Abstract
Background: There are varying dosing strategies for the administration of benzodiazepines in the setting of alcohol withdrawal. In October 2014, a symptom-based alcohol withdrawal protocol (AWP) using the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar) scale was implemented at one institution.
Objective: To evaluate the safety and efficacy of the AWP.
Methods: Retrospective chart review was completed, including patients receiving at least one dose of diazepam for alcohol withdrawal pre- and post-protocol. The primary outcome of this study was the average daily and cumulative dose of diazepam during hospital stay. Secondary outcomes included length of stay and occurrence of seizures or delirium tremens.
Results: The average daily dose and the average cumulative dose of diazepam were significantly lower in the post-protocol group (5.4 vs 12.1 mg, p < .001; 35.0 vs 77.6 mg, p < .001, respectively). Length of stay was similar between groups (6.5 vs 6.4 days, p = .91), however, duration of benzodiazepine use was decreased in the post-protocol group (2.2 vs 4.7 days, p < .001). Despite using reduced doses of benzodiazepines, there was no increase in adverse events.
Conclusions: The implementation of a symptom-based AWP using the CIWA-Ar scale was associated with a reduced average daily and cumulative dose of diazepam without any apparent safety issues.
Keywords: alcohol, benzodiazepine, protocol, withdrawal
Alcohol abuse continues to have a noteworthy impact, both in the United States and across the globe. In the United States, there are approximately 8.2 million persons currently dependent on alcohol and the lifetime prevalence of alcohol abuse or dependence is 13.6%.1,2 Furthermore, in 2011, the World Health Organization estimated that there were 2.5 million deaths worldwide related to alcohol consumption.3 While certainly not all of these deaths are related to complications of alcohol withdrawal, these statistics clearly illustrate the considerable impact alcohol has on our society.
Long-term exposure to ethanol results in changes to the neurotransmitter systems within the central nervous system (CNS). Chronic exposure leads to reduced levels of the inhibitory neurotransmitter gamma aminobutyric acid (GABA) and reduced sensitivity of the GABA receptor. In addition, chronic ethanol exposure increases activity of the excitatory neurotransmitter glutamate and increases activity of the N-methyl-D-aspartate (NMDA) receptor. These neurochemical changes remain upon abrupt discontinuation of alcohol use, leading to characteristic symptoms of alcohol withdrawal syndrome (AWS).3 Initial symptoms typically present within the first 24 hours and may include mild tremor, nausea, vomiting, and irritability. Withdrawal seizures occur approximately 48 hours after cessation and are often tonic-clonic in nature. Of those who experience an alcohol withdrawal seizure, two-thirds will experience multiple seizures and one-third will progress to delirium tremens (DTs). Hallucinosis generally presents 2 to 3 days after alcohol cessation and is characterized by the presence of predominantly auditory hallucinations. Unlike DTs, patients experiencing alcoholic hallucinosis generally remain alert and oriented with stable vital signs. The final and most fatal stage of alcohol withdrawal is DTs. Hallmark symptoms of DTs include autonomic instability, confusion and disorientation, and hallucinations (including auditory, visual, and tactile). Patients are at highest risk for DTs approximately 4 to 5 days after alcohol cessation. Without appropriate medical care, DTs are associated with a 15% to 20% mortality rate. Death typically results from physical or medical complications including arrhythmia, electrolyte abnormalities, fever, hypertension, or suicide secondary to hallucinations and delusions.4
