Abstract
Background
Breast-conserving surgery (bcs) is the preferred surgical approach for most patients with early-stage breast cancer. Frequently, concerns arise about the pathologic margin status, resulting in an average reoperation rate of 23% in Canada. No consensus has been reached about the ideal reoperation rate, although 10% has been suggested as a target. Upon undergoing reoperation, many patients choose mastectomy and breast reconstruction, which add to the morbidity and cost of patient care. We attempted to identify the cost of reoperation after bcs, and the effect that a reduction in the reoperation rate could have on the B.C. health care system.
Methods
A decision tree was constructed to estimate the average cost per patient undergoing initial bcs with two reoperation frequency scenarios: 23% and 10%. The model included the direct medical costs from the perspective of the B.C. health care system for the most common surgical treatment options, including breast reconstruction and postoperative radiation therapy.
Results
Costs ranged from a low of $8,225 per patient with definitive bcs [95% confidence interval (ci): $8,061 to $8,383] to a high of $26,026 for reoperation with mastectomy and delayed reconstruction (95% ci: $23,991 to $28,122). If the reoperation rate could be reduced to 10%, the average saving would be $1,055 per patient undergoing attempted bcs (95% ci: $959 to $1,156). If the lower rate were to be achieved in British Columbia, it would translate into a savings of $1.9 million annually.
Summary
The implementation of initiatives to reduce reoperation after bcs could result in significant savings to the health care system, while potentially improving the quality of patient care.
Keywords: Breast cancer, breast-conserving surgery, mastectomy, quality of care, costs
INTRODUCTION
Breast cancer occurs in 1 of every 9 women, making breast surgery the most common oncologic surgery performed in women in Canada. In most patients with early breast cancer, breast-conserving surgery (bcs) is the preferred approach. Although bcs is an excellent alternative to mastectomy, one potential downside is the frequent need for reoperation to address concerns about the pathologic status of the surgical margins. The frequency of re-excision shows significant variability, with institutional reviews reporting reoperation rates in the range 0%–60%1, and population-based studies reporting 17%–35%2–5. A recent report based on data from the Canadian Institute for Health Information (cihi) suggested a mean reoperation rate of 23% in Canada6, with significant interprovincial variability. Although no rate has achieved universal acceptance, many experts have suggested that the 23% rate is too high7,8, and the European Society of Breast Cancer Specialists has suggested that 10% be the goal9.
Although many patients with positive pathologic margins after bcs are candidates for re-excision, approximately half choose to have a mastectomy2,8, which might include reconstruction. The potential consequences of reoperation include social and psychological stressors, increased morbidity, and inferior cosmetic outcome10. Others have suggested that the frequency of reoperation after attempted bcs be considered for use as a quality measure in breast cancer surgery7,11,12, in part because of the aforementioned concerns. In addition, further surgery likely has significant associated economic costs, although the magnitude of those costs is poorly understood12. Hence, to quantify the impact of this potential quality measure in breast cancer surgery, we undertook a cost decision-analysis of post-bcs reoperations in a Canadian health care setting.
METHODS
To begin, a literature search was used to identify current patterns of practice. Ovid medline 1996–2015, Ovid embase 1996–2015, and PubMed medline were searched for articles dealing with breast neoplasms, mastectomy or segmental mastectomy, and reoperation (or re-excision), plus statistics, health care quality assurance, cost analyses, cost–benefit analyses, or economics. Information that best fit the context of the present study was used in the analysis.
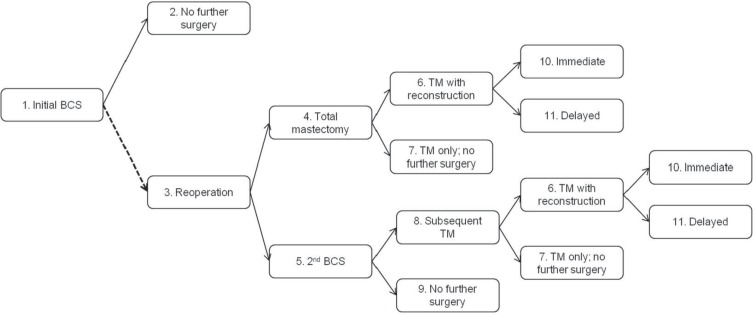
A decision tree was constructed to model the cost impact of re-excision after bcs (Figure 1). The scope of the model included the main surgical treatment options for patients after an initial bcs. Patients who do not require re-excision undergo no further surgeries. Patients requiring re-excision can undergo either a second bcs or a total mastectomy with or without immediate or delayed breast reconstruction. After a second bcs, all patients requiring further extirpative surgery are assumed to receive total mastectomy, with a probability of reconstruction equal to that for patients receiving mastectomy after the initial bcs. Table i summarizes the transition probabilities. The model tree was replicated for two scenarios: 23% re-excision, the Canadian average6, and 10% re-excision, the European Society of Breast Cancer Specialists target9.
