Abstract
Purpose: To determine the impact of a pharmacist-driven medication therapy management (MTM) program for patients receiving oral chemotherapy agents.
Methods: We assessed the impact of MTM consultations with a pharmacist for patients who were receiving a new prescription for an oral chemotherapy agent. Data were assessed for outcomes including (1) number of medication errors identified in electronic medical records (EMRs), (2) number of interventions performed by the pharmacist, (3) time spent on the MTM process, and (4) patient satisfaction. Data were compared between patients who received their oral chemotherapy agents from the onsite specialty pharmacy or from a mail-order pharmacy. The data were also examined for correlations, and logistic regression was utilized to determine the largest variant cofactor to create an equation for estimating the number of errors in a patient's EMR.
Results: Fifteen patients received an MTM consultation, and the pharmacists identified an average of 6 medication EMR errors per patient. There was an average of 3 pharmacist-led interventions per patient. Multiple significant correlations were noted between the variables: (1) total number of prescriptions a patient was taking, (2) total number of medication errors identified, (3) time spent on the MTM process, and (4) total number of interventions performed by the pharmacist. Patient satisfaction was favorable for the program.
Conclusion: The implementation of a pharmacist-driven MTM program for patients receiving a prescription for an oral chemotherapy agent had a significant impact on patient care by improving medication reconciliation, identifying drug-related problems, and strengthening pharmacist-patient interactions in the oncology clinic.
Keywords: chemotherapeutics, medication therapy management, MTM, oral antineoplastic agents, oral chemotherapy agents, oral chemotherapeutics
In recent years, there has been a large rise in the approval and utilization of oral chemotherapy agents. This is partially due to the increasing worldwide burden of cancer, which has led to greatly improved services that oncologists provide for patients diagnosed with a neoplastic disease.1–6 There has been a drastic rise in the number of new medication approvals that has added to the list of existing agents. There are now oral chemotherapy agents available for the treatment and/or chronic suppression of local and metastatic cancers, whereas previously medication options were limited.7–9 Patients may take these medications for years, in their own homes or in an outpatient clinical setting such as a community oncology center. This transition has been exciting for patients and for practitioners in the field of oncology; however, these newer oral chemotherapy agents have created numerous challenges such as nonadherence, early discontinuation, and safety concerns for patients and their caregivers. 10–14 In addition to the enormous cost, these medications have many significant adverse reactions and drug-drug or drug-food interactions, which can directly affect patient safety, adherence, and treatment success.5,14–17 Additionally, some dosing regimens are complicated, and patients can become confused about dosing instructions when these high-risk prescriptions are filled at pharmacies (such as mail-order facilities) where there is no face-to-face relationship with a pharmacist.5,18
Medication therapy management (MTM), a personalized, unique, one-on-one clinical service offered by a pharmacist, may play a critical role in helping facilitate the safe and effective use of oral antineoplastic agents. Previous studies have shown that MTM consultations impact patient care by identifying, preventing, and resolving medication-related problems.1,19 Multiple insurance agencies have acknowledged the positive impact of this service and are reimbursing pharmacies or individual pharmacists to reduce overall drug costs and decrease hospital admissions.20,21 MTM also empowers patients to take a more active role in managing their medications, which is a critical aspect of improving our health care system.22
There are limited studies examining the effects of an MTM program for patients who are undergoing antineoplastic therapy with an oral chemotherapeutic agent. This pilot study was undertaken to address the identified gap in the literature and to achieve the following goals:
Review the subjects' demographics as a single group and compare the baseline data of the individuals required to receive their chemotherapeutic agents from a specialty mail-order pharmacy to those who were able to use the clinic's outpatient pharmacy to obtain their medications.
Report and analyze the characteristics of a small group of patients diagnosed with a neoplastic disease who are undergoing treatment with an oral chemotherapeutic agent.
Compare multiple MTM data sets and describe the observed trends and ways to improve on negative outcomes in the future.
Describe the number of errors discovered through the MTM consultations in each patient's medication record via a predictive equation.
METHODS
The pilot study was conducted between February 1, 2015 and June 2, 2015 at 3 independent oncology clinics associated with a community-owned, 254-bed institution located in the northwest United States. Patients at the cancer clinics undergoing instruction for their oral chemotherapy regimen by a nurse navigator were offered a face-to-face consultation with an oncology pharmacist for MTM. Patients who opted out of their MTM consultation, did not show up for their visit, or were not offered a visit for any reason were excluded from participating in the pilot study (Figure 1).
