ABSTRACT
Testosterone is derived from Leydig cells and exerts its effects on androgen receptors to influence growth, mood, voice, and several other bodily functions. As men age, their testosterone levels decline. Human immunodeficiency virus (HIV) infection has also been associated with lowered serum testosterone levels. Subtherapeutic levels of testosterone may lead to fatigue, loss of libido, and dysphoria. Exogenous replacement of testosterone can be accomplished by several modalities (oral, topical, injection), with each having distinct advantages and disadvantages. Even though testosterone replacement has become a popular medical intervention, recent reports have made the practice increasingly controversial. Several small retrospective investigations have recently associated testosterone replacement with an increased risk of cardiovascular complications. Replacement should be used sparingly until further conclusive data regarding the efficacy and safety of testosterone become available.
Keywords: hormones, hypogonadism, steroids, testosterone
Testosterone synthesis in males starts with gonadotropin-releasing hormone (GnRH) from the hypothalamus. GnRH secretion will cause the pituitary gland to secrete luteinizing hormone, which leads to testosterone synthesis in the Leydig cells. The newly synthesized testosterone is then released from the Leydig cells into the blood at concentrations ranging from 288 to 865 ng/dL.1 Ultimately, free testosterone contributes to pharmacologic activity, which is dependent on the exact concentration of sex hormone binding. The amount of active serum testosterone is heavily reliant upon the concentration of sex hormone–binding globulin (SHBG) and albumin.2
The majority of free testosterone binds to androgen receptors, which are located on many cell types. These receptors exert their effects on mood, voice, genitalia, muscle, and other parts of the body. With regard to muscle development, testosterone acts upon multiple pathways to influence growth and ultimately directly impacts protein expression. Testosterone stimulates the release of growth hormone from the anterior pituitary, which will in turn increase secretion of insulin like growth factor-1, thus resulting in increased protein synthesis. Testosterone also possesses anti-glucocorticoid effects, which can act to preserve lean muscle mass.3
While the majority of free testosterone (90%–95%) will bind to androgen receptors to exert pharmacologic effect prior to being metabolized, smaller concentrations will follow 1 of 2 metabolic pathways (Figure 1). Five percent to 8% of free testosterone is reduced to dihydrotestosterone (DHT) via 5α reductase.1,4 DHT may still agonize androgen receptors. A much smaller percent of free testosterone (0.3%–0.5%) is metabolized by the enzyme aromatase. Aromatase will actively transform testosterone to various metabolites, in particular estrone and estradiol. These steroids in turn may bind to and agonize estrogen receptors.
Figure 1.
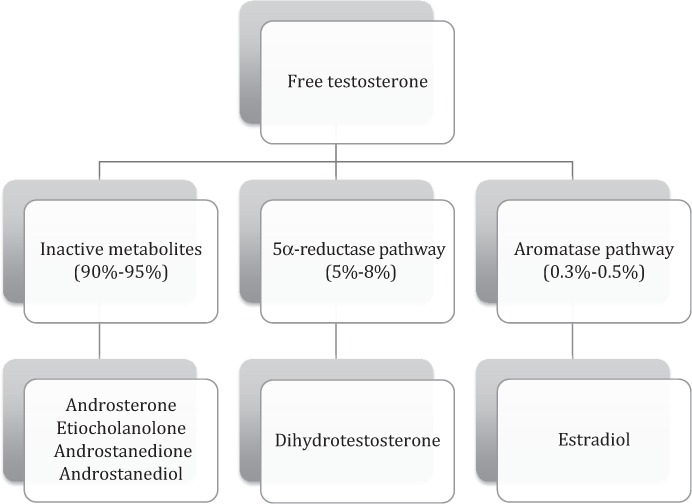
Testosterone metabolism.