Benzodiazepines have long been established as the first-line treatment option for the management of AWS. This class of medications has demonstrated reduction in the occurrence of seizures and DTs when compared to placebo.2 Benzodiazepines exert their pharmacologic action at the type A GABA receptor, which is present in high concentrations in both the cortex and limbic system. The binding of GABA to this ligand-gated chloride channel results in hyperpolarization of the neuronal membrane and is associated with inhibitory signaling pathways. Benzodiazepines serve to enhance the typical inhibitory activity of GABA at these receptors. The net result of this action is reduced neuronal excitability; in the setting of alcohol withdrawal, this results in a reduced rate of alcohol withdrawal seizures and DTs.5 Although there has not been any evidence to suggest one benzodiazepine is superior to any other within the class, diazepam is frequently selected for the management of AWS as its long elimination half-life (t1/2 = 20 to 100 hours) and active metabolites reduce the risk of breakthrough withdrawal symptoms or seizures between doses.2
Although commonly encountered in the hospital setting, there is a lack of standardization for the treatment and monitoring of AWS. There are varying dosing strategies for the administration of benzodiazepines in patients experiencing AWS, including loading doses, tapering doses, or the administration of doses based on withdrawal symptoms (symptom-based dosing strategies). However, few studies have been published comparing outcomes with these various strategies, and practices vary widely between, and within, institutions.2,6–8 Assessment of AWS also varies, although many institutions have adopted the Clinical Institute Withdrawal Assessment for Alcohol-Revised scale (CIWA-Ar) scale. The CIWA-Ar is a well-validated scale that assesses 10 symptoms related to alcohol withdrawal including nausea or vomiting, tremor, sweating, anxiety, agitation, headache, orientation, and auditory, visual, or tactile disturbances.6 Multiple studies indicate that use of CIWA-Ar reduces overall benzodiazepine dose and mean daily dose of benzodiazepines, but they have not shown any impact on length of stay (LOS).6,9,10
Prior to October 2014, there was no standardized method of administering diazepam at the Lexington VA Medical Center to prevent or treat alcohol withdrawal. Instead, alcohol withdrawal was managed at the discretion of the prescribing practitioner. This typically resulted in the use of either a scheduled diazepam taper over 5 to 7 days or the use of a symptom-driven dosing strategy based on a previously unvalidated symptom-based scale that evaluated only 4 symptoms: tremor, postural hypotension, diaphoresis, and tachycardia. The use of these different strategies resulted in patients receiving widely varying doses of diazepam for varying durations. In addition, there was the potential to over-treat patients receiving a scheduled taper of diazepam.
After a literature search was performed to identify successful alcohol withdrawal protocols at other institutions, a facility-wide, symptom-based alcohol withdrawal protocol was created by a multidisciplinary committee comprised of physicians, pharmacists, and nurses and was implemented in October 2014.11–13 The goal was to standardize the treatment of AWS at our facility (Figure 1). A key component of this protocol was the transition to the use of CIWA-Ar to monitor for AWS, as the protocol called for the administration of diazepam based on nursing assessment of CIWA-Ar scores. The administration of diazepam was going to be nursing-driven, so extensive staff development was provided to the facility nursing staff clinical nurse educators prior to implementing the protocol. Additionally, a document was created to provide recommendations for nursing staff and providers about how to use the CIWA-Ar scale as objectively as possible. This document was posted on the facility's intranet along with a copy of the protocol and a copy of the CIWA-Ar scale for nurses to use as a reference.
Figure 1.
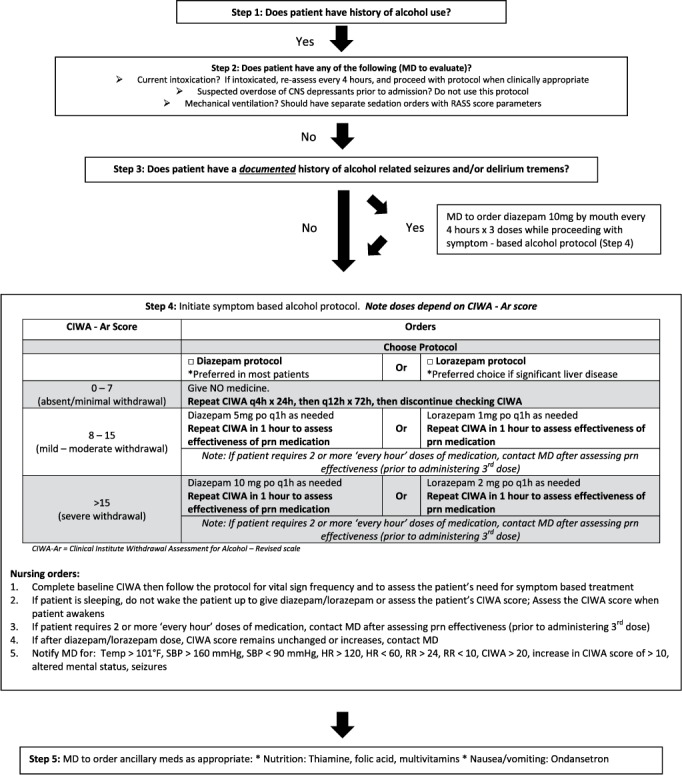
Symptom-based alcohol withdrawal protocol. CNS = central nervous system; h = hour; HR = heart rate; MD = physician; PO = oral; prn = as needed; RASS = Richmond Agitation-Sedation Scale; RR = respiratory rate; q1h = every hour; q4h = every 4 hours; q12h = every 12 hours.