Figure 1.
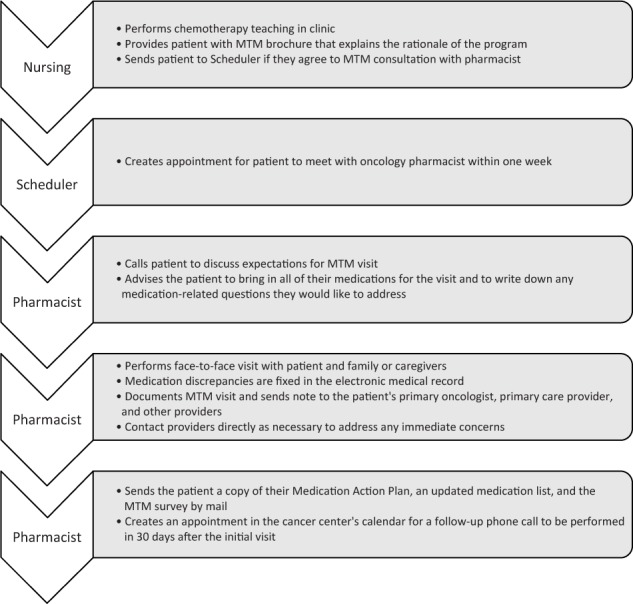
Medication therapy management (MTM) process.
Nurse navigators and pharmacists who participated in this pilot study were required to attend an MTM seminar and a staff meeting about incorporating the process into regular clinic workflow. The nurse navigators were all certified by the Oncology Nursing Society. Pharmacists were required to complete at least one training session with the primary investigator prior to conducting MTM consultations alone. Some pharmacists were already integrated into the oral chemotherapy process through regular development of patient and nursing education handouts or new drug presentations.
One day prior to the face-to-face MTM consultation, the oncology pharmacist contacted the patient by phone to explain the purpose of MTM; to request that all current prescription medications, over-the-counter (OTC) agents, and supplements be brought in; and to encourage the patient to write down all questions and/or concerns for discussion. The MTM consultation was performed on the following day by an oncology pharmacist, with or without the primary investigator present.
The protocol for the project was not reviewed by the institutional review board at the study site due to the objectives being described as quality control. All patients at the study site undergoing treatment with an oral chemotherapy agent were offered an MTM visit and had the right to decline it. Included patients verbally approved their participation and attended an MTM session before the final data were collected. Results of the study were examined retrospectively with all personal health information de-identified before a decision was made to author a manuscript. The institution allowed the review of all de-identified patient data for outcomes and results. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.23
Data were extracted retrospectively by the primary investigator through manual chart review of the electronic medical record (EMR) system. De-identified patient data collected included age, gender, creatinine clearance, cancer type, and the type of pharmacy providing the oral chemotherapeutic agent(s) (mail-order vs onsite specialty pharmacy). Additional data collected on a per-patient basis for the outcome analysis included the number of active medications, number of medication errors identified during the reconciliation process, number of interventions performed by the pharmacist, and time spent face-to-face with the patient. Time allocated to the entire MTM process included preparation prior to the visit, the MTM visit itself, and documentation/follow-up. Interventions performed by the pharmacist included oral chemotherapy education, safe handling techniques for hazardous drugs, oral chemotherapy dosage adjustments, medication interaction mitigation recommendations, immunization recommendations, and a multitude of accepted recommendations impacting medication therapy and/or patient safety.24
All MTM encounters were documented by the pharmacist and forwarded to the primary oncologist and the patient's primary care physician, as well as other providers as needed. The patient was mailed a typed copy of the updated medication list, a medication action plan, and a 10-question survey about the MTM process.
Data were transformed and basic statistics were analyzed using Microsoft Excel within Microsoft Office Professional Plus 2010 (version 14.0.7140.5002; Redmond, WA) before the patient information was transferred to IBM SPSS Statistics (version 23; IBM, Armonk, NY). Between-group differences were analyzed using a 2-tailed Student's t test in Microsoft Excel; differences were reported as decimals with a p value of less than .05 being considered significant. SPSS was then utilized to run the more complex statistical tests such as the Pearson correlation coefficient (PCC), logistic regression, and analysis of variance (ANOVA). PCC was used to analyze the majority of the recorded data as the individual measured components within the data field were compared to identify significant correlations. A linear regression model was run using ANOVA; the dependent variable was the number of medication record errors that were found and addressed during the MTM encounter. Other various regression models were analyzed prior to writing the final equation.