Men with low testosterone commonly complain of decreased libido, dysphoria, fatigue, and mild depression. Normal testosterone blood concentrations for males range from 300 to 1,000 ng/dL.5 The results of the Massachusetts Male Aging Study demonstrated that, on average, total testosterone levels decrease by approximately 0.8% with every year, whereas free testosterone levels decline at a rate of about 2% per year.2
Androgen replacement in males has become common, although it remains controversial due to a lack of efficacy data and emerging safety issues. It is interesting to note that estrogen hormone replacement therapy (HRT) was once very common in females, with an estimated 40% of postmenopausal women in the US using HRT just prior to the publication of the initial findings of the Women's Health Initiative (WHI) in 2004. This study examined risks and benefits of HRT in healthy postmenopausal women aged 50 to 79 years. The risks of HRT with conjugated equine estrogens and medroxyprogesterone seemed to outweigh any benefits in terms of the amelioration of vasomotor symptoms.6 Data from the WHI trial did not support the use of estrogen plus progestin or estrogen alone for long-term chronic disease prevention. As a result, estrogen HRT is now utilized on a much more conservative basis and only when indicated for the management of moderate to severe menopausal symptoms.
TESTOSTERONE DEFICIENCY
Testosterone deficiency affects 30% of men aged 40 to 79 years, with its prevalence increasing with advancing age.5 This phenomenon has been accelerated, as individual life expectancies continue to increase as do the prevalence of chronic diseases, such as diabetes. A major contributing factor to low testosterone is hypogonadism. Hypogonadism may be influenced and/or potentiated by elevated levels of SBG. Historically, testosterone deficiency in older men was believed to affect quality of life without any influence on morbidity and/or mortality. Recent populationbased studies have correlated low testosterone with the potential development of many disease states. A systematic review conducted by Ding et al7 examined the relative risk of developing diabetes in relation to levels of endogenous sex hormones. Men with low testosterone less than 449.6 ng/dL were 42% more likely to develop type 2 diabetes compared to men with testosterone levels between 449.6 to 605.2 ng/dL (relative risk [RR], 0.58; 95% CI, 0.39–0.87). Another population-based longitudinal study focused on 794 males aged 50 to 91 years whose baseline testosterone levels were followed from the mid-1980s through July 2004. Of the 794 men, 538 deaths occurred at an 11.8-year follow-up. Males with testosterone levels less than 241 ng/dL were 40% (hazard ratio [HR], 1.4; 95% CI, 1.14-1.71]) more likely to die.8 This study demonstrated a probable association of increased mortality risk for males with a testosterone insufficiency defined as less than 241 ng/dL.
TESTOSTERONE AND HUMAN IMMUNE DEFICIENCY VIRUS INFECTION
As previously mentioned, testosterone levels naturally decline with age, with about 20% of men over 60 year and roughly 50% of men over 80 years having below normal levels of testosterone when compared with younger men.9 Among individuals infected with human immunodeficiency virus (HIV), 30% to 50% suffer from testosterone deficiency.10 The exact pathogenesis of hypogonadism in HIV is unclear and is likely multifaceted. Proposed pathogenic mechanisms to explain hypogonadism in this population have included malnutrition, increased cytokine levels and associated inflammation, opportunistic infections, altered SHBG levels, and use of antiretroviral therapy.11 Exact mechanisms explaining how antiretroviral therapy might influence testosterone levels have not been fully elucidated. Some have reported that the protease inhibitors may actually increase serum testosterone levels by reducing the hormone's endogenous metabolism. One study reported increases in testosterone levels resulting from hindered metabolism brought on by the protease inhibitor class of antiretrovirals.11 Alternatively, protease inhibitors have been associated with lowered testosterone levels as a result of lipohypertrophy, which may cause more of the hormone to undergo conversion to estradiol via aromatase. HIV-infected patients with AIDS-related muscle wasting appear to have a higher prevalence of hypogonadism, likely from inappropriately low luteinizing hormone levels.3 As antiretroviral therapy has significantly altered the course of HIV disease, life expectancies for infected patients have continued to increase. Thus low testosterone resulting from both aging and HIV infection in concert may become increasingly prevalent among infected individuals.
TESTOSTERONE REPLACEMENT PREPARATIONS
Oral testosterone replacement therapy is limited to testosterone undecylate, which has lymphatic absorption that bypasses the liver and allows for adequate systemic circulation.4 Oral therapy is convenient for administration; however, oral testosterone is highly dependent on a high-fat meal for sufficient absorption. Avoiding transient and irregular hormone peaks with testosterone undecylate requires 2 to 3 doses per day. The benefits of oral therapy include ease in dosing and ability to titrate as well as ease in drug discontinuation.
