Abstract
The prevalence of obesity and type 2 diabetes mellitus (T2DM) continues to rise, and as a result, research aimed at understanding the molecular basis for the co-morbidities has become an area of much scientific interest. Among the more recently recognized chronic complications of T2DM is the increased risk of fracture, especially hip fracture, that has been reported independent of bone mineral density (BMD). A widely used animal model to study how the development and progression of impaired glucose tolerance affect the skeleton has been the diet-induce obesity (DIO) model. As the name implies, this model employs the use of a version of high-fat diets to induce obesity and the subsequent metabolic perturbations that occur with T2DM. Although the model offers a number of advantages, the literature reveals some inconsistent results. Upon further review, discrepancies in the choice of the experimental high-fat diets and the control diets have become a point of major concern. The variability between diets and study design has made it difficult to compare data and results across studies. Therefore, this review aims to provide guidelines that should be employed when designing studies using DIO models of T2DM.
Introduction
Obesity is a condition of excess adiposity defined as a body mass index (BMI) greater than or equal to 30 kg m−2 in adults.1 In the United States, a significant increase in the prevalence of obesity has occurred over the past five decades, and current estimates indicate that ∼35% of adults and 17% of children and adolescents are obese.1 One of the most striking health consequences related to the prevalence of obesity has been the staggering increase in cases of type 2 diabetes mellitus (T2DM), and while not all type 2 diabetics are overweight or obese, the majority of the cases occur in this population. The systemic nature of impaired insulin-stimulated glucose uptake associated with T2DM predisposes adults and children to a number of health complications that can negatively impact one's quality of life.
Complications classically associated with T2DM include macro- and micro-vascular diseases, retinopathy, nephropathies and neuropathies. Over the last two decades, studies designed to determine whether T2DM influenced fracture risk based on assessment of bone density using dual-energy X-ray absorptiometry revealed mixed results, with the preponderance of the evidence indicating that patients were not at increased risk.2,3,4 However, subsequent studies with fracture as the primary outcome variable have challenged these initial findings and the clinical evidence indicates: (i) patients with T2DM have an increased risk of fracture, independent of BMD, particularly in the hip; (ii) fracture risk in T2DM is underestimated when using BMD; and (iii) fracture risk increases with increasing duration of T2DM.3,5,6,7,8,9,10,11,12
To begin to unravel the phenomenon of increased skeletal fragility in T2DM, it is imperative that the alterations in bone metabolism be investigated during the initiation and progression of glucose intolerance. Contributing factors such as inflammation, glucose availability/transport and insulin signaling have important roles in the pathogenesis of T2DM with each of these factors having the potential to alter bone metabolism. Although some aspects of the metabolic phenotype can be accomplished by culturing cells under high-glucose, high-insulin conditions13,14 (Supplementary Table 1), the relative contribution of each of these metabolic and immunological factors on the bone undoubtedly differs over time as the patient progresses from impaired glucose tolerance to the more advanced stages of glucose intolerance. Increased adiposity and the consequent increase in weight-bearing in most T2DM patients can also confound the skeletal response.15,16 Therefore, animal models provide important tools for studying the molecular aspects and pathological effects of obesity-induced changes in glucose homeostasis and progression to glucose intolerance in bone tissue. In conjunction with these animal models, in vitro model systems could prove especially important while studying mechanisms contributing to skeletal alterations.
In 1949, Ingle17 was the first to report on an obesity model in which rats were fed diet ad libitum and their physical activity, or energy expenditure, was restricted which would ultimately result in a net positive energy balance. Since then, there have been many studies aimed at characterizing the metabolic response of rodent models exposed to high-fat diets. In particular, the mouse appears to have become the most widely used rodent, presumably due to their lower cost and the availability of genetically modified models for follow-up studies. Among the different mouse strains, the C57BL/6 mouse is commonly used during diet-induced obesity studies because it mimics many of the metabolic alterations observed with obesity and T2DM in humans, including hyperinsulinemia, hyperglycemia and hypertension.18 In addition to the metabolic and cardiovascular derangements that occur, diet-induced obesity models often demonstrate a compromise in bone structure, biomechanics and metabolism with obesity and the subsequent metabolic perturbations (Table 1). It is important to note that some inconsistencies exist within the literature as to how a high-fat diet affects bone. While some of this can be attributed to the differences in study design (i.e., age at initiation and duration of treatment, gender, strain/substrain and so on), it is also apparent that major discrepancies exist in the diets being used. The following sections aim to present the key considerations when designing studies using diet-induced obesity models to study bone, and in particular to provide pertinent information relative to diet formulation with the intent of establishing guidelines for choosing high-fat and control diets.
