltage activity across the scalp encompassing the P3 time window (400–550ms) during correct response inhibition trials for young adults (top panel) and old adults (bottom panel). Sitting (single-task) and walking (dual-task) conditions are each depicted averaged across 25ms time intervals.
P3 latency for Correct Rejections
The effects of age, task load and electrode position on P3 peak latency were assessed by a three-way ANOVA. A large effect of Group was observed (F1,31 = 22.06, p < .001) reflecting the fact that the P3 peaked earlier for the young than for the older group. This suggests that older adults were generally slower to engage inhibitory processes. Additionally, a significant Task Load x Group interaction was observed (F1,31 = 23.32, p < .001) as well as a three-way Task Load x Electrode x Group interaction (F2,62 = 3.28, p < .05). Post-hoc paired comparisons between task load conditions at each electrode site revealed that P3 latency for the young differed between sitting and walking at all electrode sites (FCz: t16 = 3.73, p < .005; Cz: t16 = 4.61, p < .001; CPz: t16 = 5.21, p < .001). There were no P3 latency differences between task load conditions at any of the three electrode sites for the older group (all comparisons: p > .09). Figure 4 illustrates this effect in the waveforms of the young group whose walking P3 occurs approximately 90ms prior to the sitting P3; while the older group exhibits no latency differences between task load conditions. The interactions of Electrode x Group (p = .11) and Task Load x Electrode (p = .14) did not reach significance.
P3 amplitude for Correct Rejections
The three-way ANOVA revealed a significant Task Load x Group interaction (F1,31 = 7.35, p < .02), reflecting a load-dependent modulation in P3 amplitude. Within-group follow-up comparisons, averaged over electrode sites, showed that the young group displayed a significant decrease in P3 amplitude for walking, compared to sitting (t16 = 2.19, p < .05). In contrast, while older adults exhibited a slightly enhanced P3 over frontal scalp sites under dual-task conditions, this task-load effect was not consistent across all channels (p = .12). There was an additional interaction between Task Load and Electrode (F2,62 = 4.01, p < .05). Post-hoc comparisons (across age groups) revealed a task load difference, with significantly smaller P3 amplitude for walking compared to sitting, only over electrode CPz (t32 = 2.52, p < .05). Furthermore, there was a trend towards an Electrode x Group interaction (F2,62 = 2.74, p = .072). Finally, main effects of Task Load (p = .55), Group (p = .58) and the Task Load x Group x Electrode interaction (p = .29) did not reach significance.
P3 latency for Difference Waves
The three-way ANOVA assessing peak latency for difference waves (CRs minus Hits) revealed a significant effect of Group on P3 latency (F1,31 = 22.62, p < .001) and a significant Task Load x Group interaction (F1,31 = 5.03, p < .05). Similar to the CR condition reported above, this result reflects an earlier-occurring P3 peak during walking compared to sitting for young, but not for older adults. No other main or interaction effects were observed.
P3 amplitude for Difference Waves
Compared to the P3 amplitude for CR waveforms, we found only significant effects of Group (F1,31 = 6.54, p < .02) and Electrode (F1.23,38.271 = 14.69, p < .001) for P3 amplitude of the difference waves. This finding reflects the fact that the young group exhibited greater P3 amplitude overall compared to the older group, and that the more anterior electrode sites, FCz and Cz, also showed greater P3 amplitude, regardless of group and task load condition.
DISCUSSION
The current study examined the neural underpinnings of attentional resource allocation during dual-task walking in young and older adults. The effects of cognitive-motor interference (CMI) were assessed using Mobile Brain/Body Imaging techniques that enabled simultaneous recording of stride time and variability measures in addition to behavioral performance and cortically-generated markers of inhibitory response control. To our knowledge, this was the first application of MoBI in an aging population.