FIGURE 1.
Decision tree for the cost model. The dashed line indicates where the probability of reoperation was varied in each of the two model scenarios. All patients undergo (1) initial breast-conserving surgery (BCS) and can either (2) require no further surgery or (3) undergo reoperation with either (4) a total mastectomy (TM), or (5) a second BCS. The second BCS can be either (9) the definitive procedure, or patients can undergo (8) a second reoperation with TM. Patients undergoing TM at any point in the model can (7) receive no further surgery, or might also receive (10) immediate or (11) delayed breast reconstruction.
TABLE I.
Model transition probabilities
| Transition | Tree path | Mean (%) | 95% CI (%) | Distribution | Source |
|---|---|---|---|---|---|
| Reoperation type | |||||
| Mastectomy | 3→4 | 52.7 | 50.1 to 55.3 | Beta | de Camargo Cancela et al., 20132 |
| Mastectomy after second BCS | 5→8 | 24.8 | 21.6 to 28.1 | Beta | |
| Reconstruction after mastectomy | 4→6 | 47.3 | 42.1 to 53.1 | Beta | Pao et al., 201313 |
| Immediate (vs. delayed) reconstruction | 6→10 | 75.0 | 70.0 to 80.0 | Normal | Assumption |
CI = confidence interval; BCS = breast-conserving surgery.
Costs in the model were considered from the perspective of the single-payer B.C. health care system and included the direct medical costs associated with the surgical procedures and any adjuvant radiotherapy (rt) after bcs or mastectomy. All other care-related costs were assumed to be independent of either re-excision rate. Costs are expressed in 2012 Canadian dollars and, where necessary, are inflated using the health care component of Statistics Canada’s Consumer Price Index14.
Surgical costs had three components (Table ii):
■ professional fees for surgeons, assistants, and anesthesiologists;
■ hospital case costs, including staff, equipment and overhead; and
■ additional materials for breast reconstruction.
TABLE II.
Surgery cost components
| Procedure | Cost ($) for … | ||||
|---|---|---|---|---|---|
|
| |||||
| Professional fees | Mean hospital case costa | Additional materials | |||
|
| |||||
| Surgeon | Assistant | Anesthetist | |||
| Breast-conserving surgery | 233 | 132 | 137 | 2,941 | 0 |
| Total mastectomy | |||||
| Alone | 465 | 186 | 137 | 4,232 | 0 |
| With immediate reconstructionb | 1,342 | 427 | 426 | 8,160 | 1,855 |
| With delayed reconstructionb | 1,015 | 268 | 378 | 4,481 | 1,716 |
| With secondary reconstructionb,c | 475 | 202 | 170 | 4,481 | 478 |
|
| |||||
| Mean (%) | 95% CI (%) | Distribution | Source | ||
|
| |||||
| Proportion bilaterald | 50 | 25 to 75 | Normal | Assumption | |
| Proportion with secondary nipple reconstruction | 75 | 65 to 85 | Normal | Assumption | |
Hospital case cost is calculated using resource intensity weight (RIW) for B.C. hospitals during 2008–2012, obtained from the Discharge Abstract Database maintained by the Canadian Institute for Health Information. Mean RIW for each case-mix group was varied using a log-normal distribution; the proportion of procedures conducted as day surgery within each group was varied using a beta distribution.
The cost values shown for reconstruction procedures are mean costs weighted to reflect the relative frequency of specific reconstruction procedure types (for example, implant of tissue expander, reconstruction with local tissue flap, reconstruction with free tissue flap, etc.) observed for B.C. hospitals during 2008–2012 in the Discharge Abstract Database maintained by the Canadian Institute for Health Information. Procedure frequency was varied using a Poisson distribution.
Secondary reconstruction procedures include replacing tissue expanders with a breast prosthesis and nipple–areolar complex reconstruction, after either immediate or delayed breast reconstruction.
Costs shown are for unilateral procedures; professional fees for bilateral procedures were calculated as 50% higher than for unilateral procedures (unless otherwise indicated in the fee schedule), materials were doubled, and case costs were unchanged with the exception of total mastectomy, for which case costs specific to unilateral and bilateral procedures were available.
CI = confidence interval.
Professional costs were obtained from the B.C. Medical Services Commission fee schedule for 201215, with average duration of anesthesia based on expert opinion.