Table 1. Review of the literature assessing bone structure has primary outcome and the corresponding high-fat and control diets used.
| Study | Strain | Sex | Age | Duration | Control diet | HF Diet (HFD) | Impact of HFD on bone |
|---|---|---|---|---|---|---|---|
| Cao et al.31 | C57BL/6 | Male | 6 wk | 14 wk | 10% kcal fat - high sucrose | 45% kcal fat (lard) | ↓BV/TV (tibia) |
| Ionova-Martin et al.32 | C57BL/6 | Male | 4 wk | 19 wk | Chow - 21.6% kcal fat, 55.2% carb, 23.3% protein | 60% kcal fat (lard) | No change BMD (whole body, femur, vertebra)↑ CtTh,↓ Biomechanical properties |
| Patsch et al.34 | C57BL/6 Ja | Male | 7 wk | 3 or 24 wk | 10% kcal fat | 60% kcal fat | ↓ BMD and BV/TV (vertebra) |
| Ionova-Martin et al.33 | C57BL/6 | Male | 3 wk or 15 wk | 16 wk | 10% kcal fat | 60% kcal fat (lard) | No change BMD (whole body, femur)↓BMD (vertebra)↓Bone strength |
| Inzana et al.30 | C57BL/6 J | Male | 5 wk and 20 wk | 12 wk; switch 12 wk | 10% kcal fat | 60% kcal fat (lard) | ↓BV/TV (femur; vertebra)↓Biomechanical properties (vertebra) |
| Lu et al.35 | C57BL | Male | 17 day | 8 wk | AIN-93G (14% kcal fat) | 45% fat (corn oil) | No change BMD (tibia)↓Tibia trabecular BMD↓Biomechanical properties |
| Doucette et al.66 | C57BL/6 J | Male; female and male | 3 wk or 13 wk | 12 wk or 14 days | 10% kcal fat - high sucrose | 60% kcal fat (lard); Surwit 58.8% kcal fat (coconut oil) | No Change in BMD, BV/TV. or cortical (femur)↓TbN, ConnDens |
| Gautam et al.40 | C57BL/6 | Male and female | 4 wk | 10 wk | Chow | 60% kcal fat (lard) | ↓BV/TV (femur and tibia) |
| Brown et al.36 | C57BL/6 J | Male | 5 wk | 0-35 days post fracture | 10% kcal fat - high sucrose | 60% kcal fat (lard) | ↓Fracture healing↓Biomechanical properties |
| Fehrendt et al.37 | C57BL/6 J | Male | 4 wk | 23 wk | 10% kcal fat | 60% kcal fat | No change BMD (whole body, spine, tibia)↓TbTh (femur)↓Bone area (femur) |
| Lecka-Czernik et al.38 | C57BL/6 | Male | 12 wk | 11 wk | 12% kcal fat | 45% kcal fat (lard) | ↑ BV/TV (tibia)↑ CtAr, CtTh |
| Shu et al.65 | C57BL/6 J | Male | 5 wk | 6 or 12 wk | 10% kcal fat - high sucrose | 60% kcal fat (lard) | ↓ BV/TV (femur) |
| Rendina-Ruedy et al.22 | C57BL/6 J C57BL/6 N C3H/HeJ | Male | 8 wk | 24 wk | 10% kcal fat - sucrose matched | 45% kcal fat (lard) | ↓ BV/TV (vertebra) -BL6J and BL6 NNo change BV/TV (femur)No change BMD (whole body) |
| Rendina-Ruedy et al.39 | C57BL/6NC3H/HeJ | Male | 6 wk | 2, 8, 16 wk | 10% kcal fat - sucrose matched | 60% kcal fat (lard) | ↓ BV/TV (femur) BL6N↓ BMD (whole body)↓CortArea (femur)↓Biomechanical properties (BL6N)No change BV/TV vertebra |
Abbreviations; BMD, bone mineral density; BV/TV, bone volume per total volume; ConnDens, connectivity density; CtAr, cortical area; CtTh, cortical thickness; HFD, high-fat diet; TbN, trabecular number, TbTh, thickness; TbSp, separation; wk, week.
Strain/substrain, age of initiation and duration of treatment, and gender are also reported. Missing details indicates a lack of or inadequate information to determine based on original publication.
aIndicates that C57BL/6 J animals were reported in original publication, however mice are described from Charles River and most likely C57BL/6 N.
Materials and methods
Pre-treatment considerations
Prior to the initiation and execution of studies utilizing the diet-induced obesity model, there are several aspects of the experimental design that should be considered. First, and perhaps foremost, is the genetic background of the mouse model to be used. Although a number of different mouse strains are available, it is relatively well documented that certain strains exhibit different metabolic perturbations in response to a high-fat diet and/ or obesity. These include strains such as BALBc19 and C3H/HeJ mice20,21,22 which exhibit a mild protection or resistance from high-fat-induced metabolic disturbances (e.g., glucose intolerance, hepatic triglyceride accumulation and bone loss). In addition, more subtle and often over looked mouse substrain variations should also be considered. For example, C57BL/6 J mice have a mutation in nicotinamide nucleotide transhydrogenase (Nnt) which results in a truncated gene (in-frame deletion of exons 7–11) and absent protein expression, while C57BL/6 N mice have a functional, intact Nnt.22,23 Given Nnt's role as an inner mitochondrial membrane protein which reduces NADP+ to facilitate proton re-entry24 and its importance in maintaining glucose-stimulated insulin secretion by the β-cells,25 the choice of the C57BL/6 J compared to the C57BL/6 N strain could be significant and complicate interpretation of the results. While the intent of this review is to focus on nutritional considerations when utilizing models of diet-induced obesity, it should be noted that genetically modified mouse models that mimic various aspects of T2DM are also available and excellent reviews of these models do exist (Note 1).26,27,28