A transmucosal option for replacement includes nondissolvable bioadhesive, controlled-release buccal tablet containing testosterone (Striant).1,4,9 Striant provides testosterone absorption across the buccal membrane thus bypassing first-pass hepatic metabolism. This preparation is dosed every 12 hours, and the tablet must be removed at the end of treatment. The buccal tablets can be irritating to the gums and may cause taste disturbances in some individuals; the twice-daily dosing requirement may not be convenient for some. This formulation also carries some risk of unintended drug transference via kissing.
Testosterone for intramuscular injection is available as testosterone propionate, enantate, cipionate, and undecylate esters. Dosing amount and frequency will depend on the specific ester chosen for administration (Table 1). Testosterone propionate is dosed 2 to 3 times per week, whereas testosterone cipionate is dosed every 2 weeks.1,4 The advantages of intramuscular testosterone include self-administration by the patient into the thigh and its low cost. Disadvantages include high testosterone concentrations shortly following administration with a slow and predictable decline to hypogonadal levels. These very high peak levels followed by pronounced troughs may lead to significant fluctuations in mood and libido.
Table 1.
Available testosterone replacement modalities
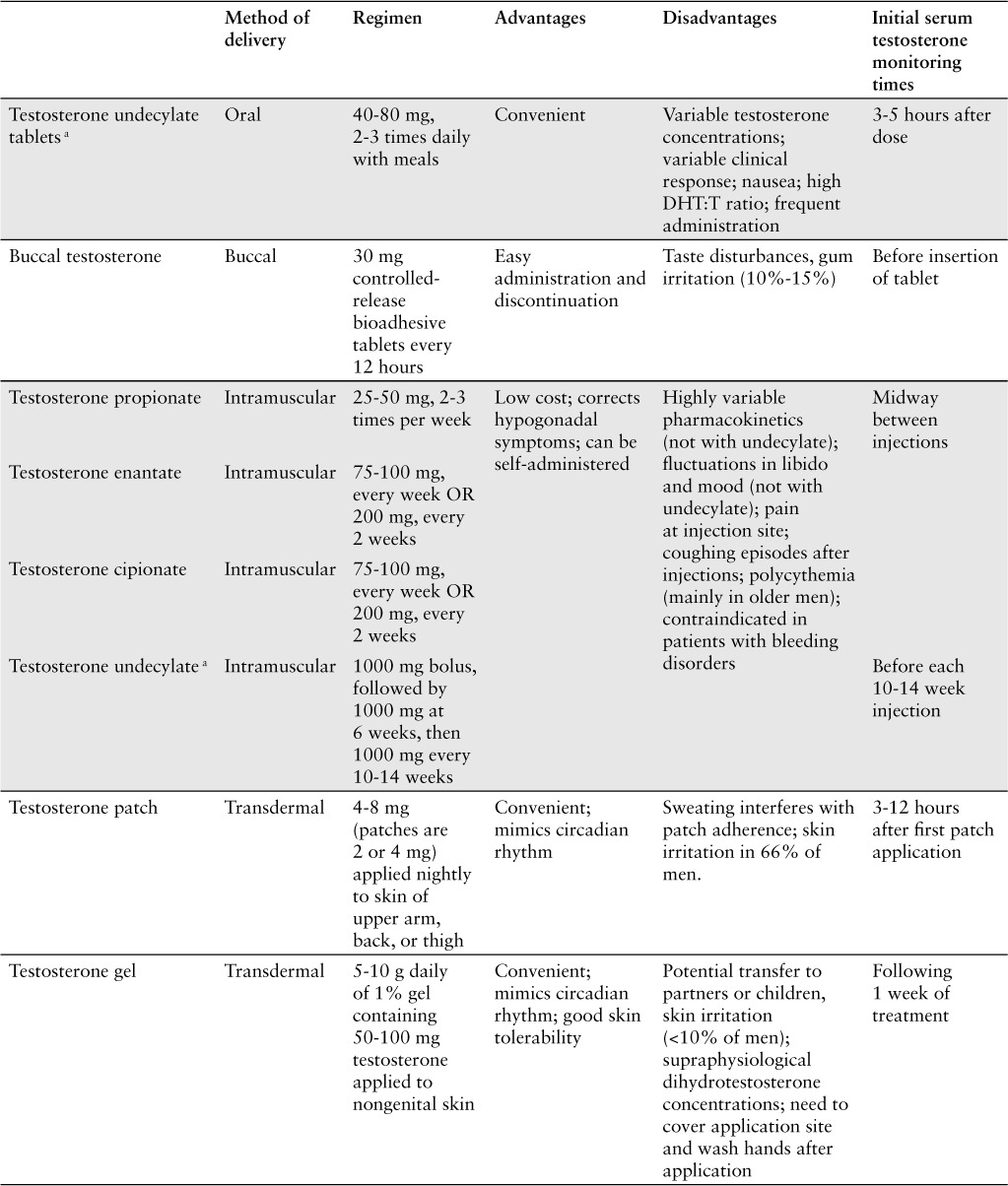
Table 1.