Case-cost was calculated using cihi’s resource intensity weight (riw) method16. Case frequency and mean riw (CMG+ Directory, 2012 version) were obtained from the B.C. Ministry of Health for all provincial hospitalizations with a most-responsible diagnosis of malignant breast cancer (code C50 in cihi’s enhanced version of the International Classification of Diseases, revision 10) with a case-mix group (cmg) of partial breast excision (cmg 388), total breast excision (unilateral, cmg 387, or bilateral, cmg 386), or breast reconstruction (cmg 385), based on B.C. data from cihi’s Discharge Abstract Database for 2008–2012. Mean riw for each cmg, weighted for the proportion of observed inpatient and day surgery procedures, was multiplied by the mean cost per weighted case for British Columbia17 to calculate an average case-cost.
The cost of materials for breast reconstruction—including tissue expander, breast prosthesis, and collagen regenerative tissue matrix—were obtained from a cost analysis conducted in British Columbia18. To calculate the mean professional and materials costs of breast reconstruction procedures, the relative frequency of procedures conducted in the province was calculated from the B.C. hospitalization data already described. Specific procedure types were identified using Canadian Classification of Health Interventions codes for total mastectomy with reconstruction (1.YM.90) and secondary breast reconstruction (1.YM.80)19. We assumed that, on average, 75% of breast reconstruction patients would undergo reconstruction of the nipple–areola complex in a subsequent procedure, and that all reconstruction procedures with implantation of a tissue expander would also include the cost of the ensuing prosthesis implantation. Costs for reconstruction of the nipple–areola complex and for the implantation procedure were both calculated using the professional fees, case-cost, and materials already described. For all total mastectomy and reconstruction costs, we assumed that, on average, 50% of the procedures would be bilateral20.
Use of adjuvant rt was calculated using aggregate data from the BC Cancer Agency (bcca) for patients diagnosed in 2008 with primary breast tumours 50 mm or less in size and no metastases (T1 or T2 and M0 according to the American Joint Committee on Cancer staging system) who had been referred to bcca treatment centres. The frequency of adjuvant rt use and the mean number of fractions delivered per patient were calculated for patients receiving either bcs or mastectomy as the definitive surgical procedure (Table iii). The proportion of patients receiving rt was adjusted to account for bcca referral rates21 and was multiplied by the mean number of fractions and the mean cost per fraction (using an estimate provided by the bcca rt department) to calculate the mean cost of rt per bcs or total mastectomy patient.
TABLE III.
Radiotherapy (RT) cost components
| Definitive surgical procedure | Proportion receiving adjuvant RT (%) | Fractions (n) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | 95% CI | Distribution | Mean | 95% CI | Distribution | |
| Breast-conserving surgery | 78.8 | 76.6 to 81.0 | Beta | 17.5 | 17.3 to 17.8 | Normal |
| Mastectomy | 33.6 | 30.3 to 36.9 | Beta | 20.6 | 20.0 to 21.3 | Normal |
CI = confidence interval.
To account for uncertainty in its parameters22, the model was analyzed probabilistically using the distributions (mean and 95% confidence intervals) provided in Tables i, ii, and iii. All transition probabilities and proportions incorporated into cost calculations (for example, the proportion of patients receiving rt) were included as beta distributions. Lognormal distributions were used for riw values; normal distributions, for the number of rt fractions; and Poisson distributions, for the frequency of reconstruction procedures. Professional fees, cost of materials, and unit costs were held fixed22. All assumed values were varied using normal distributions. The cost model was replicated 10,000 times by Monte Carlo simulation using re-excision rates of 23% and 10%. Mean and 95% confidence intervals for cost were calculated for each re-excision rate scenario and for the incremental difference between the two scenarios. Potential annual savings for the B.C. population were estimated using the approximate annual number of incident breast cancer cases receiving bcs. The model was built and analyzed in Microsoft Excel (2013 version: Microsoft Corporation, Redmond, WA, U.S.A.).
The sensitivity of the model to the distribution of reoperation procedures was investigated using deterministic sensitivity analyses. The two parameters defining the distribution of reoperation procedures were selected for the sensitivity analyses because little evidence was available locally or in the literature, and those parameters had the potential to significantly affect reoperation costs in the model.
In the first sensitivity analysis, the proportion of patients undergoing re-excision who underwent total mastectomy as their definitive procedure, on either first (Figure 1, model path 3→4) or second (model path 3→5→8) re-excision was adjusted to equal 46%, the total value reported in cihi’s breast surgery report6. In the base case, the proportion of patients receiving total mastectomy on first or second re-excision was 64%2.
In the second sensitivity analysis, the sensitivity of the model to breast reconstruction rates6 was determined by reducing the proportion of patients initially undergoing breast reconstruction to 25% from an average of 48% in the base case.