Other factors to consider prior to the start of experiments include the age of the mice at the initiation of dietary treatment, gender and duration of the treatment. While it has not been extensively studied, it appears that starting animals on the diet earlier (∼4–8 weeks of age) results in a more homogenous response (e.g., ‘responders' vs ‘non-responders').29 Inzana et al.30 directly addressed this concern and demonstrated that immature (5–week-old) cancellous bone is more susceptible to the detrimental effects of high-fat diet as opposed to skeletally mature (20-week-old) mice (Table 1). The vast majority of studies aimed to determine how diet-induce obesity impacts bone have been performed in males.22,30,31,32,33,34,35,36,37,38,39 One study that compared both genders reported that males gain more weight and lose more cancellous bone on a high-fat diet than females;40 however, there is an overall lack of knowledge as to how female bone tissue is altered in this model. Another factor to consider is the duration of the treatment. Although a high-fat diet has been shown to increase bodyweight and initiate the impairment of glucose tolerance after only 2 weeks, no skeletal alterations have been documented this early.39 However, as high-fat diet feeding continues for 8 and 16 weeks, a number of skeletal changes have been reported, including a lower femoral BV/TV, decreased whole body BMD, along with stunted tibia growth.39 It should also be emphasized that the duration of the high-fat diet feeding has significant implications on the various metabolic changes that are occurring in the development of T2DM, and these metabolic derangements should be documented. For example, obesity is an outcome variable that is often documented by monitoring body weight and body composition in these studies, but whether the animals are hyperglycemic, glucose intolerant, hyper- or hypoinsulinemic are distinct etiologies in T2DM that can have very different effects on bone metabolism. Thus, the metabolic state of the animals should be well documented (Note 2). The differences in the metabolic response occurring over time highlight the need to monitor the metabolic perturbations occurring over the course of the high-fat feeding. Since diet-induced obesity in this instance is being used as a model of early T2DM, it is important to characterize these metabolic changes, whether that is by means of fasting blood glucose and plasma insulin (Note 3) to glucose and/or insulin tolerance tests.
Experimental diets for diet-induced obesity models of T2DM
Experimental diets used in the diet-induced obesity models are those that will induce obesity and the subsequent phenotype with the co-morbidities/complications of interest, in this case T2DM. The most commonly used and widely accepted diets for these models are those that contain high amounts of fat (Note 4), often ranging from 45 to 60% kcal from fat (Note 5). Most of the studies that have examined the effects of diet-induced obesity and impaired glucose homeostasis on bone have used high-fats diets with 60% of the kcal from fat (Table 1). If the goal of the research is to produce a model that most closely mimics human consumption, then it is evident that a diet with 45% of the kcal from fat is a more reasonable choice. However, there is often greater variability in the response to the 45% fat diet from a metabolic standpoint. As such, researchers should account for this response with appropriate power calculations when designing studies to ensure adequate animal numbers and stringent metabolic profiling to document high and low responders.29 In contrast, the 60% fat diet induces obesity and loss of insulin sensitivity in a relatively short period of time (i.e., as early as 2 weeks) and has been shown to provide more reproducible results. Thus, it is important for investigators to consider the advantages and disadvantages of the 45% and 60% diets when designing studies.
Another important aspect of the diet that needs to be considered when selecting a high-fat diet is the type of fat that is to be used. Soybean oil is the fat source in the reference diet designed by the American Institute of Nutrition (AIN)-93 due to its composition of essential fatty acids, linoleic and linolenic acid. Technically, high-fat diets used in the diet-induced obesity models can be designed with additional fat from either plant or animal sources (Table 2). The most commonly used fat source in metabolic as well as bone studies (Table 1) is animal fat or lard. The fatty acid profile of lard is ∼37% saturated (sFA), 46% monounsaturated (MUFAs), and 17% polyunsaturated (PUFAs) (Note 6). Lard has particularly high amounts of palmitic, steric and oleic acid (Table 3). It is important for researchers to take note that not all high-fat diets at a given percentage of kcal are created equal and the additional fat source is of particular relevance. For example, the fatty acid profile from these lard-based high-fat diets is in stark contrast to coconut oil used in the Surwit diet (58% kcal from fat).41 Coconut oil is high in sFA, accounting for ∼90%, while MUFAs and PUFAs constitute 2% and 4%, respectively. For example, while coconut oil has been shown to increase adiposity, there is some question as to whether these animals display impaired insulin sensitivity.42 Further analysis of coconut oil's fatty acid profile reveals high amounts of lauric, myristic, capric, and caprylic acid which differ significantly from lard. The individual fatty acids derived from these different fat sources can exert very different, direct effects on tissues that are beyond the scope of this review. Nonetheless, investigators should carefully deliberate the choice of a fat source, knowing that this choice as well as the amount of fat may alter the cellular response which can particularly important when studying molecular mechanisms by which bone is responding.
Table 2. Diet formulation of American Institute Nutrition (AIN)-93 mature (M), control diet sucrose matched to the 60% kcal fat diet, control diet with additional sucrose as carbohydrate source, high-fat diet with 60% kcal from fat, and the Surwit diet.