Available testosterone replacement modalities (CONT.)
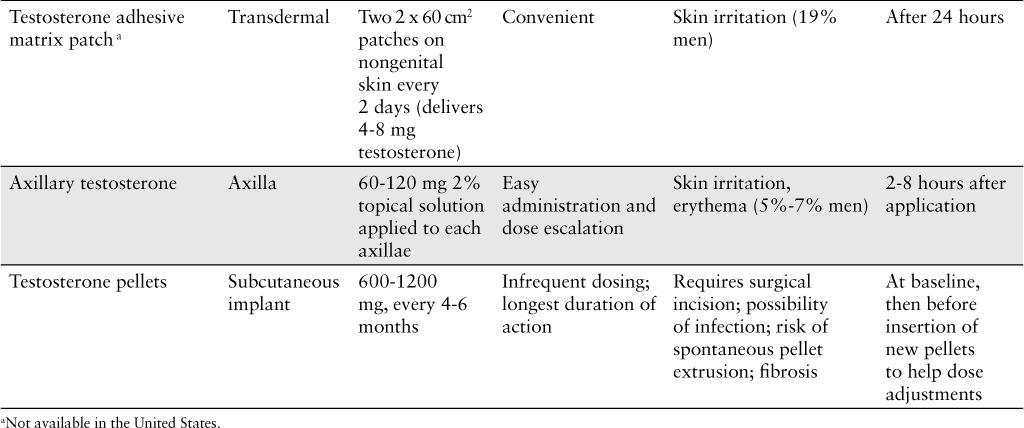
Testosterone is available transdermally as a patch or a gel applied once daily.1,9 Both are convenient dosage forms and require little manipulation to apply. Both patches and gels closely mimic physiologic circadian rhythms in terms of testosterone serum levels, resulting in fewer fluctuations as compared to other products.1 However, transdermal patches have been commonly associated with skin irritation, likely from the adhesives used within these products. Pretreatment with topical corticosteroids, such as triamcinolone cream, can reduce skin irritation without interfering with testosterone absorption.4 Transdermal gels are less likely to irritate the skin but carry some transference risk when direct skin-to-skin contact occurs. Transfer may especially be an issue with newborns and/or young children.
An additional option for topical testosterone application is the axillary product Axiron. Axiron is a testosterone spray, applied to both axilla once daily.1,4,9 This 2% ethanol-based testosterone solution is fragrance-free and available in a metered-dose pump. The formulation has been associated with skin irritation and erythema (similar to other topical testosterone preparations). An important counseling point involves the importance of deodorant or antiperspirant application prior to axillary testosterone application in order to prevent contamination of either product.
A relatively new option for testosterone replacement involves the implantation of subcutaneous testosterone pellets. This procedure is more common in Europe than in the United States.4 Implanted pellets allow for a long-lasting release of testosterone for 4 to 6 months.1,4 A significant limitation to this method of testosterone replacement is the need for surgical implantation of the pellets. Additionally, the implantation site, such as buttock or hip area, may be cosmetically unappealing and can serve as a potential locus for infection.
TREATMENT, RISKS, AND CONTROVERSY
Testosterone levels should be targeted for the mid-normal range of 400 to 700 ng/dL.2 The monitoring times for serum testosterone will differ between products being used for replacement therapy (Table 1). However, all testosterone replacement modalities should be assessed for appropriate clinical responses 3 to 6 months after treatment initiation, with subsequent dose adjustments to ensure adequate serum levels (see box, Testosterone Monitoring).