This study was performed to evaluate the impact of this facility's newly implemented symptom-driven alcohol withdrawal protocol. The goal of this study was to add to the existing literature regarding the optimal dosing strategies of benzodiazepines and the utility of the CIWA-Ar scale in the setting of AWS.
METHODS
Veterans were included in this study if they were admitted to the facility and received at least one dose of diazepam for alcohol withdrawal between February 2013 and May 2013 (pre-protocol) or November 2014 and May 2015 (post-protocol). Patients were identified using the Veterans Health Information System and Technology Architecture (VISTA) system. Patients were excluded if they received diazepam for any indication other than AWS or if they were older than 89 years of age. It should be noted that this facility's protocol also includes the option of using lorazepam in patients with advanced liver disease; however, due to its infrequent use, lorazepam was not studied. Demographic data including age, race, and gender were collected for all patients. Blood alcohol level (BAL) on presentation was also collected. Retrospective chart review was performed to determine the total dose of diazepam received during admission, LOS, and occurrence of any serious consequences of withdrawal including seizures or DTs.
The primary outcome of this study was the mean daily and cumulative dose of diazepam during hospital stay. Secondary outcomes included LOS and occurrence of seizures or DTs. The primary outcome was compared using the Student's t test. Categorical data were analyzed using a chi-square test. For all statistical tests, α was set at 0.05. This study was reviewed by the facility institutional review board and research and development committee.
RESULTS
One-hundred and seventy-four patients met the inclusion criteria. Of those patients, 79 and 95 received diazepam in the pre- and post-protocol periods, respectively. In the pre-protocol group, 49.2% of patients received scheduled benzodiazepine tapers and 50.8% of patients received symptom-driven treatment. Patient characteristics between the 2 groups were similar (Table 1), with the exception that patients were significantly less likely to be treated on the psychiatric unit in the post-protocol cohort.
Table 1.
Demographic data for patients who received a least one dose of diazepam before or after implementation of a facility-wide, symptom-based alcohol withdrawal protocol (N = 174)

The mean daily and cumulative doses of diazepam were significantly lower in the post-protocol group (Table 2). LOS was similar between groups when the entire cohort was evaluated. However, LOS for patients admitted to the psychiatric unit was reduced from 6.54 days to 4.48 days (p < .001) after implementation of the protocol. Additionally, length of treatment with diazepam was significantly shorter in the post-protocol compared to the pre-protocol group for the entire cohort.
Table 2.
Outcomes associated with implementation of a facility-wide, symptom-based alcohol withdrawal protocol a

In the pre-protocol group, there were 3 documented episodes of seizure and 2 episodes of DTs. In one patient, withdrawal seizures had begun prior to admission to the facility. Another seizure was unwitnessed, however the Veteran had a history of withdrawal seizures and was found lying on the floor in what was determined to be a post-ictal state. In the post-protocol group, there were 4 documented episodes of seizure and 1 episode of DTs. Of these, 3 patients had a history of seizure disorder and were being treated with antiepileptic medications. In 2 of these cases, medication noncompliance was suspected by prescribers and was confirmed with subtherapeutic concentrations in one of the patients.
DISCUSSION
This study illustrates the benefits of implementing a symptom-based dosing strategy of diazepam for AWS utilizing the CIWA-Ar scale. Patients required significantly lower doses of diazepam for shorter periods of time after the protocol was implemented. This is consistent with the finding of other studies, which found decreased benzodiazepine use with the use of symptom-driven alcohol withdrawal protocol, and adds additional support for the implementation of such protocols.9,13–16
This is clinically relevant because decreased benzodiazepine doses lead to a lower risk of adverse events such as falls, oversedation, and respiratory depression. In addition, patients may be better able to ambulate and participate in activities such as physical therapy, decreasing the risk for complications such as venous thromboembolism and leading to better overall outcomes. However, these outcomes were not specifically evaluated in this study.