| AIN-93M | Control (sucrose matched) | Control (high sucrose) | High fat | Surwit | ||
|---|---|---|---|---|---|---|
| Protein | kcal% | 14.7 | 20 | 20 | 20 | 16.4 |
| Carbohydrate | kcal% | 75.9 | 70 | 70 | 20 | 25.5 |
| Fat | kcal% | 9.4 | 10 | 10 | 60 | 58 |
| kcal g−1 | 3.85 | 3.85 | 3.85 | 5.24 | 5.56 | |
| Sucrose | kcal | 400 | 275 | 1400 | 275 | 700 |
| g kg−1 | 100 | 65.2 | 331.74 | 88.9 | 175 | |
| Cornstarch | kcal | 1983 | 2024.8 | 1200 | 0 | 0 |
| g kg−1 | 495.7 | 479.8 | 298.6 | 0 | 0 | |
| Maltodextrin | kcal | 500 | 500 | 140 | 500 | 680 |
| g kg−1 | 125 | 118.5 | 33.2 | 161.5 | 170 | |
| Coconut Oil | kcal | — | — | — | — | 3001 |
| g kg−1 | — | — | — | — | 333.5 | |
| Lard | kcal | — | 180 | 180 | 2205 | — |
| g kg−1 | — | 20 | 20 | 245 | — | |
| Soybean Oil | kcal | 360 | 225 | 225 | 225 | 225 |
| g kg−1 | 40 | 25 | 25 | 25 | 25 | |
| Saturated | % | 14 | 24 | 23.5 | 37.1 | 93.46 |
| Monounsaturated | % | 21 | 34.7 | 29.7 | 46 | 2.38 |
| Polyunsaturated | % | 58 | 40.2 | 46.8 | 16.9 | 4.16 |
Table 3. Fatty acid profile (%) of soybean oil, lard, and coconut oil.
| Soybean oil | Lard | Coconut oil | |
|---|---|---|---|
| Saturated | 14 | 40 | 90 |
| Lauric | — | — | 48 |
| Myristic | — | 2 | 16 |
| Palmitic | 10 | 27 | 9 |
| Caprylic | — | — | 8 |
| Capric | — | — | 7 |
| Stearic | 4 | 11 | 2 |
| Unsaturated | 81 | 59 | 9 |
| Oleic | 23 | 44 | 7 |
| Linoleic | 51 | 11 | 2 |
| Linolenic | 7 | — | — |
| Palmitoleic | — | 4 | — |
| Other | 5 | 1 | 1 |
It is also important to highlight that it is not possible to simply manipulate the percentage of kcal coming from one macronutrient without changing the relative contribution of other macronutrients. Consequently, it stands to reason that increasing the percentage of kcal from fat in the diet will require adjustments in either the percentage of kcal from carbohydrate, protein or both. High-fat diets are often formulated to maintain 20% kcal from protein and decrease the amount of carbohydrates from 64–70% to 35–20% kcal depending on whether fat accounts for 45 or 60% kcal. In a purified diet, the source of carbohydrate that is most commonly reduced to account for the increased fat is the cornstarch. These alterations in macronutrients result in what can be considered a high-fat, low carbohydrate diet, and as such, may result in the accumulation of ketone bodies. It should be noted, that the working assumption here is investigators desire to have isocaloric diets and are not merely adding fat to an adequate purified diet or chow. This practice is strongly discouraged due to the fact that the concentration of vitamins and minerals can be diluted and deficiencies can result.
When choosing an experimental diet to be used for diet-induced obesity models, key considerations include the percentage of kcal from fat, the fat source and the impact of the adjustments on other macro- and micronutrients (Table 4). These decisions can have implications on the characteristics and timing of the metabolic response that is achieved as well as the resulting bone phenotype. However, after careful consideration is given to the experimental diet, an important, but often overlooked next step, is to make sure that an appropriate control diet is selected.
Table 4. Key dietary considerations when using a diet-induced obesity (DIO) model.
| Experimental Diets |
|---|
| Amount of fat: 45% kcal from fat closely mimics high-fat diet intake by humans but increases the response variability (i.e., increase in non-responders); 60% kcal from fat more homogeneous response and most commonly used in studies focused on bone as an outcome. |
| Source of fat: Lard is the most commonly used source of saturated fats and will yield different metabolic responses compared to plant sources of saturated fats (i.e., coconut oil) and polyunsaturated or monounsaturated fats. |
| Adjustments in other nutrients: Adding fat to a diet will alter the proportion of other nutrients; purified diets allow for maintaining adequate protein (amino acid) and micronutrient status while adjusting fat. |
| Control Diets |
| Chow diets: Generally chow is cereal- or grain-based with fat, vitamins and minerals added; seasonal variations can occur; chow contains phytochemicals and is not easily modified; adding fat to chow diets is discouraged due to the dilution of protein as well as vitamins and minerals. |
| Purified diets: Improve repeatability of the study due to known ingredients and well characterized diets such as AIN-93 diet; most high-fat diets increase fat and decrease the carbohydrates with minor or no modifications in protein; purified diets are recommended for control diet. |
| Adjustments in other nutrients: To account for the absence of additional fat relative to the experimental diet other macronutrients (usually carbohydrate sources such as cornstarch or sucrose) are often manipulated; often, high amounts of sucrose can alter the metabolic response; reference AIN diets should be consulted. |
These include the amount and source of fat, and adjustments in other nutrients in both the experimental and the control diets.