The young adult group maintained their behavioral performance under increased task load (i.e., walking while performing a Go/No-Go response inhibition task), suffering no costs in terms of reaction times or accuracy. The older participants were significantly slower to perform the task both while sitting and walking, although somewhat surprisingly, their rates of successful response inhibition across both task-load conditions were numerically better than those of the younger group, although this was not a statistically robust difference. Importantly, however, only the older group exhibited a significant task-load related cost in the form of an average drop in accuracy of approximately 4% during walking compared to sitting. With regard to gait pattern, we found an increase in average stride time of 82.4ms, or 8.4% for young adults under dual-task relative to single-task load. In contrast, older adults showed no changes in stride time between single and dual-task load. One possible explanation as to why increased load affected cognitive performance in older adults but gait performance in young adults may be that older individuals adopted a postural prioritization strategy - the tendency to prioritize the maintenance of stable gait and posture over performance on the secondary task to ensure safe walking (Li et al., 2012; Lovden et al., 2008; Yogev-Seligmann, Hausdorff, & Giladi, 2012). Also in line with prioritizing gait is the finding that older adults’ stride time variability did not increase under dual-task load. Unstable gait in the form of greater stride-to-stride fluctuations under increased load have been frequently reported, particularly in older adults less able to flexibly accommodate multiple task demands, such as individuals with mild cognitive impairment or a history of falls (Hausdorff, 2007; Montero-Odasso, Verghese, Beauchet, & Hausdorff, 2012; Springer et al., 2006). However, it is not clear whether prioritization of walking was a voluntary strategy or an impairment of the older group as no explicit task prioritization instructions were given to participants. Therefore, age-associated mobility decline might in fact be reason for prioritizing the walking subtask. Going forward, more explicit instructions to prioritize both tasks equally are advised (Verghese, Kuslansky, et al., 2007).
While the drop in the older adults’ Go/No-Go performance supports the notion of dual-task costs, the young adults’ increase in stride time while executing two tasks simultaneously is not easily explained in terms of dual-task costs. As walking pace was kept constant by the speed of the treadmill, it follows that an increase in stride time under dual-task load amounts to younger adults making longer and therefore fewer steps. Maintaining balance while taking longer steps is considered to be more challenging because a person’s center of gravity is more often further from one’s base of support (Bhatt, Wening, & Pai, 2005), and longer steps have been linked to higher probability of slips (Moyer, Chambers, Redfern, & Cham, 2006). Assuming that participants operate under limited resources shared across walking and cognitive demands, making longer steps might therefore appear to be a less effective strategy. However, there is reason to contend that making longer steps could actually be an adaptive walking strategy to reduce interference with a cognitive task (CMI). Here, and in our previous report (De Sanctis et al., 2014), we argue that by increasing stride length, a direct outcome is that one executes the walking task less often (i.e., takes fewer steps) and thereby reduces instances of intertask competition under dual-task load (Li et al., 2012; Lovden et al., 2008). It could be argued that making longer steps may be easier when walking at a relatively faster speed, putting older adults at a disadvantage to implement such a strategy. However, our previous findings on dual-task walking at fast and slow speeds in young participants would indicate otherwise (De Sanctis et al., 2014). We found that young participants increased stride time under both walking speeds, possibly as a strategy to accommodate increased cognitive task load. In the slow-walking condition, young participants walked at a fixed speed of 2.4 km/h, which is in fact slower than the average walking speed of the older adults at 3.5 km/h (range: 2.4 to 4.8 km/h) in the current study. Ultimately, this indicates that such a strategy may also be applied while walking at slower speeds. Overall, behavioral results support a loss in the flexible allocation of processing resources across tasks in aging, indicative of an increased susceptibility to CMI and in line with the extant aging literature on dual-tasking (Hausdorff, Schweiger, Herman, Yogev-Seligmann, & Giladi, 2008; Holtzer, Wang, & Verghese, 2012; Montero-Odasso et al., 2012; Yogev-Seligmann et al., 2008).