Despite the decrease in benzodiazepine usage, there was no observed change in LOS for the overall cohort involved in this study, which is consistent with the findings of other studies. 9,12,14 Even though it seems incongruent to decrease benzodiazepine use, yet not affect LOS, it should be noted that this study included a mixed population of psychiatric and nonpsychiatric patients, of whom approximately 40% were admitted primarily for indications other than alcohol withdrawal. Therefore, it is possible that LOS did not change due to the need for medical or psychiatric stabilization unrelated to AWS. Although there was no overall change in LOS, patients in the post-protocol group received diazepam for fewer days compared to the pre-protocol group, indicating that duration of AWS treatment did decrease with implementation of the protocol. Faster resolution of AWS would allow the medical or psychiatric teams to more quickly shift their attention to other comorbidities or admitting diagnoses, which would presumably result in better patient outcomes, although this was not captured in this study.
Of note, a subgroup analysis found that only patients admitted to the psychiatric floor experienced a shorter LOS in the post-protocol period. These patients also were more likely to be admitted with a primary indication of AWS, both pre- and post-protocol, compared to nonpsychiatric patients (Table 3). Prior to protocol implementation, these patients may have received a diazepam taper over 5 to 7 days, although many of them may not have truly needed that extensive taper, or any doses at all, due to relatively minor symptoms. A switch to a symptom-based protocol meant that patients only received doses when they were symptomatic; thus, after implementing the protocol, these patients may not have required any doses of diazepam based on their CIWA-Ar score, resulting in fewer patients receiving diazepam and possibly faster discharges from the psychiatric unit. The patients on the nonpsychiatric units, however, may have been admitted with a primary diagnosis unrelated to alcohol withdrawal, which required the same duration of therapy regardless of how the AWS was treated. This theory seems to be supported by the fact that the study identified a shift in location of treatment for patients needing management of AWS. Prior to implementing the protocol, 64.6% of patients who received at least one dose of diazepam were more likely to be treated on the psychiatric floor; this decreased to 43.2% post-protocol.
Table 3.
Percentage of patients admitted for primary indication of alcohol withdrawal syndrome on psychiatric vs nonpsychiatric units before and after implementation of symptom-based alcohol withdrawal protocol

It is notable that the implementation of the protocol was associated with a reduction in benzodiazepine dosing without impacting safety outcomes. This study observed no change in the frequency of seizures or DTs. Three of the seizures that occurred post-protocol were in patients with a documented seizure disorder. In 2 cases, medication noncompliance was suspected by the provider, with documented subtherapeutic concentrations in one case. These reports suggest that factors other than the protocol may have contributed to the occurrence of seizures. However, it should be mentioned that due to the relative infrequency of these serious outcomes, a larger sample size may be needed in order to detect a statistically significant change in seizures or DTs, if one were to exist.
There are some limitations of this study. First, this study did not account for patients who may have elected to discharge prior to completion of the detoxification process. This may have resulted in artificially low cumulative doses of diazepam, however this could have also occurred in the pre-protocol group, so it is unlikely that this had a large impact on the results. Additionally, this study did not evaluate the presence of comorbid medical conditions or the use of adjunct medications such as antiemetics, antiepileptics, or clonidine, which may have impacted patients' CIWA-Ar scores. Because this study was performed at a Veterans Affairs hospital and evaluated a mostly male population, the results may not be generalizable to all patient populations. This transition was also accompanied by significant staff development by clinical nurse educators. Some institutions may not have the resources for this level of training, and therefore this nurse-driven protocol may not be feasible at all institutions. Finally, this study evaluated only one benzodiazepine, diazepam; as a result, the outcomes of this study may not be generalizable to all other agents within the class.
On the other hand, this study does have a number of strengths. It adds to the available literature, suggesting that symptom-based protocol, using CIWA-Ar, decreases the use of benzodiazepines. In addition, as this protocol was utilized hospital-wide by all services, including surgical, neurological, psychiatric, critical care, and acute care services, it may serve as a useful tool to other hospitals, looking to institute a hospital-wide AWP.