Control diets for diet-induced obesity models
As with any experimental treatment, the control cohort should be designed in such a way as to minimize variation. Although this seems like a trivial concept, the fact that controversy and debate still exists on the topic of control diets relative to diet-induced obesity models, underscores the complexity of the issue. Ideally, once an experimental, high-fat diet has been determined, an appropriate control diet should minimize differences with the experimental diet to key dietary variables of interest (i.e., fat and carbohydrate content and source). Excessive deviation between the control and experimental diet such as the sources macro- and micronutrients are derived from will certainly confound data interpretation and the conclusions that can be drawn. Laboratory rodent diets are generally divided into two categories; chow or unpurified and purified and semi-purified diets (Note 7). From those basic descriptors it is intuitive that the appropriate control for a purified high-fat diet would at the very least be a purified control diet. Unfortunately the literature suggests the profound differences between purified diets and chow are not always appreciated.43
While the decision of using chow vs. purified diets presents a real dilemma for some scientists, purified control vs. purified high-fat diets can also pose serious problems as well. Purified control diets should mimic the experimental high-fat control as closely as possible, however, the amount of fat and the carbohydrate source must be altered. These control diets often have 10% kcal from fat, often coming from both soybean oil and lard. Since the fat proportion is lower (10% kcal) and the protein is the same (20% kcal) compared to the defined high-fat diet, the carbohydrate proportion must account for 70% of kcal. This is achieved by increasing the amount of cornstarch or sucrose in the diet. Although both ‘control' diets are readily available to laboratories, it is important to note that matching the sucrose amount to that of the high-fat diet and substituting the remaining carbohydrates with cornstarch appears to be the most appropriate formulation (Note 8). These diets are specifically designed so that when the same amount of calories are consumed by the experimental and control groups the same amount of sucrose is consumed. In summary, diet-induced obesity models of T2DM can be a very useful and powerful tool, however, care must be taken when designing and carrying out these studies. This review aimed to bring those key considerations to the forefront of discussion in an attempt to establish some guidelines to facilitate the reproducibility between studies and ability to compare results across data sets; and to highlight key nutritional aspects of the diet-induced obesity models.
Notes
Note 1
Other animal models used in metabolic research include L-SACC, TALLYHO/JngJ, KK-Ay (yellow Kuo Kondo), ob/ob, and db/db, mice as they develop severe obesity and subsequent metabolic derangements (i.e., hyperglycemia and glucose intolerance). Interestingly, the skeletal phenotype of each of these models is variable. The L-SACC mice, for example, demonstrate higher bone mass due to both decreased bone formation and bone resorption,44 while TALLYHO/JngJ mice have lower BMD and diminished peak bone mass.45,46 The KK-Ay mouse appears to be more complicated as BMD and cortical bone are increased, while trabecular bone is decreased.47,48 Perhaps even more complex is the skeletal phenotype reported from ob/ob and db/db mice. The ob/ob mice were initially reported to have high bone mass,49 further studies revealed that while these mice have increased trabecular bone in the vertebra, and they also exhibited decrease cortical bone.50,51,52 In addition, the db/db mice which exhibit a more severe metabolic phenotype compared to the ob/ob, have impaired longitudinal bone growth, decreased cortical bone, and increased trabecular bone.53,54,55,56
Note 2
Prior to focusing on diet, there is one other point that should be emphasized. It is important to monitor food intake throughout the course of diet-induced obesity studies. Although this may not seem critical, when drawing conclusions based on treatment groups it can be a valuable data. An example of this would be if an experimental group on the high-fat diet is not increasing bodyweight, a conclusion could be drawn that is merely that. However, other options could be that the animals are not consuming as much food and their caloric intake is lower or, if food intake is adequate, energy expenditure could be elevated. These bits of information help to gain full, well-rounded insight into how the animals are responding.
Note 3
An additional variable that may complicate study-to-study comparisons is the duration of the fast prior to testing. Clinically, fasting often implies an ‘overnight' fast, 8–12 h. As such, this same approach is often applied to laboratory settings and rodent models; however, this may not be appropriate.57 Fasting mice can experience alterations in a number of key parameters, including hormones, hepatic energy metabolism and body temperature.58 It is also important to highlight that mice consume approximately two-thirds of their total food intake during the night or scotophase. Therefore, fasting mice for 5–6 h instead of overnight may be more comparable to humans.
Note 4
It is essential to note that the subjective terms ‘high' and ‘low' in regards to nutrients are given value by comparisons to an established reference diet. For example, ‘high' fat diets used in diet-induced obesity models are meaningless until a ‘normal' or reference dietary fat intake is established for laboratory rodents. As such, these standards have been defined by the American Institute of Nutrition (AIN) to include AIN-76(A), AIN-93G, and AIN-93M. Relevant to this particular topic, the fat source was changed from corn oil to soybean oil in 1993 as it is the only single source of dietary fat that provides sufficient amounts and adequate balance of the essential FAs linoleic and linolenic acid.59,60 In the AIN-93G diet, ∼16 of kilocalories (kcal) are from fat, while ∼10% kcal from fat as an energy source in the AIN-93M diet. Therefore, based on these references we are able to deem diets as ‘high' fat based on the amount of kcal from fat. Due to the rigor that has been exercised during the development and refinement of AIN diets,59,61,62 these formulas are typically the basis that all other experimental diets are formulated around.
Note 5
It should be noted that diet formulas may differ in how macronutrient amounts are described or reported. For example, some may list fat as g kg−1 diet, which is not % kcal. For the conversion of macronutrients by weight to energy density, Atwater factors are used to assign 9 kcal g−1 for fat and 4 kcal g−1 for carbohydrates and proteins.
Note 6
Although the exact stability of this diet is unknown, diets high in PUFAs are especially susceptible to oxidation. Due to the high-fat content of these diets it is often recommended to store the diet at −20 °C and change the diet 2–3 times per week to minimize rancidity. Companies that formulate diets report that users often find that when the diet is changed less often the daily consumption is decreased, which is disconcerting given the dependence of the model on food and calorie intake.