RESULTS
A substantial difference was observed in the cost associated with each of the surveyed surgical procedures, exclusive of radiation charges (Table iv). The mean health care costs for bcs and mastectomy were $3,443 [95% confidence interval (ci): $3,420 to $3,465] and $6,088 (95% ci: $5,552 to $6,617) respectively; those for mastectomy with immediate or delayed reconstruction were $19,229 (95% ci: $17,945 to $20,628) and $20,185 (95% ci: $18,169 to $22,236) respectively.
TABLE IV.
Summary of model costs
| Procedure | Tree node | Costs ($) | |
|---|---|---|---|
|
| |||
| Mean | 95% CI | ||
| Breast-conserving surgery (BCS) | 1, 5 | 3,443 | 3,420 to 3,465 |
| RT after BCS | 2, 9 | 4,807 | 4,617 to 4,931 |
| Total mastectomy | |||
| Alone | 7 | 6,088 | 5,552 to 6,617 |
| With immediate reconstruction | 10 | 19,229 | 17,945 to 20,628 |
| With delayed reconstruction | 11 | 20,185 | 18,169 to 22,236 |
| RT after mastectomy | 7, 10, 11 | 2,387 | 2,163 to 2,662 |
CI = confidence interval; RT = radiotherapy; TM = total mastectomy.
When considering the total pathway costs in the decision tree, including the use of rt, a definitive initial bcs cost an average of $8,225 per patient (95% ci: $8,061 to $8,383). Reoperation with bcs only cost nearly as much as reoperation with mastectomy only [mean cost: $11,667 (95% ci: $11,499 to $11,832) and $11,930 (95% ci: $11,346 to $12,529) respectively]. Those pathway costs were substantially less than the total pathway costs for patients who underwent either immediate ($25,080; 95% ci: $23,809 to $26,518) or delayed ($26,026; 95% ci: $23,991 to $28,122) reconstruction.
Using the probabilities included in the decision tree and a baseline reoperation rate of 23%, the average cost per initial bcs patient is estimated to be $10,091 (95% ci: $9,868 to $10,314; Table v). If the reoperation rate were to be lowered to 10%, with all other factors kept constant, the average cost would fall to $9,036 (95% ci: $8,868 to $9,204). The difference translates into a potential cost saving of $1,055 per patient (95% ci: $959 to $1,156) undergoing attempted bcs. Put another way, the potential saving per reoperation avoided is $8,118 (95% ci: $7,378 to $8,897). If applied at a population level within British Columbia, the likely result would be an annual saving of at least $1.9 million.
TABLE V.
Mean costs and potential savings for patients initially treated with breast-conserving surgery in British Columbia
| Variable | Cost per patient ($) | Savings ($) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | 95% CI | Per patient | Population | |||
|
|
|
|||||
| Mean | 95% CI | Mean | 95% CI | |||
| Canadian average (23% reoperation) | 10,091 | 9,868 to 10,314 | Reference | Reference | ||
| Target (10% reoperation) | 9,036 | 8,868 to 9,204 | 1,055 | 959 to 1,156 | 1,899,000 | 1,726,000 to 2,081,000 |
CI = confidence interval.
The model was moderately sensitive to the parameters defining reoperation procedures. Of the reoperation costs, a significant portion is attributable to the selection of mastectomy, with subsequent reconstruction. Reducing the use of mastectomy to 46% among all patients undergoing re-excision lowered the potential per-patient saving to $924 (95% ci: $852 to $997). If the proportion of mastectomy patients subsequently undergoing breast reconstruction were to be reduced by roughly one half, to 25%, the potential per-patient saving from a reduction in the re-excision rate fell to $802 (95% ci: $743 to $863).
DISCUSSION
Most patients with early-stage breast cancer are eligible for and can be safely treated with bcs. Reoperation rates are highly variable1–5, and although there is no evidence that re-excision is detrimental to overall survival23, it likely causes patient anxiety, increases surgical complication rates24, and contributes to poorer cosmetic results10,25. It might also delay the initiation of adjuvant chemotherapy. As an alternative, many patients elect to undergo mastectomy with or without reconstruction, which is a vastly different clinical course, associated with higher complications26,27. Moreover, the need for secondary procedures has a significant economic cost, which is of particular concern in a publicly funded health care system.
Little information is available about the costs associated with surgical interventions in general—and breast surgery in particular12. Studies performed soon after the introduction of bcs found that the procedure was more expensive than mastectomy, largely because of the fees associated with adjuvant radiation28–31. To our knowledge, only two other centres have compared the costs associated with the 3 major surgical interventions in breast cancer: namely, bcs, mastectomy, and mastectomy with reconstruction.