Testosterone Monitoring
Testosterone level at baseline, then at 3–6 months, then annually.
Target testosterone level should be in mid-normal range of 400–700 ng/dL.
Measure baseline bone mineral density and repeat every 1–2 years.
Prostate specific antigen at baseline, then at 3–6 months, then annually.
Hematocrit at baseline, then at 3–6 months; >54% suggests overtreatment or potential abuse.
Assess for adverse effects including: acne, male pattern baldness, gynecomastia, new or worsening sleep apnea, inhibited spermatogenesis, and formulation-specific adverse effects at regular follow-ups.
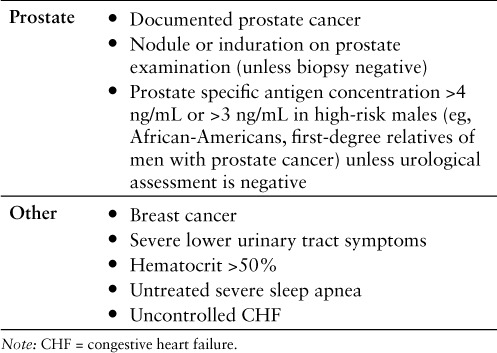
Testosterone replacement therapy is not recommended for every male (Table 2). The exogenous administration of testosterone can hasten the development of prostate cancer; however, this supposition is somewhat controversial. A 5-year retrospective study of 81 hypogonadal men receiving testosterone replacement therapy (75 received testosterone gel, 4 received injection, 1 received a patch, and 1 received another unspecified formulation) reported an increase in PSA levels and incidence of prostate cancer. The exact dose of testosterone that each subject was receiving was not reported. The average baseline PSA level for all men was 1.32 ng/mL. For the 77 men who did not develop prostate cancer, mean PSA levels at 1, 2, 3, 4, and 5 years were 1.49 (p = .42), 1.35 (p = .89), 1.31 (p = .97), 1.10 (p = .58), and 1.43 ng/mL (p = .82), respectively. There was no statistically significant difference in PSA levels from baseline to any of the yearly intervals. In the 4 men with prostate cancer, there was an increase in mean PSA level from baseline to 18 months of 1.8 ng/mL (p = .15), whereas the mean PSA level increased from baseline by 3.2 ng/mL (p < .05) at the 3-year follow-up. However, the incidence of prostate cancer in testosterone-receiving males was similar to that of the general population.12 Few conclusive studies regarding testosterone replacement and prostate cancer are available, so caution should be taken. Men aged 40 years and older who have a prostate specific antigen (PSA) level greater than 0.6 ng/mL should have a digital rectal examination (DRE) at baseline as well as regular PSA assays at 3 to 6 months and then in accordance with current prostate cancer screening guidelines. A rise in PSA greater than 1.4 ng/mL per year with the detection of prostate abnormalities on DRE warrants a complete urologic consultation.12 For men who have a history of or who are newly diagnosed with prostate cancer, testosterone replacement should be avoided.
Table 2.
Contraindications to testosterone replacement
Erythrocytosis is frequently a common adverse event associated with testosterone replacement therapy. Calof et al13 conducted a meta-analysis of randomized, placebo-controlled trials involving adverse effects of testosterone replacement in middle-aged and older men. The studies differed in terms of testosterone formulations (patch, injectable esters, gel, and oral undecanoate) and doses. This meta-analysis concluded that men receiving testosterone replacement are roughly 4 times more likely to develop erythrocytosis (odds ratio [OR], 3.69; 95% CI, 1.82–7.51) compared to men who did not receive testosterone replacement. This increase in red blood cells may make the blood more viscous and predispose individuals to thrombosis. Elevated hematocrit levels have been associated with an increased risk for cardiovascular events, such as myocardial infarction (MI), stroke, and coronary heart disease.2 When prescribing testosterone, hematocrit levels should be evaluated at baseline, at 3 to 6 months, and then every year thereafter. Levels greater than 54% may imply overtreatment, abuse, or some other underlying pathology.9
The reported effects of testosterone replacement therapy on lipids have been mixed. A meta-analysis by Fernández-Balsells et al14 focused on adverse events of testosterone replacement therapy (including oral, transdermal, and intramuscular formulations). In terms of lipid abnormalities, the authors found correlation between testosterone therapy and a slight reduction in high-density lipoprotein (HDL), with a weighted mean difference of −0.49 mg/dL (95% CI, −0.85 to −0.13). Effects on low-density lipoprotein (LDL) were not significant. Until more definitive studies are available, it is hard to discern the exact effects of testosterone replacement therapy on lipid profiles.
