Abstract
Background: A shortage of indigotindisulfonate sodium has led to a search for an alternative visualizing agent.
Objective: The primary objective of this study was to evaluate the potency and sterility of 10% sodium fluorescein, USP solutions stored in sterile polypropylene syringes and refrigerated.
Methods: Four samples of 10% fluorescein injection, USP were aseptically drawn up in 3 mL polypropylene syringes and stored in a refrigerator at an average temperature of 3.9°C for 7 days. After 7 days, the samples were cultured for microbial growth. Four other samples were assayed by UV/VIS spectroscopy. Concentration measurements were made at day 0 and at day 7. The pH was also measured at day 0 and day 7.
Results: There was no statistical difference between the mean sodium fluorescein concentration at day 0 and at day 7 (α = 0.05, p = .622). There was no statistical difference in the pH of the samples at day 0 and at day 7 (α = 0.05, p = .0689). There was no evidence of microbial growth in any of the samples for the duration of the study period.
Conclusions: The findings of this study demonstrate that a sterile solution of 10% sodium fluorescein, USP retained its potency and showed no signs of microbial growth for a period of 7 days when refrigerated and stored in sterile polypropylene syringes.
Keywords: cystoscopy, hysterectomy, intraoperative cystoscopy, sodium fluorescein
Fluorescence is a type of luminescence that occurs when molecules, known as flurophores, absorb electromagnetic energy exciting them to a higher energy state. As the molecules return to their original energy level, they emit light that can be visualized using special instruments.1
Fluorescence-based optical imaging technology is widely used in gynecological and urological procedures. In fact, routine intraoperative use of cystoscopy for women undergoing major gynecological surgery has been proposed as a secondary preventive measure for urinary tract injury.2 The American Association of Gynecological Laproscopists (AAGL) advances liberal use of cystoscopy with laparoscopic hysterectomy.3
Indigotindisulfonate sodium has been used with cystoscopy to detect urinary tract injury and to verify ureteral efflux during pelvic surgery. The package insert indicates that it is primarily used in locating ureteral orifices during cystoscopy and urethral catheterization. 4
In June 2014, the US Food and Drug Administration announced the shortage of indigoundisulfonate sodium. Manufacturing delays and shortage of the active ingredient were the reasons cited.5 The shortage of indigoundisulfonate sodium prompted the search for alternative agents with comparable visualization and safety profile. To date, several alternative agents have been explored, but the majority of them have been considered suboptimal by some physicians. Agents that have been tried include 50% dextrose, sterile water, methylene blue-tinged saline, intravenous methylene blue, and oral phenazopyridine.6 The agent that is ultimately used will depend on the type of procedure and physician preference.
An alternative contrast agent that has been proposed is 10% sodium fluorescein, USP.7 Sodium fluorescein is supplied as a 5 mL sterile, unpreserved, single-use aqueous solution that has a pH of 8.0 to 9.8. Sodium fluorescein responds to light between the wavelengths of 465 to 490 nm and fluoresces light at wavelengths of 520 to 530 nm.8 The molecule is excited by blue light and emits light that appears yellowish-green. In clinical practice, it has been described as a brilliant yellow color.
At our institution, the pharmacy department is faxed a surgery list indicating the number of surgery cases requiring 10% sodium fluorescein. This is done one day in advance of the surgical procedure. On the morning of surgery, pharmacy personnel draws up a 0.5 mL sample of 10% sodium fluorescein in a sterile 3 mL polypropylene syringe and takes it to the operating room receiving area. The remaining portion, 4.5 mL, is discarded. If another case presents that day, the same process is carried out. It is a wasteful, costly, and time-consuming process.
The objective of this study was to evaluate the potency and sterility of 10% solutions of sodium fluorescein, from single-dose vials, stored in sterile polypropylene syringes and refrigerated. If potency and sterility can be demonstrated, the time-consuming and costly process of discarding medication would be minimized or avoided.
MATERIALS AND METHODS
All chemicals used were of analytical grade. The 10% sodium fluorescein for injection, USP (NDC 0065-0092-65; lot: 245661f; lot 253996f) was purchased from Alcon Laboratories, Inc (Fort Worth, TX). The sodium fluorescein powder (F6377; lot: SLBL547OV) was purchased from Sigma-Aldrich (St. Louis, MO). The Tris(hydroxymethyl)aminomethane hydrochloride buffer (lot: 148024) was purchased from Fisher (USA).
Fluorescein is an extremely fluorescent compound. To use UV/VIS spectrophotometry, the fluorescein standards prepared and the study samples had to undergo a significant, 20,000–fold, dilution step. This dilution step was necessary to avoid detector saturation and allow for proper reading. In addition, a calibration curve using sodium fluorescein powder was generated. In quantitative chemical analysis, a calibration curve is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown sample to a set of standard samples of known concentration. In our study, the calibration curve was used to verify the concentration of our 10% sodium fluorescein, USP samples at day 0 and at day 7.