One study identified patients from a state cancer registry and reported that the costs associated with bcs were higher than those associated with mastectomy, and not surprisingly, that reconstruction was more expensive than either of the other two procedures29. Those findings are similar to ours, except that their costs were roughly double ours for each intervention (bcs: $21,582 vs. $8,225; mastectomy: $16,122 vs. $11,930; reconstruction: $31,047 vs. $25,080). Their cost calculations were presented in U.S. dollars indexed to the year 1992 and included all procedures within 2 years of the index operation; in contrast, our study is presented in Canadian dollars for the year 2012. The second study also found that, compared with mastectomy, bcs was associated with higher charges ($26,330 vs. $9,780), but interestingly, with less for reconstruction ($22,720), suggesting that the radiation treatment greatly influenced the charges31.
Additional limitations arise in comparing those studies with ours. The two U.S. studies are highly influenced by variable charges, which occur routinely in the United States32. Within Canada, physician fees and hospital-related costs are both more likely to reflect the direct costs associated with health care and are thus subject to much less variability. The hospital costs in the present study are based on riws from Canadian micro-costing data and average case costs from actual B.C. hospital expenditures16. The fee schedule for all publicly paid physicians in British Columbia (under fee-for-service) is fixed in any given year15 and reflects the direct cost to the public payer. Moreover, the two U.S. studies are inappropriate for population-based analysis, given that both included only patients with insured services and low rates of breast conservation, with one excluding half the patients because of inconsistencies in claims29. The U.S. reports are also not contemporaneous; the patients underwent surgery more than 20 years ago (when hospital stays for individuals undergoing mastectomy were prolonged), and details about the types of reconstruction performed are lacking31. Finally, the two reports pre-date modern breast cancer treatment, with no reporting or consideration of rt for individuals undergoing mastectomy.
Surgery represents the single most important intervention in the care of patients with nonmetastatic breast cancer, and it is responsible for a significant component of the initial health care cost associated with the treatment of breast cancer33. Given the significant variation in the need for reoperation after an initial attempt at bcs, the reoperation rate becomes a potential area of focus in the pursuit of health care cost reductions. A number of factors determine the need for reoperation, including disease3,5,34–37, patient-related considerations3–5, and potential influences of the surgeon2,35,38,39 and the institution3–5,8. Although changing disease-and patient-related factors is difficult, there are structural and process-related measures that could potentially be implemented to reduce the need for reoperation. Possibilities include the use of intraoperative imprint cytology1, cavity margin shaving40, and other technologies41,42. Others have focused on the effects of education initiatives43 or guidelines44. Recent efforts to standardize the approach to addressing pathologic margin status also have the potential to effect reoperation rates45. Although no clear single solution has emerged, high variability suggests this intervention could be a useful quality measure7,11,12, or at least a factor that affects patient satisfaction and cost46,47.
In Canada, 23% of breast cancer patients require at least 1 reoperation after attempted bcs6. Although no consensus on the optimal reoperation rate has been reached, several international bodies have, as noted earlier, suggested that reducing the number of repeat operations is a necessity7–9, with one organization suggesting 10% as a goal9. A number of institutional publications have reported reoperation rates close to that benchmark1,8,34, suggesting that it is achievable. However, any effort to move toward that goal has to take into account other considerations. First, the amount of tissue resected during bcs must not be excessive or the cosmesis associated with the procedure could be adversely affected. Second, placing too much emphasis on limiting the reoperation rate might potentially sway some surgeons to perform a mastectomy rather than attempt a bcs. Thus, any structured attempt to lower the reoperation rate must also consider the cosmesis associated with the procedure and both the initial and the overall mastectomy rate.
Assuming that other breast surgery quality metrics can be maintained, lowering the number of reoperations after attempted bcs has significant economic implications. Based on our decision analysis, if the current reoperation rate in Canada were to be lowered to 10% from 23%, the average savings per patient in the population undergoing initial attempted bcs would be $1,055. That amount is likely an underestimate because it does not take into account the costs associated with any complications that might occur out of hospital26,27 or any secondary procedures for complications, which are more likely in patients who choose mastectomy and reconstruction48. The economic implications are significant, especially in a publicly funded universal health care system, given that the potential savings are at least $1.9 million annually in the province of British Columbia alone. The same argument also supports the assertion that a focus on quality in general has a positive impact on cost reduction12, and that the provision of cost-effective care is increasingly considered an important component of quality health care49.