RESULTS
The trial consisted of a total of 15 patients who were prescribed an oral chemotherapeutic medication. The median age of patients included in the study was 75 years, including 8 males (53.3%) and 7 females (46.7%) (Table 1). There was a significantly higher percentage of males than females among patients who received their oral chemotherapy from the institution's pharmacy, compared to those who, as required by their pharmacy benefits manager, used a mail-order service (100% vs 22.2%, respectively; p < .001) (Table 1). A significantly higher median creatinine clearance of 85.5 mL/min was observed in the patients who picked up their medications from the institutional pharmacy compared to a median creatinine clearance of 52 mL/min for those in the mail-order group (p = .03) (Table 1). Study patients were receiving a median of 12 medications; patients in the institutional pharmacy group were on a median of 11.5 medications, whereas those receiving their medications via a mail-order pharmacy were on a median of 13 medications (Table 1).
Table 1.
Total study population demographics whole and grouped by where the individual patients were allowed to receive their oral chemotherapy agents (mail-order vs institutional pharmacy) at the time of the medication therapy management (MTM) encounter
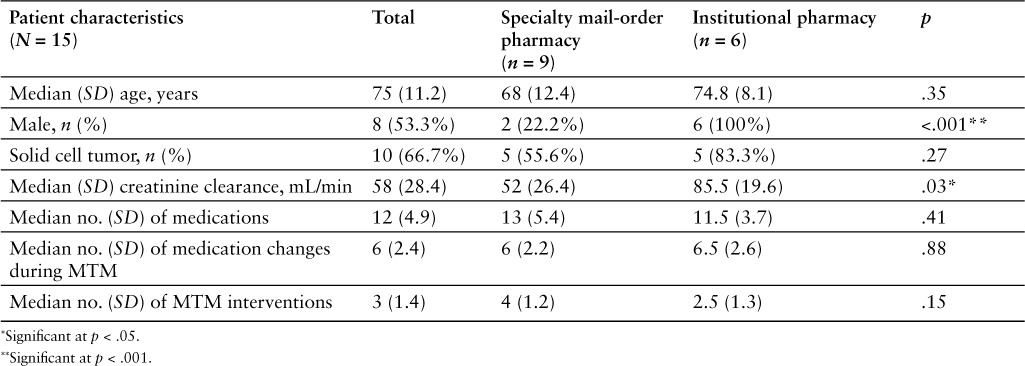
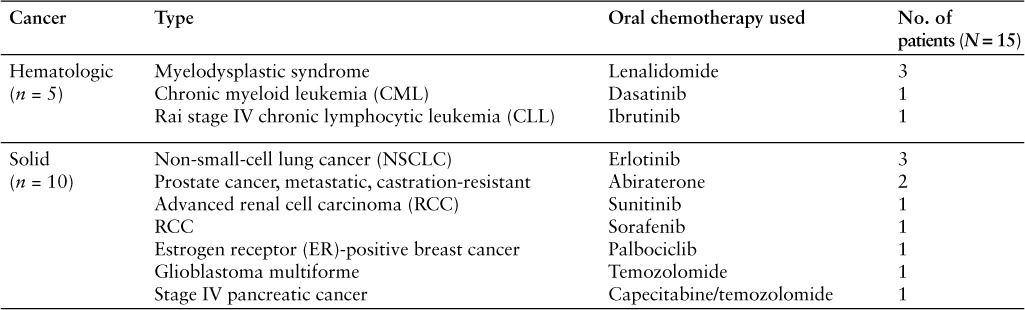
Of the total study patient population, there were significantly more patients diagnosed with a solid cell cancer (n = 10), such as renal cell carcinoma, than those diagnosed with a hematologic malignancy (n = 5), such as myelodysplastic syndrome (p = .015) (Table 2). However, there was no significant difference in the percentage of patients diagnosed with a solid cell tumor when pharmacy types were compared. Solid cell cancer patients constituted 83.3% of patients receiving medications from an institutional pharmacy and 55.6% of patients receiving medications from a specialty mail-order pharmacy (p = .27) (Table 1). The most common medication utilized for the treatment of solid cell cancer was erlotinib (n = 3), followed closely by abiraterone (n = 2). The most common medication used in the hematologic malignancies group was lenalidomide (n = 3). All other oral chemotherapeutic medications were used by one patient each (Table 2).