Note 7
An unpurified diet, or ‘chow' as it is commonly referred to, is a grain based diet that is inexpensive to manufacture and palatable to rodents. These rudimentary qualifications of chow are the primary reason it is used as a background or ‘maintenance' diet. Interpreting this diet as a control diet however, poses many problems including; (1) diets are often ‘closed' formulas, meaning that the exact amount of each ingredient is unknown to the purchaser; (2) many compounds are inseparable from the next; (3) the content of these plant materials will naturally fluctuate from batch to batch; (4) the exceptionally high and variable amounts of phytoestrogens; and (5) extremely high-fiber content and presence of toxic heavy metals.
Note 8
Some investigators account for the lower fat (10% kcal), and disregard the high addition of sucrose to the diet. This commercially available ‘control' diet has ∼332 g sucrose per kg diet or 35% kcal sucrose, compared with the 7% kcal sucrose found in the 60% high-fat diet and 10% kcal sucrose from the AIN-93M. As such, these high sucrose ‘control' diets have been shown to exhibit mild metabolic derangements including glucose intolerance and non-alcoholic fatty liver disease (NAFLD).63 This is particularly important when using diet-induced obesity models, as many of these perturbations are relevant to the condition being tested. For example, high-fat diet leads to impaired glucose tolerance, and as such, is commonly used as a tool for studying T2DM. In this scenario if the ‘control' group exhibits glucose intolerance as well, there is no system to study how impaired glucose tolerance impacts said condition or co-morbidity. Furthermore, it is possible that the higher sucrose in this diet increases food intake due to palatability and preference, which can also confound results (Table 4).
Conclusion
Matthew Ricci and Edward Ulman64 sum it up best when they stated that all scientists feeding an animal some type of diet should add nutritional scientist to their job description. Though this is true for the vast majority of studies, it is especially critical when the experimental model is diet dependent. While it's important to ensure adequate nutritional status, aside from the target of the dietary modification when manipulating any diet, one must understand what ingredient is being altered and have an appreciation for how this may impact the other ingredients. Fortunately, many diet manufacturers offer excellent nutritional guidance and advice, but ultimately the responsibility rests on researchers to ensure the diet is appropriate for the model.
The diet-induced obese mouse model provides a valuable tool that portrays the complexity of the physiological environment that occurs in the development of and increasing duration of glucose intolerance. The decision process that researchers must undergo when designing studies to investigate the skeletal response to decreased insulin sensitivity resulting from diet-induced obesity have been described herein and a working model is provided (Figure 1). Each of these decisions can be made following consideration of the aspects of T2DM that is to be studied and recognition that not all high-fat diets will elicit the same response. Although there are a number of factors that can affect the outcomes of these studies, the choice of the diet, both experimental and control, is critical and should be selected based on considerations highlighted in this review.
Figure 1.
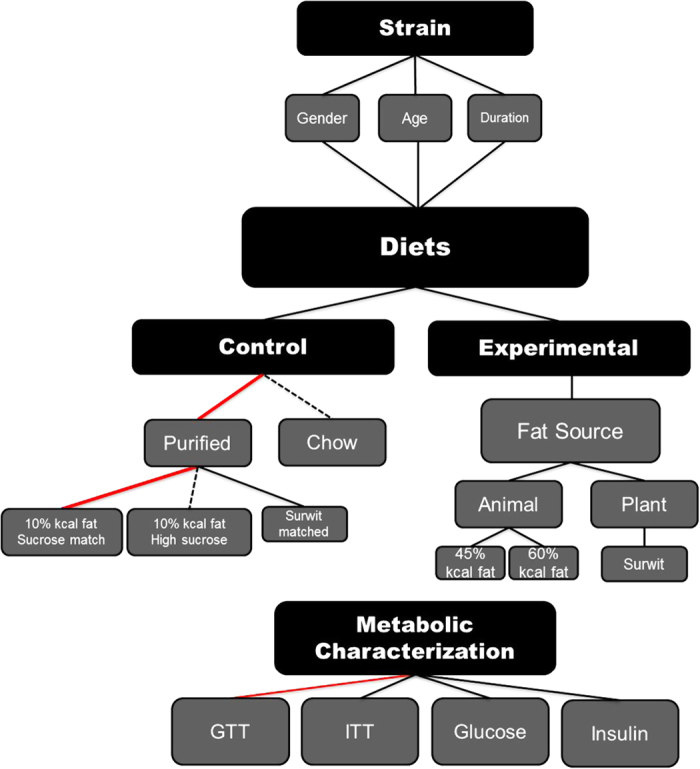
Schematic diagram outlining the considerations researchers must when using diet-induced obesity models for T2DM. Red lines depict prefered and justified selections, while hashed lines are discouraged.
Supplementary Material
Acknowledgments
Supported by the Oklahoma Center for the Advancement of Science and Technology and the Oklahoma Agricultural Experiment Station.
Footnotes
The authors declare no conflict of interest.