We turn now to the neural measures of response inhibition and cognitive control. Our previous work using MoBI in young adults provided evidence for the implementation of substantial dual-task modifications in both walking behavior and concurrent brain measures of response inhibition processes, including increased stride time, decreased N2 amplitude and an earlier and more frontally distributed P3 (De Sanctis et al., 2014). These outcomes were interpreted to reflect a flexible redeployment of cognitive-motor processes and set the stage for the current work in which we predicted that older adults would show a reduced ability to engage these adaptive processes. More specifically, as motor load increased, we predicted a drop in performance accuracy and a delay and attenuation of ERPs underlying successful response inhibition. These predictions, however, were only partially supported by the data. While ERP patterns in young adults showed substantial changes between the sitting and walking conditions, the same comparison in older adults yielded minimal variation. Thus, to a first approximation, young adults showed clear evidence for neural reconfiguration in response to increasing dual-task demands, whereas older adults showed, for the most part, a distinct lack of such flexibility. More precisely, in young adults, the enhanced N2 component following correctly withheld responses was strongly reduced in amplitude when load increased under dual-task walking conditions. Older adults, in contrast, displayed a markedly different pattern. An age-related topographical shift was seen, with a more posterior distribution for the N2 under both single and dual-task conditions, but in contrast to the findings in young adults, this N2 showed minimal amplitude modulation as a function of task load. Contrary to CRs, the N2 evoked during Hit trials revealed far fewer distinctions between age groups. Here, a load-related N2 reduction in amplitude was evident in both groups. Furthermore, the N2 for both CRs as well as Hit trials was substantially delayed for the older adults compared to the young. This may be indicative of a general delay in cognitive processing mechanisms with increasing age, a finding that is in agreement with previous literature (Salthouse, 2005). Finally, the difference waves (see Figure 4) most clearly highlight the distinction between the dynamic processing mechanisms recruited by the young, in contrast to the relatively static processing mode employed by the older group. The young show a robust N2 amplitude reduction while walking, particularly over centro-parietal sites, while the older adult waveforms show a remarkable lack of load-dependent modulation. The largely overlapping time course of the difference waves for the older group, throughout early and later processing stages, most clearly indicates a less flexible reallocation of cognitive resources during dual-task walking in aging.
Subsequently, correctly withheld responses produced a P3 component showing a distinct latency shift over a widespread scalp area in young adults, peaking approximately 90ms earlier under increased task load. In contrast, no latency differences were found for the older group, although a very modest increase in P3 amplitude over frontal scalp areas was observed for this group while walking. Finally, while Hit trials evoked negligible P3-like activity, overall amplitude was smaller in young and larger in older adults under increased task load. Results indicate more pronounced age-related differences during CR trials, possibly due to a relatively higher processing load required in order to inhibit rather than execute a prepotent motor response.
Taking both the performance data and neural measures into account, what can we conclude from the current results? It is clear that in response to the increased demands of performing the Go/No-Go task under walking conditions, young adults made online adjustments to both their physical behavior by increasing stride length and to the way in which response inhibition processes were deployed in the brain. These adjustments were associated with essentially perfect maintenance of performance levels on the cognitive control task. In contrast, older adults showed no changes in their physical behavior and what differences were observed in the deployment of response inhibition processes were extremely modest and only emerged during later P3-related stages of processing. This lack of flexibility, in turn, was accompanied by a significant, albeit modest, decrement in performance of the cognitive task.
Only a small number of previous studies have evaluated task-evoked ERPs in the context of motor-related dual-task load. In one investigation, Hahn and colleagues instructed participants to prioritize a driving-like tracking task and investigated the effects of age and P3-related activity on a secondary visual attention task. Older adults showed a greater degree of dual-task motor interference compared to young. They also failed to exhibit the pattern shown in young adults of increased P3 amplitude for target compared to non-target stimuli, possibly indicating that when cognitive resources were taxed under increased load, older adults dedicated comparable attentional resources to all stimulus types regardless of relevance (Hahn, Wild-Wall, & Falkenstein, 2011). However, since only dual-task conditions were considered in this experiment, it is unclear if the age-related differences in P3 can be fully attributed to increased load. Other investigations have reported effects of increased task load on the timing of ERPs, specifically delays in P3-related processes (Bomba & Singhal, 2010; Fujiyama, Garry, Martin, & Summers, 2010; Matthews, Garry, Martin, & Summers, 2006). Matthews et al. (2006) combined a bimanual motor with a visual task, requiring foot responses to infrequently presented visual target stimuli. They observed increased P3 latency for visual targets when the motor task was prioritized compared to when the visual task was prioritized (Matthews et al., 2006). In a similar design, Fujiyama and colleagues compared the performance of young and older adults on an interlimb coordination task combined with visual oddball discrimination. The P3 evoked by the visual task was reduced in amplitude and longer latencies were observed during dual-task conditions for both groups, while P3 latencies in older adults were further delayed compared to those of the young (Fujiyama et al., 2010).