Table 2.
Types of malignancies included in the study and the agents used for cancer chemotherapy
MTM encounters resulted in a median of 6 medication changes performed by the clinical pharmacist; there was no significant difference in the number of medication changes between those who received their oral chemotherapeutic medications from the institutional pharmacy versus a mail-order pharmacy (6.5 vs 6 changes, respectively; p = .88) (Table 1). There was also a median of 3 pharmacist-led medical interventions performed during each MTM session; patients who were able to pick up their medications at the clinic's specialty pharmacy had fewer interventions per MTM session than did those who received their medications from a mail-order pharmacy (2.5 vs 4, respectively; p = .15) (Table 1). The most common intervention was the recommendation of a vaccination, such as a Streptococcus pneumonia vaccine (eTable 1).
There were multiple correlations noted throughout the study period; many of which were related to the total amount of time spent on the MTM process (eTable 2). The following 4 endpoints were significantly impacted by various study factors:
Number of errors identified in a patient's EMR (Table 3A)
Total number of prescribed medications for a single patient (Table 3B)
Total amount of time that was spent on the MTM encounter (Table 3C)
Time directly spent with a patient during the MTM session (Table 3D)
Table 3.
Correlates evaluated during the pilot study
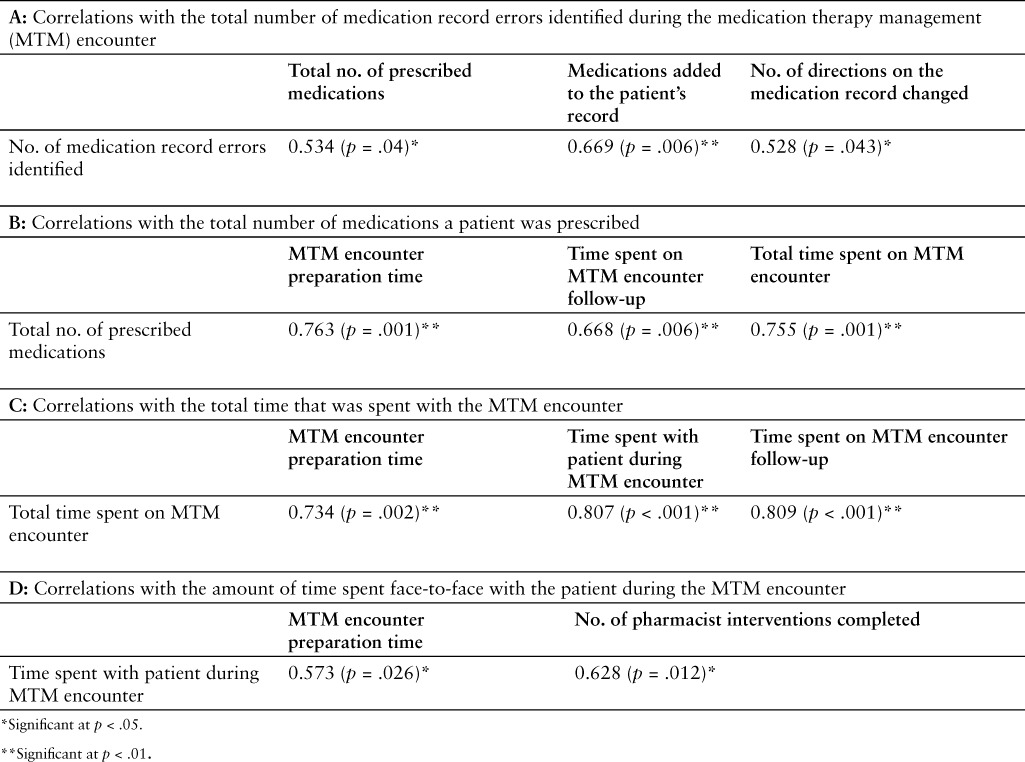
Table 3A shows a positive relationship between the number of medication errors identified during the MTM session and the total number of medications that the study participant was prescribed (PCC = 0.534; p = .04), the number of medications that were added to the patient's EMR (PCC = 0.669; p = .006), and the number of prescription directions that required correction on an individual's EMR (PCC = 0.528; p = .043). The total number of medications that an individual patient was prescribed (Table 3B) was significantly related to the amount of time that pharmacists spent preparing for the MTM encounter (PCC = 0.763; p = .001), the total amount of time that was spent on the follow-up after the MTM encounter (PCC = 0.668; p = .006), and the total amount of time that was spent on the entire MTM process (PCC = 0.755; p = .001). The total amount of time that was spent on the entire MTM process (Table 3C) was significantly related to the number of medications that an individual patient was prescribed (PCC = 0.734; p = .002), the amount of time that was spent face-to-face with the patient during the MTM session (PCC = 0.807; p < .001), and the amount of time that the pharmacist spent following up after completion of the MTM encounter (PCC = 0.809; p < .001). Finally, the amount of time directly spent with the patient during the MTM session (Table 3D) was significantly related to the amount of time spent preparing for the MTM encounter (PCC = 0.573; p = .026) and the number of interventions that the pharmacist completed during the follow-up to an MTM encounter (PCC = 0.628; p = .012).