As with any decision analysis, ours has limitations based on available information. First, it might not be possible to reduce the reoperation rate to 10% on a population level. Most publications addressing efforts to improve outcomes are usually reported at the institutional or regional level, with many showing variable success. For example, at an institutional level, a randomized trial of routine cavity shaving reduced the reoperation rate for margin clearance to 10% from 21%40. At a regional level, efforts that used workshops and periodic audit and feedback to influence a larger and more diverse group of surgeons yielded moderate success in reducing the number of pathologically positive margins after bcs43. In addition, the joint guidelines released by the Society for Surgical Oncology and the American Society for Radiation Oncology in 2014 are likely to lower the baseline Canadian re-excision rate below its current 23%; however, the impact of their approach has yet to be determined. If adopted on a global scale, it has significant potential cost savings45. Nonetheless, our study shows that there are significant economic implications of measures that reduce the need to reoperate after attempted bcs. The present work could be useful in comparative effectiveness research considering interventions that might potentially lower the reoperation rate and the costs associated with those interventions.
A second limitation is that the use of reconstruction appears to significantly affect the costs associated with reoperation, primarily because of its high resource intensity, which introduces some uncertainty about the magnitude of the potential cost savings. When the reconstruction rate used in the model was halved, the cost saving per patient declined by roughly 20%, to $802 from $1,055. There are significant disparities in access to reconstruction in Canada50, suggesting that the potential cost savings from reductions in re-excision would vary across regions. Also, evidence from other countries suggests that the use of breast reconstruction is on the increase and is likely to increase in Canada as well51, implying potentially greater cost savings with a reduction in re-excision rates. Furthermore, the costs associated with reconstruction are likely to have been underestimated, because health care costs arising from out-of-hospital complications, readmissions for complications, and further revision surgery (which is moderately frequent) were not factored in20,48,52.
Finally, our model does not consider the long-term outcomes of breast surgery, such local recurrence, survival, or quality of life, which would be required for a comprehensive cost-effectiveness or cost–utility analysis22. The final pathologic status of surgical margins is associated with risk of recurrence53; re-excision alone is not54. By focusing only on cost, the analysis presented here implicitly assumes that the long-term outcomes of breast surgery are independent of the re-excision rate. Economic evaluation of any measures designed to reduce the re-excision rate would need to confirm that assumption.
CONCLUSIONS
The necessity to reoperate after attempted bcs is considered by some to be a quality measure, and it has significant cost implications. Our study demonstrates that efforts to lower the reoperation rate could result in significant resource savings, and the cost estimates presented could be of use in future comparative effectiveness research. Further investigation into the role of guidelines and education in lowering the reoperation rate, and into the potential impact of lower rates on costs and improved quality of care for breast cancer patients is needed.
ACKNOWLEDGMENTS
The authors thank Malcolm Paterson (Director, Clinical Initiatives) and Krista Clement (librarian) at the bcca’s Sindi Ahluwalia Hawkins Centre for the Southern Interior for their research and editorial assistance. The Canadian Centre for Applied Research in Cancer Control is funded by the Canadian Cancer Society Research Institute (grant no. 019789)
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Esbona K, Li Z, Wilke LG. Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: a systematic review. Ann Surg Oncol. 2012;19:3236–45. doi: 10.1245/s10434-012-2492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Camargo Cancela M, Comber H, Sharp L. Hospital and surgeon caseload are associated with risk of re-operation following breast-conserving surgery. Breast Cancer Res Treat. 2013;140:535–44. doi: 10.1007/s10549-013-2652-5. [DOI] [PubMed] [Google Scholar]
- 3.Wilke LG, Czechura T, Wang C, et al. Repeat surgery after breast conservation for the treatment of stage 0 to ii breast carcinoma: a report from the National Cancer Data Base, 2004–2010. JAMA Surg. 2014;149:1296–305. doi: 10.1001/jamasurg.2014.926. [DOI] [PubMed] [Google Scholar]