A stock solution of 10% (w/v) fluorescein was prepared from sodium fluorescein powder, USP. A 50 μL sample of this solution was diluted to a final volume of 1 L using a volumetric flask to obtain a 5 mcg/mL solution of fluorescein. The dilution was done with a 50 mM Tris(hydroxymethyl)aminomethane hydrochloride buffer adjusted to a pH of 8.13 to maintain stability. Study solutions were also diluted in 50mM Tris(hydroxymethyl)aminomethane hydrochloride buffer (pH 8.13).
From the 5 mcg/mL solution of fluorescein, fluorescein equivalent standards were prepared in triplicates in the following concentrations, 5, 2.5, 1.25 and 0.5 mcg/mL, and spectrophotometrically measured at 490 nm. The absorbances were plotted versus concentration to determine linearity (r = 0.999, α = 0.05, p = .0001). Absorbance measurements were obtained using Eppendorf Biospectrometer Kinetic UV-Vis spectrophotometer. Figure 1 depicts the absorbance versus concentration for each standard.
Figure 1.
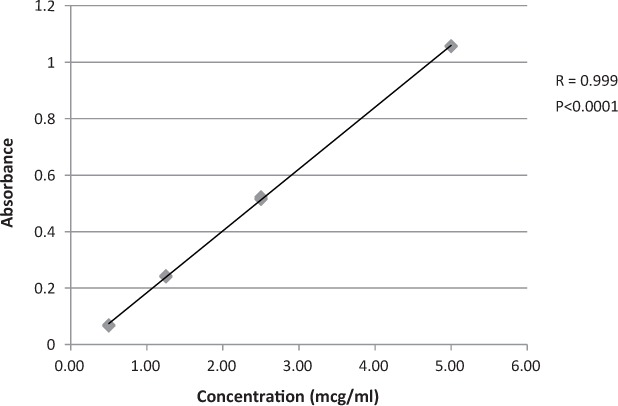
Ultraviolet absorbance versus concentration for fluorescein equivalent standards. Mean = triplicate determination of 4 standards.
Sterility Testing
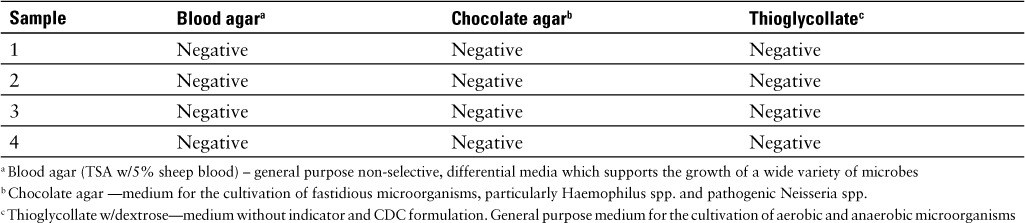
Sample collection for the microbial testing was carried out in an ISO class 6 room under and ISO class 5 laminar flow hood in accordance with the USP <797> guidelines for compounding sterile compounds. 9 Four 1 mL samples of 10% sodium fluorescein, USP were drawn up in sterile 3 mL polypropylene syringes. The samples were refrigerated, under fluorescent lights, at an average temperature of 3.9°C for 7 days then sent to the microbiology department for microbial growth testing. The sample volume used to inoculate the samples was 1.0 mL and the incubation temperature was 33°C to 36°C. The samples were incubated for 7 days. The microbiology test results and characteristics of each media are provided in Table 1.
Table 1.
Microbial growth data; 10% sodium fluorescein, USP (equivalent to fluorescein 10%w/v)
Physical Evaluation
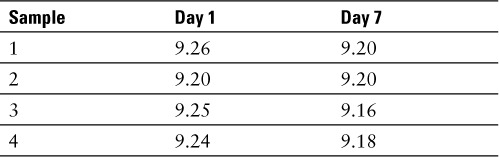
The physical stability of each sample was carried out by visual inspection against black and white background. All samples were evaluated for any visible signs of particulate matter, cloudiness, and color change. None were observed for the duration of the study. In addition, the pH of each sample was measured at baseline and at day 7. The pH measurements were obtained using an Acccumet AR-20 pH meter. The pH results are provided in Table 2.