In addition to the significant correlations, there were multiple trends noted. The total amount of time spent preparing for the MTM encounter trended toward an association with the time spent face-to-face with the patient (PCC = 0.441; p = .1). Also, the number of medication direction modifications made by the pharmacist was marginally linked with the total number of medications that the patient was prescribed (PCC = 0.486; p = .079). Finally, the total amount of time spent on the entire MTM process trended toward correlation with the total number of interventions that the pharmacist made (PCC = 0.49; p = .064).
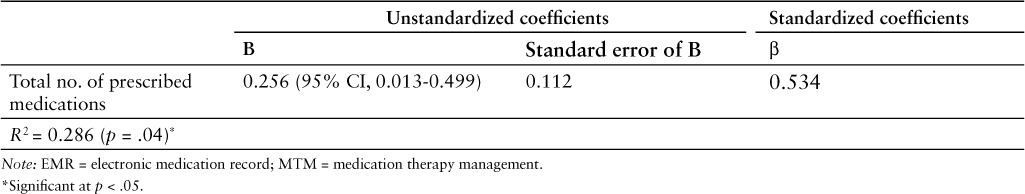
A linear regression model was used to develop an equation to predict the number of EMR errors that could potentially be identified and corrected by the clinical pharmacist conducting the MTM session (Table 4). A total of 28.6% of the variance in the number of medication errors identified by the pharmacist during the MTM meeting was attributable to the total number of medications that the patient was prescribed (R2 = 0.286; standard error [SE] = 2.145). The unstandardized coefficient (B) for the total number of medications that the individual patients were prescribed was found to be 0.256 (95% CI, 0.013 to 0.499; SE = 0.0112; p = .04). The standardized coefficient (β) for the number of medications that a patient was prescribed was found to be 0.534, while the constant's unstandardized coefficient was 3.337 (95% CI, 0.07–6.594; SE = 1.508; p = .045). Based on the regression model, the number of medication errors in a patient's medical record could be predicted by the following equation:
Table 4.
Regression model for predicting the number of medication based EMR errors that can be identified during an MTM session
The overall patient satisfaction of the pilot study was measured using a mail-in survey, completed by 7 of the 15 participants (46.7%) (eTable 3). Based on survey data, patients felt that the majority of their questions and concerns were answered during the MTM consultation. The participants felt that any possible medication interaction was addressed. Patients expressed that they knew the person to call if they had questions or problems with their oral chemotherapeutic medications, that they knew exactly how to take their medications, and that they were more comfortable taking their medications following the MTM visit with the pharmacist. The patients perceived the MTM consultation to be educational and the pharmacist to be professional, pleasant, and helpful. Finally, the participants felt that the MTM session with a pharmacist played an important role in the management of their respective neoplastic diseases and that they would recommend it to anyone newly starting an oral chemotherapeutic medication.
DISCUSSION
The increased utilization of oral chemotherapy and the ongoing revolution in oncology pharmacy workflow has encouraged pharmacists to find new ways to optimize medication therapy and improve patient safety. Patients who are prescribed these high-risk medications often have multiple health care providers and numerous prescriptions, which can affect their chemotherapy regimen. Many of these patients are obligated to use a mail-order pharmacy, which fails to provide personal interaction with the pharmacist dispensing the drug. This may create situations in which patients are filling prescriptions for oral chemotherapy agents that are expensive and often have complicated dosing regimens. In response to these factors, we set out to determine the benefits of MTM for patients starting a new prescription to evaluate the potential impact on medication reconciliation, clinical interventions, and patient satisfaction.