It is noteworthy that no previous study has reported a reduction in P3 latency under increased task load, in contrast to the current results where a distinct shortening of the P3 latency was observed for younger adults. Of course, this P3 latency reduction cannot be considered in isolation but must be construed in the context of the large reduction in N2 amplitude that accompanies it. In our prior work, we interpreted this as a shift in processing strategy from a mostly automatic mode of operation represented by the strong No-Go N2 during single-task load, to a more conscious evaluative, and presumably more effortful, process represented by the earlier P3 under dual-task load. On the other hand, our older participants appeared to deploy essentially the same processing strategies under all task load conditions.
In addition, a trend towards a more frontally distributed P3 topography was observed in older adults, which was largely load-independent (i.e., evident during both sitting and walking conditions). Anteriorization of P3 in aging has been regularly reported in the literature (Anderer, Semlitsch, & Saletu, 1996; De Sanctis, Gomez-Ramirez, Sehatpour, Wylie, & Foxe, 2009; Fabiani & Friedman, 1995; Friedman, Simpson, & Hamberger, 1993; Friedman & Simpson, 1994) to implicate the engagement of additional prefrontal cortical resources in compensation for age-related cognitive decline. Our age-associated findings during the P3 timeframe might therefore suggest that older adults’ recruitment of additional frontal control regions is required to reduce and prevent even higher costs resulting from increased cognitive motor interference.
In conclusion, the MoBI approach provides an excellent methodology by which neuroscientists can interrogate the underlying neurophysiology of cognitive control processes in the context of real-time measures of gait, posture and other physical parameters. In this way, we can move beyond the somewhat artificial constraints of traditional EEG and ERP work, providing a considerably higher degree of ecological validity to the work we conduct. This is especially useful in the case of aging where the relationship between decline in cognitive flexibility and measures of gait and posture disturbances are well-established (Verghese, Wang, Lipton, Holtzer, & Xue, 2007). MoBI allows for an integrated assessment of these two domains and we anticipate that it will have significant utility in the early identification of older individuals who are at risk for injurious falls, a leading cause of morbidity in this population (Stevens, 2005). The present results indicate a clear lack of flexibility, both in terms of adjusting physical behavior and in reconfiguring cognitive control mechanisms at the neural level, in a cohort of healthy older individuals. It will be of significant interest to contrast these processes in elderly individuals with and without a history of falls in future work to see if these measures can distinguish between these groups. It will also be of interest to assess other domains of cognitive control, such as task-set reconfigurations (Foxe, Murphy, & De Sanctis, 2014; Wylie, Javitt, & Foxe, 2003) or the maintenance of attentional focus (O’Connell, Dockree, Robertson, et al., 2009), since these control processes may require greater metacognitive resources and may well lead to greater CMI effects.
Supplementary Material
Highlights.
Dual-task performance in aging during active walking was explored
High-density brain electrical activity was recorded from freely walking individuals
Mobile Brain-Body Imaging (MOBI) allows for gait and neuro-cognitive assessment in naturalistic settings
Cognitive flexibility to manage dual-task walking interference was reduced in aging
Acknowledgments
The primary source of funding for this work was provided by a pilot grant from the Einstein-Montefiore Institute for Clinical and Translational Research (UL1-TR000086) and the Sheryl & Daniel R. Tishman Charitable Foundation. Participant recruitment and scheduling was performed by The Human Clinical Phenotyping Core at Einstein, a facility of the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (RFK-IDDRC) which is funded by a center grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD P30 HD071593). We would like to express our sincere gratitude to the participants for giving their time to this effort. Additionally, we thank Dr. Gizely Andrade, Mr. Gregory Peters and Ms. Eva-Maria Schlichtmann for their efforts and support during data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Adjusted df as assumption of sphericity is violated
AUTHOR CONTRIBUTIONS
JJF and PDS were responsible for initial study concept and design. BRM and JSB were responsible for participant recruitment, phenotyping and coordinating data collection. JSB and PDS took primary responsibility for setup and development of the MoBI system. All authors contributed to data analysis and data interpretation. BRM wrote the first draft of the manuscript. JJF, PDS and JSB provided extensive editorial input throughout the process, and critical revisions of the manuscript for important intellectual content. All authors critically reviewed the content of the paper and approved the final version for publication.