- 4.Spilsbury K, Semmens JB, Saunders CM, Hall SE, Holman CD. Subsequent surgery after initial breast conserving surgery: a population based study. ANZ J Surg. 2005;75:260–4. doi: 10.1111/j.1445-2197.2005.03352.x. [DOI] [PubMed] [Google Scholar]
- 5.Jeevan R, Cromwell DA, Trivella M, et al. Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ. 2012;345:e4505. doi: 10.1136/bmj.e4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canadian Institute for Health Information (cihi) Breast Cancer Surgery in Canada, 2007–2008 to 2009–2010. Ottawa, ON: CIHI; 2012. [Google Scholar]
- 7.Landercasper J, Attai D, Atisha D, et al. Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: the American Society of Breast Surgeons consensus conference. Ann Surg Oncol. 2015;22:3174–83. doi: 10.1245/s10434-015-4759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talsma AK, Reedijk AM, Damhuis RA, Westenend PJ, Vles WJ. Re-resection rates after breast-conserving surgery as a performance indicator: introduction of a case-mix model to allow comparison between Dutch hospitals. Eur J Surg Oncol. 2011;37:357–63. doi: 10.1016/j.ejso.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Del Turco MR, Ponti A, Bick U, et al. Quality indicators in breast cancer care. Eur J Cancer. 2010;46:2344–56. doi: 10.1016/j.ejca.2010.06.119. [DOI] [PubMed] [Google Scholar]
- 10.Parvez E, Cornacchi SD, Hodgson N, et al. A cosmesis outcome substudy in a prospective, randomized trial comparing radioguided seed localization with standard wire localization for nonpalpable, invasive, and in situ breast carcinomas. Am J Surg. 2014;208:711–18. doi: 10.1016/j.amjsurg.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 11.McCahill LE, Privette A, James T, et al. Quality measures for breast cancer surgery: initial validation of feasibility and assessment of variation among surgeons. Arch Surg. 2009;144:455–62. doi: 10.1001/archsurg.2009.56. [DOI] [PubMed] [Google Scholar]
- 12.Landercasper J, Tafra L. The relationship between quality and cost during the perioperative breast cancer episode of care. Breast. 2010;19:289–96. doi: 10.1016/j.breast.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Pao JS, Kuusk U, Cheema A, Dingee C, McKevitt E. Assessment of a Canadian breast cancer centre against European and American quality standards [abstract 144] CMAJ. 2013;56(suppl 3):S132. [Google Scholar]
- 14.Statistics Canada . Table 326-0021: Consumer Price Index, annual (2002=100) [health and personal care component] Ottawa, ON: Statistics Canada; 2013. [Available at: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=3260021; cited 28 October 2013] [Google Scholar]
- 15.B.C. Medical Services Commission (msc) MSC Payment Schedule. Victoria, BC: MSC; 2012. [Google Scholar]
- 16.Pink GH, Bolley HB. Physicians in Health Care Management: 3. case mix groups and resource intensity weights: an overview for physicians. CMAJ. 1994;150:889–94. [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Institute for Health Information (cihi) Hospital Financial Performance Indicators, 2009–2010. Ottawa, ON: CIHI; 2012. [Google Scholar]
- 18.Jansen LA, Macadam SA. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part ii. A cost analysis. Plast Reconstr Surg. 2011;127:2245–54. doi: 10.1097/PRS.0b013e3182131c6b. [DOI] [PubMed] [Google Scholar]
- 19.Canadian Institute for Health Information (cihi) Canadian Classification of Health Interventions. Vol. 3. Ottawa, ON: CIHI; 2012. tabular list. [Google Scholar]
- 20.Abedi N, Ho AL, Knox A, et al. Predictors of mastectomy flap necrosis in patients undergoing immediate breast reconstruction: a review of 718 patients. Ann Plast Surg. 2016;76:629–34. doi: 10.1097/SAP.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 21.BC Cancer Agency (bcca) Referral Rates for Cancer Cases Diagnosed in 2012. Vancouver, BC: BCCA; 2014. Cancer Surveillance and Outcomes. [Google Scholar]
- 22.Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 23.Vos EL, Jager A, Verhoef C, Voogd AC, Koppert LB. Overall survival in patients with a re-excision following breast conserving surgery compared to those without in a large population-based cohort. Eur J Cancer. 2015;51:282–91. doi: 10.1016/j.ejca.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Xue DQ, Qian C, Yang L, Wang XF. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012;38:375–81. doi: 10.1016/j.ejso.2012.02.179. [DOI] [PubMed] [Google Scholar]
- 25.Wazer DE, DiPetrillo T, Schmidt-Ullrich R, et al. Factors influencing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1992;10:356–63. doi: 10.1200/JCO.1992.10.3.356. [DOI] [PubMed] [Google Scholar]
- 26.Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: a claims-based analysis. Ann Surg. 2016;263:219–27. doi: 10.1097/SLA.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyfer B, Chatterjee A, Chen L, et al. Early postoperative outcomes in breast conservation surgery versus simple mastectomy with implant reconstruction: a nsqip analysis of 11,645 patients. Ann Surg Oncol. 2016;23:92–8. doi: 10.1245/s10434-015-4770-2. [DOI] [PubMed] [Google Scholar]