CONCLUSIONS
The implementation of a symptom-based alcohol withdrawal protocol using the CIWA-Ar scale at a single institution was associated with a reduced mean cumulative and daily doses of diazepam. The use of lower benzodiazepine doses did not appear to result in an increase in the frequency of seizures or DTs. Although there was no change in the LOS, a shorter duration of treatment with benzodiazepines was observed.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest. This material is the result of work supported with resources and the use of facilities at the Lexington VA medical facility. The contents of this manuscript do not represent the views of the US Department of Veterans Affairs or the United States Government.
REFERENCES
- 1. Kosten TR, O'Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003; 348( 18): 1786– 1795. [DOI] [PubMed] [Google Scholar]
- 2. Maldonado JR, Nguyen LH, Schader EM, Brooks JO. Benzodiazepine loading versus symptom-triggered treatment of alcohol withdrawal: A prospective, randomized clinical trial. Gen Hosp Psychiatr. 2012; 34: 611– 617. [DOI] [PubMed] [Google Scholar]
- 3. Cooper E, Vernon J. The effectiveness of pharmacological approaches in the treatment of alcohol withdrawal syndrome (AWS): A literature review. J Psychiatr Ment Health Nurs. 2013: 20( 7); 601– 612. [DOI] [PubMed] [Google Scholar]
- 4. Maldonado JR. An approach to the patient with substance use and abuse. Med Clin N Am. 2013; 94: 1169– 1205. [DOI] [PubMed] [Google Scholar]
- 5. Griffin CE, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013; 13: 214– 223. [PMC free article] [PubMed] [Google Scholar]
- 6. Reoux JP, Miller K. Routine hospital alcohol detoxification practice compared to symptom triggered management with an objective withdrawal scale (CIWA-Ar). Am J Addict. 2000; 9( 2): 135– 144. [DOI] [PubMed] [Google Scholar]
- 7. Daeppen JB, Gache P, Landry U. et al. Symptom – triggered vs fixed – schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002; 162( 10): 1117– 1121. [DOI] [PubMed] [Google Scholar]
- 8. Muzyk AJ, Leung JG, Nelson S. et al. The role of diazepam loading for the treatment of withdrawal syndrome in hospitalized patients. Am J Addict. 2013; 22( 2): 113– 118. [DOI] [PubMed] [Google Scholar]
- 9. DeCarolis DD, Rice KL, Ho L. et al. Symptom-driven lorazepam protocol for treatment of severe alcohol withdrawal delirium in the intensive care unit. Pharmacotherapy. 2007; 27: 510– 518. [DOI] [PubMed] [Google Scholar]
- 10. Ng K, Dahri K, Chow I. et al. Evaluation of an alcohol withdrawal protocol and a preprinted order set at a tertiary care hospital. Can J Hosp Pharm. 2011; 64: 436– 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sullivan JT. et al. Assessment of alcohol withdrawal the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989; 1353– 1357. [DOI] [PubMed] [Google Scholar]
- 12. See S. et al. Implementation of a symptom-triggered benzodiazepine protocol for alcohol withdrawal in family medicine inpatients. Hosp Pharm. 2009; 44: 881– 887. [Google Scholar]
- 13. Grafenreed KM, Lobo B, Sands C, Yates M. Development of an alcohol withdrawal delirium prophylaxis protocol in a community teaching hospital. Am J Health Syst Pharm. 2004; 61: 1151– 1155. [DOI] [PubMed] [Google Scholar]
- 14. Stanley KM, Worrall CL, Lunsford SL, Simpson KN, Miller JG, Spencer AP. Experience with an adult alcohol withdrawal syndrome practice guideline in internal medicine patients. Pharmacotherapy. 2005; 25( 8): 1073– 1083. [DOI] [PubMed] [Google Scholar]
- 15. Taheri A, Dahri K, Chan P. et al. Evaluation of a symptom-triggered protocol approach to the management of alcohol withdrawal syndrome in older adults. J Am Geriatr Soc. 2014; 62( 8): 1551– 1555. [DOI] [PubMed] [Google Scholar]
- 16. Cassidy EM, O'Sullivan I, Bradshaw P. et al. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: A comparison with the standard fixed dose benzodiazepine regimen. Emerg Med J. 2012; 29: 802– 804. [DOI] [PubMed] [Google Scholar]