Recently the publication of several studies has called into question the safety of testosterone replacement in terms of the incidence of MI and/or stroke. This increased risk may be especially significant among patients with preexisting comorbidities. Although no study has directly linked testosterone replacement therapy to cardiovascular death, several potential mechanisms that would explain a possible link have been described. Testosterone increases levels of the platelet aggravator thromboxane A2, increasing risk of stroke and other thrombotic events.15 Additionally, the testosterone metabolite DHT has been shown to enhance monocyte activation in the epithelium.15 This enhanced monocyte activation promotes atherosclerosis through its effects on inflammatory cytokines and matrix metalloproteinases, furthering the potential risk of stroke. Testosterone can also worsen sleep apnea, which is a known risk factor for both atherosclerosis and MI.15
The Testosterone in Older Men with Mobility Limitations trial (TOM) sought to examine the safety of testosterone replacement therapy in patients with a high prevalence of preexisting cardiovascular disease.16 The study was prematurely discontinued, because a significantly increased risk of cardiovascular events was detected among patients receiving testosterone. This randomized, parallel group, placebo-controlled, double-blinded trial included men 65 years or older who had a total serum testosterone level between 100 and 350 ng/dL. Subjects also had limitations in mobility (defined as “difficulty walking 2 blocks on a flat incline or climbing 10 steps”) and as having a score of 4 to 9 on the Short Physical Performance Battery (scale from 0–12, with 12 being best performance). The study excluded men with uncontrolled hypertension, unstable angina, or MI within 3 months of enrollment. Also excluded were any subjects with New York Heart Association Class 3 or Class 4 congestive heart failure, prostate or other active cancers, severe lower urinary tract symptoms, and/or untreated severe obstructive sleep apnea. Any patients using glucocorticoid or anabolic steroid treatment were also excluded as were those with a HGBA1C greater than 8.5%, a hematocrit greater than 48%, a prostate specific antigen level higher than 4 ng/mL, and/or a body mass index (BMI) greater than 40. Participants were stratified by age and randomly assigned to receive 10 g of transdermal gel containing either placebo or 100 mg of testosterone, applied once daily for 6 months. Two weeks after therapy initiation, if the testosterone levels were below 500 ng/dL, the dose of drug was increased to 15 g daily. If testosterone levels were greater than 1,000 ng/dL, the dose was decreased to 5 g daily. The primary outcome was any change in baseline strength involving leg press exercises. Secondary outcomes included changes in baseline chest press strength, speed while walking 50 meters, stair climbing speed/power, and a lift/lower test. A total of 209 participants were enrolled in the trial. The study was initiated in September 2005 and prematurely terminated in December 2009. Participants in the testosterone-receiving group were found to be more statistically significantly likely to experience cardiovascular events (OR, 5.8; 95% CI, 1.2–28.4). The TOM trial was limited by the exclusion of men younger than 65 years. Additionally, the study sample had a high prevalence of preexisting comorbidities (eg, hypertension, diabetes, hyperlipidemia, obesity) that likely increased risk of cardiovascular adverse events.
Etminan et al17 conducted a study using a health plan claims database provided by IMS Health to determine the rate of new-onset MIs potentially associated with testosterone replacement therapy. This retrospective case-control study identified 30,066 cases of MI and 120,264 controls. Among the MI cohort, 515 had used testosterone replacement therapy within the past year, with the most common treatment modality being the transdermal gel, followed by intramuscular injections and then transdermal patches. Unlike in the TOM trial, testosterone replacement therapy was not associated with an increased risk of MI (RR, 1.01; 95% CI, 0.89–1.16). However, a very marginal MI risk was associated with first time use of testosterone in naive patients (RR, 1.41; 95% CI, 1.06–1.87). With regard to specific testosterone formulations, the transdermal gel (RR, 1.49; 95% CI, 1.02–2.18) was associated with a statistically significant increased incidence of MI among first-time users as compared to other formulations. The strength of this retrospective study lies in its large sample size of MI cases along with the largest cohort of MI patients with documented testosterone exposure. Although the study failed to demonstrate a correlation between current testosterone use and MI, it did demonstrate a slight increased risk among first-time users.