References
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief 2013; 1–8. [PubMed] [Google Scholar]
- Rishaug U, Birkeland KI, Falch JA, Vaaler S. Bone mass in non-insulin-dependent diabetes mellitus. Scand J Clin Lab Invest 1995; 55: 257–262. [DOI] [PubMed] [Google Scholar]
- van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A et al. Bone density in non-insulin-dependent diabetes mellitus. The Rotterdam Study. Ann Intern Med 1995; 122: 409–414. [DOI] [PubMed] [Google Scholar]
- Stolk RP, van Daele PL, Pols HA, Burger H, Hofman A, Birkenhager JC et al. Hyperinsulinemia and bone mineral density in an elderly population: The Rotterdam Study. Bone 1996; 18: 545–549. [DOI] [PubMed] [Google Scholar]
- Valerio G, Galle F, Mancusi C, Di OV, Guida P, Tramontano A et al. Prevalence of overweight in children with bone fractures: a case control study. BMC Pediatr 2012; 12: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 2001; 86: 32–38. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 2001; 24: 1192–1197. [DOI] [PubMed] [Google Scholar]
- de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: The Rotterdam Study. Osteoporos Int 2005; 16: 1713–1720. [DOI] [PubMed] [Google Scholar]
- Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: the Nurses' Health Study. Diabetes Care 2006; 29: 1573–1578. [DOI] [PubMed] [Google Scholar]
- Farr JN, Drake MT, Amin S, Melton LJ III, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res 2013; 29: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole ZA, Harvey NC, Kim M, Ntani G, Robinson SM, Inskip HM et al. Increased fat mass is associated with increased bone size but reduced volumetric density in pre pubertal children. Bone 2012; 50: 562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding A, Taylor RW, Jones IE, McAuley KA, Manning PJ, Williams SM. Overweight and obese children have low bone mass and area for their weight. Int J Obes Relat Metab Disord 2000; 24: 627–632. [DOI] [PubMed] [Google Scholar]
- Thomson MJ, Williams MG, Frost SC. Development of insulin resistance in 3T3-L1 adipocytes. J Biol Chem 1997; 272: 7759–7764. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Scribner KA. In-vivo and in-vitro models of type 2 diabetes in pharmaceutical drug discovery. Diabetes Obes Metab 1999; 1: 75–86. [DOI] [PubMed] [Google Scholar]
- Kuruvilla SJ, Fox SD, Cullen DM, Akhter MP. Site specific bone adaptation response to mechanical loading. J Musculoskelet Neuronal Interact 2008; 8: 71–78. [PubMed] [Google Scholar]
- Suva LJ, Gaddy D, Perrien DS, Thomas RL, Findlay DM. Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr Osteoporos Rep 2005; 3: 46–51. [DOI] [PubMed] [Google Scholar]
- INGLE DJ. A simple means of producing obesity in the rat. Proc Soc Exp Biol Med 1949; 72: 604. [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6 J mouse: physiological and molecular characteristics. Physiol Behav 2004; 81: 243–248. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013; 56: 1129–1139. [DOI] [PubMed] [Google Scholar]
- Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 2007; 50: 1267–1276. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina-Ruedy E, Hembree KD, Sasaki A, Davis MR, Lightfoot SA, Clarke SL et al. A comparative study of the metabolic and skeletal response of C57BL/6 J and C57BL/6 N mice in a diet-induced model of type 2 diabetes. J Nutr Metab 2015; 2015: 758080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Reifsnyder PC, Malcolm RD, Lucas CA, MacGregor GR, Zhang W et al. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity (Silver Spring) 2010; 18: 1902–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek JB, Rydstrom J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J 1988; 254: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman H, Shimomura K, Horner E, Cox RD, Ashcroft FM. Nicotinamide nucleotide transhydrogenase: a key role in insulin secretion. Cell Metab 2006; 3: 35–45. [DOI] [PubMed] [Google Scholar]
- Fajardo RJ, Karim L, Calley VI, Bouxsein ML. A review of rodent models of type 2 diabetic skeletal fragility. J Bone Miner Res 2014; 29: 1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Rosen CJ. Energy excess, glucose utilization, and skeletal remodeling: new insights. J Bone Miner Res 2015; 30: 1356–1361. [DOI] [PubMed] [Google Scholar]
- Leiter EH. Selecting the ‘right' mouse model for metabolic syndrome and type 2 diabetes research. Methods Mol Biol 2009; 560: 1–17. [DOI] [PubMed] [Google Scholar]
- Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R et al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 2010; 59: 2178–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana JA, Kung M, Shu L, Hamada D, Xing LP, Zuscik MJ et al. Immature mice are more susceptible to the detrimental effects of high fat diet on cancellous bone in the distal femur. Bone 2013; 57: 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone 2009; 44: 1097–1104. [DOI] [PubMed] [Google Scholar]
- Ionova-Martin SS, Do SH, Barth HD, Szadkowska M, Porter AE, Ager JW III et al. Reduced size-independent mechanical properties of cortical bone in high-fat diet-induced obesity. Bone 2010; 46: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionova-Martin SS, Wade JM, Tang S, Shahnazari M, Ager JW III, Lane NE et al. Changes in cortical bone response to high-fat diet from adolescence to adulthood in mice. Osteoporos Int 2011; 22: 2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch JM, Kiefer FW, Varga P, Pail P, Rauner M, Stupphann D et al. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism 2011; 60: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XM, Zhao H, Wang EH. A high-fat diet induces obesity and impairs bone acquisition in young male mice. Mol Med Rep 2013; 7: 1203–1208. [DOI] [PubMed] [Google Scholar]