One of the most critical findings in this study was the statistical impact that pharmacists had on the medication reconciliation process. Our data illustrate that the oncology pharmacist was able to make an average of 6 medication changes per patient in their EMR. This significant number of errors could have an impact on the identification of drug interactions, drug-related problems, and potentially inappropriate medications. This process allowed us to provide patients with an accurate medication list they could use during their transitions of care. We used a regression model to develop an equation to predict the number of medication errors in a patient's EMR; this could be of use to a provider who wants to identify patients who would most likely benefit from an MTM visit with a pharmacist prior to starting a new oral chemotherapy agent.
In our pilot study of 15 patients, pharmacists performed a median of 3 interventions per patient; some of these were critical for patient safety. In one of the consultations, a frail 79-year-old female who had been experiencing dizziness and recent falls in the middle of the night was found to be taking zolpidem 12.5 mg extended-release and diazepam 5 mg for her insomnia. Neither medication was on her medication list, and they were potentially inappropriate per Beer's Criteria.25 In another consultation performed for a patient who had recently completed multiple cycles of lenalidomide (21 days on and 7 days off) prior to the study, it was identified that the patient was only taking rivaroxaban for her deep vein thrombosis on the active days of her chemotherapy cycle, rather than on a daily basis. Two out of 15 patients (13.3%) in this study had their chemotherapy held or their dosage adjusted as a result of poor creatinine clearance identified during the MTM visit. Both patients had already received the oral chemotherapy agent from a mail-order pharmacy and started taking their prescriptions at the highest prescribed dose, which is 250% higher than their renal-adjusted dose. For all of these scenarios, it is possible that these critical errors could have gone undetected and posed significant patient harm without the MTM consultation.
Based on follow-up survey data, the MTM sessions were well received by the patients. A total of 7 patients mailed in their responses; the surveys strongly indicated that patients felt that the MTM session was important for the management of their cancer and that they would recommend it to any patient starting an oral chemotherapy agent. Many patients stated that they had been looking for a service like MTM because they were overwhelmed by their medication regimens and wanted to ensure they were avoiding drug interactions and side effects as much as possible.
Our data show multiple statistically significant links between important variables in an MTM session, such as the total number of medication errors in the EMR, total number of active medications, time spent on the MTM, and the number of interventions performed. This information could be used in the future to schedule an appropriate amount of time for an MTM session. The correlation between the total amount of time spent on the MTM session and the number of interventions performed may be useful for implementing a similar program in an oncology clinic.
The most evident limitations to this study include the small sample size, the single institution of study, and lack of intensive standardized training for pharmacists conducting the MTM. Variance in the background training or work experience of the pharmacists may have affected clinical interventions and patient interaction. There was no control group in this study, and the 2 arms were divided up solely based on the patient's insurance or pharmacy benefit manager who covered the oral chemotherapy agent. In the future, randomized, multicenter studies should be performed to decrease potential bias and to expand patient demographics.
To determine the full benefits of an MTM program for patients receiving a new prescription for an oral chemotherapy agent, multiple long-term and large sample sized studies are needed. It would be useful to evaluate the effects of MTM on patient outcomes such as overall survival and disease-free progression. Additionally, it would be interesting to evaluate the effects on oral chemotherapy medication adherence and chemotherapy-induced side effects. Due to the expense of oral chemotherapy agents, it would also be important to evaluate a cost-benefit analysis of utilizing the time of a pharmacist to perform such MTM consultations.
Despite the limitations to our study, we have experienced phenomenal success with this project. Our oncology clinic has changed their pharmacist scheduling to accommodate a new shift that focuses on improving inpatient and ambulatory care services through multidisciplinary inpatient rounding, regular MTM appointments, and other patient education activities. As a result, we have seen a 6-fold increase in the number of MTM visits per month and we are starting to expand into other specialty services such as rheumatology. We have completed a lean-process meeting pertinent to our process and continue to receive patient satisfaction surveys to identify areas of improvement.