CONFLICT OF INTEREST STATEMENT
All authors of this paper declare that they have no conflict-of-interest, financial or otherwise, that would bias their contributions to this work.
References
- Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2011;35(3):715–728. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Anderer P, Semlitsch HV, Saletu B. Multichannel auditory event-related brain potentials: effects of normal aging on the scalp distribution of N1, P2, N2 and P300 latencies and amplitudes. Electroencephalogr Clin Neurophysiol. 1996;99(5):458–472. doi: 10.1016/s0013-4694(96)96518-9. [DOI] [PubMed] [Google Scholar]
- Beurskens R, Bock O. Age-related deficits of dual-task walking: a review. Neural Plast. 2012;2012:131608. doi: 10.1155/2012/131608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurskens R, Helmich I, Rein R, Bock O. Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. Int J Psychophysiol. 2014;92(3):122–128. doi: 10.1016/j.ijpsycho.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Bhatt T, Wening JD, Pai YC. Influence of gait speed on stability: recovery from anterior slips and compensatory stepping. Gait Posture. 2005;21(2):146–156. doi: 10.1016/j.gaitpost.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Bock O. Dual-task costs while walking increase in old age for some, but not for other tasks: an experimental study of healthy young and elderly persons. J Neuroeng Rehabil. 2008;5:27. doi: 10.1186/1743-0003-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin Neurophysiol. 2001;112(12):2224–2232. doi: 10.1016/s1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Bomba MD, Singhal A. ERP evidence of early cross-modal links between auditory selective attention and visuo-spatial memory. Brain Cogn. 2010;74(3):273–280. doi: 10.1016/j.bandc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Ageing and gait variability--a population-based study of older people. Age Ageing. 2010;39(2):191–197. doi: 10.1093/ageing/afp250. [DOI] [PubMed] [Google Scholar]
- Castermans T, Duvinage M. Corticomuscular coherence revealed during treadmill walking: further evidence of supraspinal control in human locomotion. J Physiol. 2013;591(Pt 6):1407–1408. doi: 10.1113/jphysiol.2012.247593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castermans T, Duvinage M, Cheron G, Dutoit T. About the cortical origin of the low-delta and high-gamma rhythms observed in EEG signals during treadmill walking. Neurosci Lett. 2014;561:166–170. doi: 10.1016/j.neulet.2013.12.059. [DOI] [PubMed] [Google Scholar]
- De Sanctis P, Butler JS, Green JM, Snyder AC, Foxe JJ. Mobile brain/body imaging (MoBI): High-density electrical mapping of inhibitory processes during walking. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1542–1545. doi: 10.1109/EMBC.2012.6346236. [DOI] [PubMed] [Google Scholar]
- De Sanctis P, Butler JS, Malcolm BR, Foxe JJ. Recalibration of inhibitory control systems during walking-related dual-task interference: a mobile brain-body imaging (MOBI) study. Neuroimage. 2014;94:55–64. doi: 10.1016/j.neuroimage.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sanctis P, Gomez-Ramirez M, Sehatpour P, Wylie GR, Foxe JJ. Preserved executive function in high-performing elderly is driven by large-scale recruitment of prefrontal cortical mechanisms. Hum Brain Mapp. 2009;30(12):4198–4214. doi: 10.1002/hbm.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Gandras K, Debener S. Towards a truly mobile auditory brain-computer interface: exploring the P300 to take away. Int J Psychophysiol. 2014;91(1):46–53. doi: 10.1016/j.ijpsycho.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Debener S, Minow F, Emkes R, Gandras K, de Vos M. How about taking a low-cost, small, and wireless EEG for a walk? Psychophysiology. 2012;49(11):1617–1621. doi: 10.1111/j.1469-8986.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dockree PM, Kelly SP, Robertson IH, Reilly RB, Foxe JJ. Neurophysiological markers of alert responding during goal-directed behavior: a high-density electrical mapping study. Neuroimage. 2005;27(3):587–601. doi: 10.1016/j.neuroimage.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Doi T, Makizako H, Shimada H, Yoshida D, Ito K, Kato T, Suzuki T. Brain atrophy and trunk stability during dual-task walking among older adults. J Gerontol A Biol Sci Med Sci. 2012;67(7):790–795. doi: 10.1093/gerona/glr214. [DOI] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56(2):165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Duvinage M, Castermans T, Petieau M, Seetharaman K, Hoellinger T, Cheron G, Dutoit T. A subjective assessment of a P300 BCI system for lower-limb rehabilitation purposes. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:3845–3849. doi: 10.1109/EMBC.2012.6346806. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol. 1993;35(2):123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32(6):579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Inhibition-related ERP components: Variation with modality, age, and time-on-task. Journal of Psychophysiology. 2002;16(3):167–175. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Murphy JW, De Sanctis P. Throwing out the rules: anticipatory alpha-band oscillatory attention mechanisms during task-set reconfigurations. Eur J Neurosci. 2014;39(11):1960–1972. doi: 10.1111/ejn.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Simpson G, Hamberger M. Age-related changes in scalp topography to novel and target stimuli. Psychophysiology. 1993;30(4):383–396. doi: 10.1111/j.1469-8986.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Simpson GV. ERP amplitude and scalp distribution to target and novel events: effects of temporal order in young, middle-aged and older adults. Brain Res Cogn Brain Res. 1994;2(1):49–63. doi: 10.1016/0926-6410(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Garry MI, Martin FH, Summers JJ. An ERP study of age-related differences in the central cost of interlimb coordination. Psychophysiology. 2010;47(3):501–511. doi: 10.1111/j.1469-8986.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17(4):1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gramann K, Ferris DP, Gwin J, Makeig S. Imaging natural cognition in action. Int J Psychophysiol. 2014;91(1):22–29. doi: 10.1016/j.ijpsycho.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramann K, Gwin JT, Bigdely-Shamlo N, Ferris DP, Makeig S. Visual evoked responses during standing and walking. Front Hum Neurosci. 2010;4:202. doi: 10.3389/fnhum.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramann K, Gwin JT, Ferris DP, Oie K, Jung TP, Lin CT, Makeig S. Cognition in action: imaging brain/body dynamics in mobile humans. Rev Neurosci. 2011;22(6):593–608. doi: 10.1515/RNS.2011.047. [DOI] [PubMed] [Google Scholar]
- Gramann K, Jung TP, Ferris DP, Lin CT, Makeig S. Toward a new cognitive neuroscience: modeling natural brain dynamics. Front Hum Neurosci. 2014;8:444. doi: 10.3389/fnhum.2014.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Gwin JT, Gramann K, Makeig S, Ferris DP. Electrocortical activity is coupled to gait cycle phase during treadmill walking. Neuroimage. 2011;54(2):1289–1296. doi: 10.1016/j.neuroimage.2010.08.066. [DOI] [PubMed] [Google Scholar]
- Hahn M, Wild-Wall N, Falkenstein M. Age-related differences in performance and stimulus processing in dual task situation. Brain Res. 2011;1414:66–76. doi: 10.1016/j.brainres.2011.07.051. [DOI] [PubMed] [Google Scholar]
- Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193(3):445–454. doi: 10.1007/s00221-008-1643-y. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM. Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Hum Mov Sci. 2007;26(4):555–589. doi: 10.1016/j.humov.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1335–1343. doi: 10.1093/gerona/63.12.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164(4):541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010;65(10):1086–1092. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoellinger T, Petieau M, Duvinage M, Castermans T, Seetharaman K, Cebolla AM, Cheron G. Biological oscillations for learning walking coordination: dynamic recurrent neural network functionally models physiological central pattern generator. Front Comput Neurosci. 2013;7:70. doi: 10.3389/fncom.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011;66(8):879–887. doi: 10.1093/gerona/glr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20(2):215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor Control. 2012;16(1):64–80. doi: 10.1123/mcj.16.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R, De Sanctis P, Mahoney JR, Sehatpour P, Murphy CF, Gomez-Ramirez M, Foxe JJ. Cognitive control in late-life depression: response inhibition deficits and dysfunction of the anterior cingulate cortex. Am J Geriatr Psychiatry. 2010;18(11):1017–1025. doi: 10.1097/JGP.0b013e3181d695f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killane I, Donoghue OA, Savva GM, Cronin H, Kenny RA, Reilly RB. Relative association of processing speed, short-term memory and sustained attention with task on gait speed: a study of community-dwelling people 50 years and older. J Gerontol A Biol Sci Med Sci. 2014;69(11):1407–1414. doi: 10.1093/gerona/glu140. [DOI] [PubMed] [Google Scholar]