- Brown ML, Yukata K, Farnsworth CW, Chen DG, Awad H, Hilton MJ et al. Delayed fracture healing and increased callus adiposity in a C57BL/6 J murine model of obesity-associated type 2 diabetes mellitus. PLoS ONE 2014; 9: e99656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrendt H, Linn T, Hartmann S, Szalay G, Heiss C, Schnettler R et al. Negative influence of a long-term high-fat diet on murine bone architecture. Int J Endocrinol 2014; 2014: 318924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Stechschulte LA, Czernik PJ, Dowling AR. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol Cell Endocrinol 2015; 410: 35–41. [DOI] [PubMed] [Google Scholar]
- Rendina-Ruedy E, Graef JL, Davis MR, Hembree KD, Gimble JM, Clarke SL et al. Strain differences in the attenuation of bone accrual in a young growing mouse model of insulin resistance. J Bone Miner Metab 2015; 34: 380–394. [DOI] [PubMed] [Google Scholar]
- Gautam J, Choudhary D, Khedgikar V, Kushwaha P, Singh RS, Singh D et al. Micro-architectural changes in cancellous bone differ in female and male C57BL/6 mice with high-fat diet-induced low bone mineral density. Br J Nutr 2014; 111: 1811–1821. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6 J mice. Diabetes 1988; 37: 1163–1167. [DOI] [PubMed] [Google Scholar]
- Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 2006; 36: 485–501. [DOI] [PubMed] [Google Scholar]
- Warden CH, Fisler JS. Comparisons of diets used in animal models of high-fat feeding. Cell Metab 2008; 7: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Kaw M, Harris MT, Ebraheim N, McInerney MF, Najjar SM et al. Decreased osteoclastogenesis and high bone mass in mice with impaired insulin clearance due to liver-specific inactivation to CEACAM1. Bone 2010; 46: 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won HY, Lee JA, Park ZS, Song JS, Kim HY, Jang SM et al. Prominent bone loss mediated by RANKL and IL-17 produced by CD4+ T cells in TallyHo/JngJ mice. PLoS ONE 2011; 6: e18168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ, Van VM, Motyl K, Karim L, Brooks DJ, Louis L et al. Early-onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse. Endocrinology 2014; 155: 3806–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Miura T, Yamashita T, Ando N, Nakao H, Ishihara E et al. Characteristics of diabetic osteopenia in KK-Ay diabetic mice. Biol Pharm Bull 2012; 35: 438–443. [DOI] [PubMed] [Google Scholar]
- Kolli V, Stechschulte LA, Dowling AR, Rahman S, Czernik PJ, Lecka-Czernik B. Partial agonist, telmisartan, maintains PPARgamma serine 112 phosphorylation, and does not affect osteoblast differentiation and bone mass. PLoS ONE 2014; 9: e96323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 2000; 100: 197–207. [DOI] [PubMed] [Google Scholar]
- Ealey KN, Fonseca D, Archer MC, Ward WE. Bone abnormalities in adolescent leptin-deficient mice. Regul Pept 2006; 136: 9–13. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 2004; 34: 376–383. [DOI] [PubMed] [Google Scholar]
- Matsunuma A, Kawane T, Maeda T, Hamada S, Horiuchi N. Leptin corrects increased gene expression of renal 25-hydroxyvitamin D3-1 alpha-hydroxylase and -24-hydroxylase in leptin-deficient, ob/ob mice. Endocrinology 2004; 145: 1367–1375. [DOI] [PubMed] [Google Scholar]
- Lorentzon R, Alehagen U, Boquist L. Osteopenia in mice with genetic diabetes. Diabetes Res Clin Pract 1986; 2: 157–163. [DOI] [PubMed] [Google Scholar]
- Hosokawa T. Altered bone metabolism in db/db mice. Nihon Ronen Igakkai Zasshi 1992; 29: 540–548. [DOI] [PubMed] [Google Scholar]
- Takeshita N, Mutoh S, Yamaguchi I. Osteopenia in genetically diabetic DB/DB mice and effects of 1alpha-hydroxyvitamin D3 on the osteopenia. Basic Research Group. Life Sci 1995; 56: 1095–1101. [DOI] [PubMed] [Google Scholar]
- Williams GA, Callon KE, Watson M, Costa JL, Ding Y, Dickinson M et al. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res 2011; 26: 1698–1709. [DOI] [PubMed] [Google Scholar]
- Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab 2008; 295: E1323–E1332. [DOI] [PubMed] [Google Scholar]
- Jensen TL, Kiersgaard MK, Sorensen DB, Mikkelsen LF. Fasting of mice: a review. Lab Anim 2013; 47: 225–240. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76 A rodent diet. J Nutr 1993; 123: 1939–1951. [DOI] [PubMed] [Google Scholar]
- Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76 A diet. J Nutr 1997; 127: 838 S–83841. [DOI] [PubMed] [Google Scholar]
- Reeves PG. AIN-76 diet: should we change the formulation? J Nutr 1989; 119: 1081–1082. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr 1993; 123: 1923–1931. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi M, Sakanaka M, Kimura Y. Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice. J Nutr 2006; 136: 582–587. [DOI] [PubMed] [Google Scholar]
- Ricci MR, Ulman EA. Laboratory animal diets: a critical part of in vivo research. Animal Lab News 2005; 4: 1–6. [Google Scholar]
- Shu L, Beier E, Sheu T, Zhang H, Zuscik MJ, Puzas EJ et al. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif Tissue Int 2015; 96: 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette CR, Horowitz MC, Berry R, Macdougald OA, Aunuciado-Koza R, Koza RA et al. A high-fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6 J mice. J Cell Physiol 2015; 230: 2032–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