Supplementary Material
REFERENCES
- 1. Ramalho de Oliveira D, Brummel AR, Miller DB. Medication therapy management: 10 years of experience in a large integrated health care system. J Manag Care Pharm. 2010; 16( 3): 185– 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weingart SN, Spencer J, Buia S. et al. Medication safety of five oral chemotherapies: A proactive risk assessment. J Oncol Pract. 2011; 7( 1): 2– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khandelwal N, Duncan I, Ahmed T. et al. Impact of clinical oral chemotherapy program on wastage and hospitalizations. J Oncol Pract. 2011; 7( 3 suppl): e25s– e29s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bubalo J, Warden BA, Wiegel JJ. et al. Does applying technology throughout the medication use process improve patient safety with antineoplastics? J Oncol Pharm Pract. 2014; 20( 6): 445– 460. [DOI] [PubMed] [Google Scholar]
- 5. Wong SF, Bounthavong M, Nguyen C. et al. Implementation and preliminary outcomes of a comprehensive oral chemotherapy management clinic. Am J Health Syst Pharm. 2014; 71( 11): 960– 965. [DOI] [PubMed] [Google Scholar]
- 6. Fitzmaurice C, Dicker D, Pain A. et al. The global burden of cancer 2013. JAMA Oncol. 2015; 1( 4): 505– 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma S, Saltz LB. Oral chemotherapeutic agents for colorectal cancer. Oncologist. 2000; 5( 2): 99– 107. [DOI] [PubMed] [Google Scholar]
- 8. Aisner J. Overview of the changing paradigm in cancer treatment: Oral chemotherapy. Am J Health Syst Pharm. 2007; 64( 9 suppl 5): S4– 7. [DOI] [PubMed] [Google Scholar]
- 9. Hematology/oncology (cancer) approvals & safety notifications. www.fda.gov. Last update August 18 2015. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm. Accessed August 21, 2015.
- 10. Barron TI, Connolly R, Bennett K. et al. Early discontinuation of tamoxifen: A lesson for oncologists. Cancer. 2007; 109( 5): 832– 839. [DOI] [PubMed] [Google Scholar]
- 11. Owusu C, Buist DS, Field TS. et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008; 26( 4): 549– 555. [DOI] [PubMed] [Google Scholar]
- 12. Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009; 59( 1): 56– 66. [DOI] [PubMed] [Google Scholar]
- 13. Hershman DL, Kushi LH, Shao T. et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010; 28( 27): 4120– 4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lau PM, Stewart K, Dooley M. The ten most common adverse drug reactions (ADRs) in oncology patients: Do they matter to you? Support Care Cancer. 2004; 12( 9): 626– 633. [DOI] [PubMed] [Google Scholar]
- 15. Curtiss FR. Pharmacy benefit spending on oral chemotherapy drugs. J Manag Care Pharm. 2006; 12( 7): 570– 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Segal EM, Flood MR, Mancini RS. et al. Oral chemotherapy food and drug interactions: A comprehensive review of the literature. J Oncol Pract. 2014; 10( 4): e255– e268. [DOI] [PubMed] [Google Scholar]
- 17. Oral chemotherapy: What you need to know. www.cancer.org. Last update July 17, 2014. http://www.cancer.org/treatment/treatmentsandsideeffects/treatmenttypes/chemotherapy/oral-chemotherapy. Accessed August 26, 2015.
- 18. Bindler RJ. Inpatient and outpatient pharmacy monitoring of oral antineoplastic medications [Editorial]. Hosp Pharm. 2015; 50( 2): 91– 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holle LM, Puri S, Clement JM. Physician-pharmacist collaboration for oral chemotherapy monitoring: Insights from an academic genitourinary oncology practice [published online ahead of print April 21, 2015]. J Oncol Pharm Pract. [DOI] [PubMed] [Google Scholar]
- 20. The hidden value of MTM. www.mirixa.com. Last update 2012. http://www.mirixa.com/#/mtm/overview. Accessed August 26, 2015.
- 21. Pharmacy: Overview. www.outcomesmtm.com. Last update 2015. http://www.outcomesmtm.com/pharmacy-overview.aspx. Accessed August 26, 2015.
- 22. Moczygemba LR, Barner JC, Brown CM. et al. Patient satisfaction with a pharmacist-provided telephone medication therapy management program. Res Social Adm Pharm. 2010; 6( 2): 143– 154. [DOI] [PubMed] [Google Scholar]
- 23. World Medical Association. . World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013; 310( 20): 2191– 2194. [DOI] [PubMed] [Google Scholar]
- 24. Goodin S, Griffith N, Chen B. et al. Safe handling of oral chemotherapeutic agents in clinical practice: Recommendations from an international pharmacy panel. J Oncol Pract. 2011; 7( 1): 7– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fick DM, Semla TP, Beizer J. et al. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults [published online ahead of print October 8, 2015]. J Am Geriatr Soc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