Table 2.
pH readings; 10% sodium fluorescein, USP (equivalent to fluorescein 10% w/v)
Potency Testing
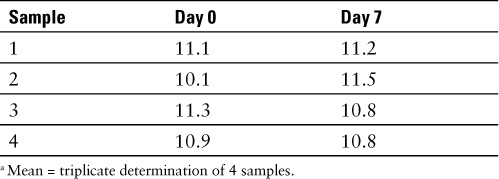
Potency was determined by UV/VIS spectroscopy. Four test samples of 10% sodium fluorescein, USP were assayed for potency. All test samples were read in triplicate and a mean concentration was reported for each one. Test samples were diluted in 50 mM Tris(hydroxymethyl)aminomethane hydrochloride buffer (pH 8.13). These samples were diluted to a final concentration of 2.5 mcg/mL prior to assaying. An initial reading of each sample was made at day 0. The samples were subsequently refrigerated at an average temperature of 4.1°C for 7 days. At day 7 the samples were assayed and the results were compared to those a baseline. The concentration readings, in fluorescein equivalents, are provided in Table 3.
Table 3.
10% sodium fluorescein, USP (equivalent to fluorescein 10% w/v) a
DISCUSSION
Most health care settings are very complex institutions with unique and often specialized departments. The pharmacy department is a prime example of a very specialized area within a hospital. Like any other department within a hospital, the pharmacy department is always seeking ways to reduce waste and improve efficiency. The findings of this study have enabled our pharmacy department to achieve both goals. Whenever a dose of 10% sodium fluorescein, USP is requested, the entire contents of a 5 mL vial are drawn up in 0.5 mL doses. Those doses are stored in the refrigerator for up to 7 days. This enables our pharmacy department to quickly and efficiently dispense this medication. Furthermore, it can potentially eliminate the considerable amount of waste that results from discarding unused portion and also decrease the cost associated with dispensing and stocking this medication. Unlike many other medications that can be ordered as a single dose vial, when an order is placed for a vial of 10% sodium fluorescein, it must be purchased as an entire box of 12 vials. The average wholesale price of sodium fluorescein is $50.22 per vial or $602.64 per box.
A drug utilization review was carried out at our facility. A week-by-week analysis between April 13, 2015 and December 31, 2015 showed that there were 125 doses of sodium fluorescein dispensed. Sixty-two vials were utilized to meet those requests. If we apply the findings of this study with a 7-day storage period, the actual number of vials that would have been needed would have been 23 vials. This represents a 63% decrease with an associated cost-savings of $1,958.58 for that time period. The decision to use 0.5 mL of 10% sodium fluorescein was solely based on physician preference. Doses of 0.25 mL may also be used.
CONCLUSION
Potency and sterility are major factors in the compounding and storage of pharmaceutical compounds. The United States Pharmacopeia has established guidelines where, under certain conditions, it is permissible to repackage single use vials into smaller doses.9 The importance of following these established guidelines cannot be overstated. It is also incumbent on all pharmacists and institutions to verify compliance requirements before repackaging any single dose vial. The findings of this study demonstrate that a 10% solution of sodium fluorescein, USP may be drawn up in sterile polypropylene syringes and stored refrigerated for 7 days. These samples retained their potency and remained microbial free.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of Nikki Bentley, MT, AMT, and Regina Stamey, MT, who provided the microbiology expertise for this study. The authors declare no conflicts of interest.
REFERENCES
- 1. Bennett T. Fundamentals of fluorescein angiography. Ophthalmic Photographers' Society Website. 2011. http://www.opsweb.org/ December 2015.
- 2. Gilmour D. Urinary tract injury in gynecologic surgery: evaluation and management. : Up To Date [internet database]. Wolters Kluwer Health; 2014. http://www.uptodate.com/ December 2015. [Google Scholar]
- 3. AAGL Advancing Minimally Invasive Gynecology Worldwide. . AAGL Practice Report; Practice guidelines for intraoperative cystoscopy in laparoscopic hysterectomy. J Minim Invasive Gynecol. 2012; 19: 407. [DOI] [PubMed] [Google Scholar]
- 4. Akorn. . Indigo Carmine injection [product information]. Lake Forest, IL: Akorn, Inc; June 2012. [Google Scholar]
- 5. Indigo Carmine Shortage. . American Society of Health-System Pharmacists Drug Shortage Resource Management Center. Website. http://www.ashp.org/. June 30, 2015.
- 6. Doyle PJ, Lipestskaia L, Duecy E. et al. Sodium fluorescein use during intraoperative cystoscopy. Obstet Gynecol. 2015; 125: 548. [DOI] [PubMed] [Google Scholar]
- 7. Doyle PJ, Lipestskaia L, Duecy E. et al. Sodium fluorescein use during intraoperative cystoscopy. Obstet Gynecol. 2015; 125: 548. [DOI] [PubMed] [Google Scholar]
- 8. Alcon. . Sodium fluorescein injection [product information]. Fort Worth, TX: Alcon Laboratories, Inc.; July 2009. [Google Scholar]
- 9. Pharmaceutical Compounding-Sterile Preparations. General Information Chapter <797>. The United States Pharmacopeia, 39th rev., and the National Formulary, 34 ed. Rockville, MD: The United States Pharmacopeial Convention; 2016: 626– 670. [Google Scholar]