Conversely, some recent reports have found little association between replacement therapy and cardiovascular effects. Baillargeon et al18 examined the risk of venous thromboembolism (VTE) associated with exposure to testosterone replacement therapy in men aged 40 years and older. This case-control study of 30,572 men who were 40 years old and older was conducted from January 1, 2007 to December 31, 2012. Cases were defined as men who received a primary diagnosis of VTE and were administered anticoagulant treatment within 60 days of diagnosis. Of the 30,572 men in this study, 7,643 were identified as cases and 22,929 as controls. Men exposed to testosterone therapy 15 days before the index date were not at increased risk for VTE (OR, 0.9; 95% CI, 0.73–1.12). In a subanalysis of testosterone formulations, none was associated with an increased risk of VTE (topical: OR, 0.80; 95% CI, 0.61–10.41; transdermal: OR, 0.91; 95% CI 0.38–2.16; intramuscular: OR, 1.15; 95% CI, 0.80–1.64).
In another recent study, Basaria et al19 sought to determine the impact of long-term testosterone replacement therapy on the progression of atherosclerosis. This double-blind, placebo-controlled, randomized, parallel group trial involved 308 men 60 years or older with low or low-normal testosterone levels (100–400 ng/dL). Of these 308 men, 156 randomized participants were to receive 7.5 g of 1% testosterone gel and 152 were randomized to receive placebo daily for 3 years. The primary outcomes of this trial were a measure of common carotid artery intima-media thickness and coronary artery calcium, and secondary outcomes included sexual function and health-related quality of life. Using multidetector-row computed tomography, the researchers assessed the total coronary artery calcium by taking multiple images of the coronary arteries. From the images, the arterial calcium was defined as a plaque of 3 or more pixels (area ≥1.02 mm2) with a density greater than 130 Hounsfield units. These lesions where multiplied by a density factor derived from Hounsfield units and subsequently summed together to determine the total calcium score. The results for change in carotid artery intima-media thickness in the placebo group and testosterone group were 0.010 mm per year and 0.012 mm per year, respectively (mean difference, 0.0002 mm/year; 95% CI, −0.003 to 0.003; p = .89). The rate of change in coronary artery calcium score for the placebo group and testosterone group were 41.4 Agatston units/year and 31.4 Agatston units/year, respectively (adjusted mean difference of −10.8 Agatston units/year; 95% CI, −45.7 to 24.2; p = .54). The authors concluded that testosterone therapy did not appear to effect underlying pathology that is normally associated with accelerated cardiovascular disease. Neither sexual function nor quality of life differed between the 2 treatment groups.
Several trials regarding testosterone replacement therapy have demonstrated an increased risk of cardiovascular events, such as stroke and MI. However, some trials have found no association between testosterone replacement therapy and cardiovascular risk. Given these conflicting reports, it is ultimately up to the clinician and patient to determine whether the benefits of testosterone treatment may outweigh any potential risks.
RECOMMENDATIONS
For patients with serum testosterone levels less than 300 ng/dL, replacement therapy may be considered if hypogonadal symptoms greatly interfere with quality of life. If symptoms are mild and do not affect quality of life, testosterone replacement should be deferred so as to avoid any potential cardiovascular risks and other adverse effects (eg, acne, gynecomastia, etc). For those receiving testosterone replacement therapy, counseling regarding the potential risks of increased MI or stroke must be provided. Continual monitoring is required to ensure adequate treatment and prevention of adverse effects (see box, Testosterone Monitoring). Research regarding the role of replacement therapy has reported varying results. Some studies (ie, TOM trial) were prematurely halted due to detection of an increased incidence of cardiovascular events. Other studies have demonstrated only small increases in MI risk among testosterone-receiving naive patients. Some studies have found no link between replacement therapy and the underlying pathology associated with accelerated cardiovascular disease or VTE. Nevertheless, benefits of testosterone replacement therapy should clearly outweigh risks especially in males with a prior history of MI or coronary artery disease. Until studies provide a definitive answer, testosterone replacement therapy should be used with caution and at the lowest possible dose.