- Knoblauch RLPM, Nitzburg M. Field studies of pedestrian walking speed and start-up time. Transp Res Rec. 1996;1538:27–38. (Pedestrian and Bicycle Research) [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9(4):491–512. [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW, Arpin DJ. Stride-time variability and sensorimotor cortical activation during walking. Neuroimage. 2012;59(2):1602–1607. doi: 10.1016/j.neuroimage.2011.08.084. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instructional manual. Technical report A-8. gainesville, FL: University of Florida; 2008. [Google Scholar]
- Li KZ, Abbud GA, Fraser SA, Demont RG. Successful adaptation of gait in healthy older adults during dual-task treadmill walking. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19(1–2):150–167. doi: 10.1080/13825585.2011.628375. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15(3):417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- Lovden M, Schaefer S, Pohlmeyer AE, Lindenberger U. Walking variability and working-memory load in aging: a dual-process account relating cognitive control to motor control performance. J Gerontol B Psychol Sci Soc Sci. 2008;63(3):P121–P128. doi: 10.1093/geronb/63.3.p121. [DOI] [PubMed] [Google Scholar]
- Lucci G, Berchicci M, Spinelli D, Taddei F, Di Russo F. The effects of aging on conflict detection. PLoS One. 2013;8(2):e56566. doi: 10.1371/journal.pone.0056566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. Ten simple rules for designing ERP experiments. In: Handy TC, editor. Event-related potentials: A methods handbook. 2004. pp. 17–32. [Google Scholar]
- Makeig S, Gramann K, Jung TP, Sejnowski TJ, Poizner H. Linking brain, mind and behavior. Int J Psychophysiol. 2009;73(2):95–100. doi: 10.1016/j.ijpsycho.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews A, Garry MI, Martin F, Summers J. Neural correlates of performance tradeoffs and dual-task interference in bimanual coordination: an ERP investigation. Neurosci Lett. 2006;400(1–2):172–176. doi: 10.1016/j.neulet.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Herman T, Brozgol M, Dorfman M, Sprecher E, Schweiger A, Hausdorff JM. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One. 2012;7(6):e40297. doi: 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Kubota K. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14(5):1186–1192. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60(11):2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morie KP, Garavan H, Bell RP, De Sanctis P, Krakowski MI, Foxe JJ. Intact inhibitory control processes in abstinent drug abusers (II): a high-density electrical mapping study in former cocaine and heroin addicts. Neuropharmacology. 2014;82:151–160. doi: 10.1016/j.neuropharm.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Moyer BE, Chambers AJ, Redfern MS, Cham R. Gait parameters as predictors of slip severity in younger and older adults. Ergonomics. 2006;49(4):329–343. doi: 10.1080/00140130500478553. [DOI] [PubMed] [Google Scholar]
- Nolan H, Butler JS, Whelan R, Foxe JJ, Bulthoff HH, Reilly RB. Neural correlates of oddball detection in self-motion heading: a high-density event-related potential study of vestibular integration. Exp Brain Res. 2012;219(1):1–11. doi: 10.1007/s00221-012-3059-y. [DOI] [PubMed] [Google Scholar]
- Nolan H, Whelan R, Reilly RB, Bulthoff HH, Butler JS. Acquisition of human EEG data during linear self-motion on a Stewart platform; 4th International IEEE/EMBS Conference on Neural Engineering; 2009. pp. 585–588. [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Turin A, Ward S, Foxe JJ, Robertson IH. Two types of action error: electrophysiological evidence for separable inhibitory and sustained attention neural mechanisms producing error on go/no-go tasks. J Cogn Neurosci. 2009;21(1):93–104. doi: 10.1162/jocn.2009.21008. [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Robertson IH, Bellgrove MA, Foxe JJ, Kelly SP. Uncovering the neural signature of lapsing attention: electrophysiological signals predict errors up to 20 s before they occur. J Neurosci. 2009;29(26):8604–8611. doi: 10.1523/JNEUROSCI.5967-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Giard MH, Echallier JF. Mapping of scalp potentials by surface spline interpolation. Electroencephalogr Clin Neurophysiol. 1987;66(1):75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- Plummer-D’Amato P, Altmann LJ, Reilly K. Dual-task effects of spontaneous speech and executive function on gait in aging: exaggerated effects in slow walkers. Gait Posture. 2011;33(2):233–237. doi: 10.1016/j.gaitpost.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Erickson KI, Colcombe SJ, Kim JS, Voss MW, Kramer AF. Age-related differences in the involvement of the prefrontal cortex in attentional control. Brain Cogn. 2009;71(3):328–335. doi: 10.1016/j.bandc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis PM, Hebenstreit F, Gabsteiger F, von Tscharner V, Lochmann M. Methodological aspects of EEG and body dynamics measurements during motion. Front Hum Neurosci. 2014;8:156. doi: 10.3389/fnhum.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Normal rates of cognitive change in successful aging: the freedom house study. J Int Neuropsychol Soc. 2005;11(7):899–909. doi: 10.1017/s135561770505109x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19(4):532–545. doi: 10.1037/0894-4105.19.4.532. [DOI] [PubMed] [Google Scholar]
- Silva AMCB, da Cunha JRR, da Silva JPC. Estimation of pedestrian walking speeds on footways. Proceedings of the Institution of Civil Engineers-Municipal Engineer. 2014;167(1):32–43. [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21(7):950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- Srygley JM, Mirelman A, Herman T, Giladi N, Hausdorff JM. When does walking alter thinking? Age and task associated findings. Brain Res. 2009;1253:92–99. doi: 10.1016/j.brainres.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JA. Falls among older adults--risk factors and prevention strategies. J Safety Res. 2005;36(4):409–411. doi: 10.1016/j.jsr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Miyai I, Ono T, Kubota K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. Neuroimage. 2008;39(2):600–607. doi: 10.1016/j.neuroimage.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Theill N, Martin M, Schumacher V, Bridenbaugh SA, Kressig RW. Simultaneously measuring gait and cognitive performance in cognitively healthy and cognitively impaired older adults: the Basel motor-cognition dual-task paradigm. J Am Geriatr Soc. 2011;59(6):1012–1018. doi: 10.1111/j.1532-5415.2011.03429.x. [DOI] [PubMed] [Google Scholar]
- Townsend JT, Ashby FG. The stochastic modeling of elementary psychological processes. New York: Cambridge University Press; 1983. [Google Scholar]
- Uehara K, Higashi T, Tanabe S, Sugawara K. Alterations in human motor cortex during dual motor task by transcranial magnetic stimulation study. Exp Brain Res. 2011;208(2):277–286. doi: 10.1007/s00221-010-2478-x. [DOI] [PubMed] [Google Scholar]
- Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA, Wang C. Motoric cognitive risk syndrome: Multicountry prevalence and dementia risk. Neurology. 2014 doi: 10.1212/WNL.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Holtzer R, Katz M, Xue X, Buschke H, Pahor M. Walking while talking: effect of task prioritization in the elderly. Arch Phys Med Rehabil. 2007;88(1):50–53. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68(4):412–418. doi: 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78(9):929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher E, Falkenstein M, Wild-Wall N. Age related strategic differences in processing irrelevant information. Neurosci Lett. 2011;487(1):66–69. doi: 10.1016/j.neulet.2010.09.075. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Falkenstein M, Schwarz M, Muller T, Beste C. Effects of aging, Parkinson’s disease, and dopaminergic medication on response selection and control. Neurobiol Aging. 2011;32(2):327–335. doi: 10.1016/j.neurobiolaging.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther. 1990;70(6):340–347. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ. Task switching: a high-density electrical mapping study. Neuroimage. 2003;20(4):2322–2342. doi: 10.1016/j.neuroimage.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–342. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov Disord. 2012;27(6):765–770. doi: 10.1002/mds.24963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.