CONCLUSION
Testosterone replacement is often prescribed for individuals with subtherapeutic levels who may or may not also be experiencing associated symptomology. Replacement can be accomplished through use of a variety of formulations, each with its own distinctive advantages and disadvantages. Any patient prescribed testosterone replacement should be assessed for prostate cancer risk through the combined use of a PSA level and DRE. Recently several small studies have reported a potential association between testosterone replacement and cardiovascular complications. Until larger and more definitive studies are completed, clinicians should be advised to use testosterone replacement conservatively and only after careful assessment of cardiovascular risk.
REFERENCES
- 1. Corona G, Rastrelli G, Forti G, Maggi M. Update in testosterone therapy for men. J Sexual Med. 2011; 8: 639– 654. [DOI] [PubMed] [Google Scholar]
- 2. Cunningham GR, Toma SM. Why is androgen replacement in males controversial? J Clin Endocrinol Metabol. 2010; 96: 38– 52. [DOI] [PubMed] [Google Scholar]
- 3. Dudgeon W, Phillips K, Carson J. et al. Counteracting muscle wasting in HIV-infected individuals. HIV Med. 2006; 7: 299– 310. [DOI] [PubMed] [Google Scholar]
- 4. Basaria S. Male hypogonadism. Lancet. 2014; 383: 1250– 1263. [DOI] [PubMed] [Google Scholar]
- 5. Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med. 2011; 124( 7): 578– 587. [DOI] [PubMed] [Google Scholar]
- 6. Manson J. Current recommendations: What is the clinician to do? Fertility Sterility. 2014; 101( 4): 916– 921. [DOI] [PubMed] [Google Scholar]
- 7. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2006; 295: 1288– 1299. [DOI] [PubMed] [Google Scholar]
- 8. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metabol. 2008; 93: 68– 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abadilla KA, Dobs AS. Topical testosterone supplementation for the treatment of male hypogonadism. Drugs. 2012; 72( 12): 1591– 1603. [DOI] [PubMed] [Google Scholar]
- 10. Rochira V, Zirilli L, Orlando G. et al. Premature decline of serum total testosterone in HIV-infected men in the HAART-Era. PLoS ONE 2011; 6( 12): 1– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crum NF, Furtek KJ, Olson PE. et al. A review of hypogonadism and erectile dysfunction among HIV-infected men during the pre- and post-HAART eras: Diagnosis, pathogenesis, and management. AIDS Patient Care STDS. 2005; 19: 655– 671. [DOI] [PubMed] [Google Scholar]
- 12. Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009; 103: 1179– 1183. [DOI] [PubMed] [Google Scholar]
- 13. Calof OM, Singh AB, Lee ML. et al. Adverse events associated with testosterone supplementation of older men. J Gerontol A Biol Sci Med Sci. 2005; 60: 1451– 1457. [DOI] [PubMed] [Google Scholar]
- 14. Fernández-Balsells HM, Murad MH, Melanie L. et al. Adverse effects of testosterone therapy in adult men: A systematic review and meta-analysis. J Clin Endocrinol Metabol. 2010; 95: 2560– 2575. [DOI] [PubMed] [Google Scholar]
- 15. Vigen R, O'Donnell CI, Barón AE. et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013; 310( 17): 1829– 1836. [DOI] [PubMed] [Google Scholar]
- 16. Basaria S, Coviello AD, Travison TG. et al. Adverse events associated with testosterone administration. N Engl J Med. 2010; 363( 2): 109– 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Etminan M, Skeldon SC, Goldenberg SL. et al. Testosterone therapy and risk of myocardial infarction: A pharmacoepidemiologic study. Pharmacotherapy. 2015; 35: 72– 78. [DOI] [PubMed] [Google Scholar]
- 18. Baillargeon J, Urban RJ, Morgentaler A. et al. Risk of venous thromboembolism in men receiving testosterone therapy. Mayo Clinic Proceed. 2015; 90( 8): 1038– 1045. [DOI] [PubMed] [Google Scholar]
- 19. Basaria S, Harman S, Travison TG. et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: A randomized clinical trial. JAMA. 2015; 314( 6): 570– 581. [DOI] [PubMed] [Google Scholar]