- 28.Barlow WE, Taplin SH, Yoshida CK, Buist DS, Seger D, Brown M. Cost comparison of mastectomy versus breast-conserving therapy for early-stage breast cancer. J Natl Cancer Inst. 2001;93:447–55. doi: 10.1093/jnci/93.6.447. [DOI] [PubMed] [Google Scholar]
- 29.Desch CE, Penberthy LT, Hillner BE, et al. A sociodemographic and economic comparison of breast reconstruction, mastectomy, and conservative surgery. Surgery. 1999;125:441–7. doi: 10.1016/S0039-6060(99)70012-7. [DOI] [PubMed] [Google Scholar]
- 30.Norum J, Olsen JA, Wist EA. Lumpectomy or mastectomy? Is breast conserving surgery too expensive? Breast Cancer Res Treat. 1997;45:7–14. doi: 10.1023/A:1005804101106. [DOI] [PubMed] [Google Scholar]
- 31.Palit TK, Miltenburg DM, Brunicardi FC. Cost analysis of breast conservation surgery compared with modified radical mastectomy with and without reconstruction. Am J Surg. 2000;179:441–5. doi: 10.1016/S0002-9610(00)00383-4. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Herrin J, Soulos PR, et al. The role of patient factors, cancer characteristics, and treatment patterns in the cost of care for Medicare beneficiaries with breast cancer. Health Serv Res. 2016;51:167–86. doi: 10.1111/1475-6773.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21:281–93. doi: 10.3747/co.21.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kryh CG, Pietersen CA, Rahr HB, Christensen RD, Wamberg P, Lautrup MD. Re-resection rates and risk characteristics following breast conserving surgery for breast cancer and carcinoma in situ: a single-centre study of 1575 consecutive cases. Breast. 2014;23:784–9. doi: 10.1016/j.breast.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Landercasper J, Whitacre E, Degnim AC, Al-Hamadani M. Reasons for re-excision after lumpectomy for breast cancer: insight from the American Society of Breast Surgeons Mastery (sm) database. Ann Surg Oncol. 2014;21:3185–91. doi: 10.1245/s10434-014-3905-1. [DOI] [PubMed] [Google Scholar]
- 36.Lovrics PJ, Cornacchi SD, Farrokhyar F, et al. Technical factors, surgeon case volume and positive margin rates after breast conservation surgery for early-stage breast cancer. Can J Surg. 2010;53:305–12. [PMC free article] [PubMed] [Google Scholar]
- 37.Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol. 2008;15:1297–303. doi: 10.1245/s10434-007-9777-x. [DOI] [PubMed] [Google Scholar]
- 38.Blair SL, O’Shea KE, Orr RK. Surgeon variability in treating nonpalpable breast cancer: surgical oncology as a value-added specialty. Ann Surg Oncol. 1998;5:28–32. doi: 10.1007/BF02303760. [DOI] [PubMed] [Google Scholar]
- 39.Zork NM, Komenaka IK, Pennington RE, Jr, et al. The effect of dedicated breast surgeons on the short-term outcomes in breast cancer. Ann Surg. 2008;248:280–5. doi: 10.1097/SLA.0b013e3181784647. [DOI] [PubMed] [Google Scholar]
- 40.Chagpar AB, Killelea BK, Tsangaris TN, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med. 2015;373:503–10. doi: 10.1056/NEJMoa1504473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnabel F, Boolbol SK, Gittleman M, et al. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol. 2014;21:1589–95. doi: 10.1245/s10434-014-3602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krekel NM, Haloua MH, Lopes Cardozo AM, et al. Intraoperative ultrasound guidance for palpable breast cancer excision (cobalt trial): a multicentre, randomised controlled trial. Lancet Oncol. 2013;14:48–54. doi: 10.1016/S1470-2045(12)70527-2. [DOI] [PubMed] [Google Scholar]
- 43.Lovrics P, Hodgson N, O’Brien MA, et al. Results of a surgeon-directed quality improvement project on breast cancer surgery outcomes in South-Central Ontario. Ann Surg Oncol. 2014;21:2181–7. doi: 10.1245/s10434-014-3592-y. [DOI] [PubMed] [Google Scholar]
- 44.Reames BN, Shubeck SP, Birkmeyer JD. Strategies for reducing regional variation in the use of surgery: a systematic review. Ann Surg. 2014;259:616–27. doi: 10.1097/SLA.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moran MS, Schnitt SJ, Giuliano AE, et al. on behalf of the Society of Surgical Oncology and the American Society for Radiation Oncology Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages i and ii invasive breast cancer. J Clin Oncol. 2014;32:1507–15. doi: 10.1200/JCO.2013.53.3935. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz T, Degnim AC, Landercasper J. Should re-excision lumpectomy rates be a quality measure in breast-conserving surgery? Ann Surg Oncol. 2013;20:3180–3. doi: 10.1245/s10434-013-3206-0. [DOI] [PubMed] [Google Scholar]
- 47.Haloua MH, Krekel NM, Coupe VM, et al. Ultrasound-guided surgery for palpable breast cancer is cost-saving: results of a cost–benefit analysis. Breast. 2013;22:238–43. doi: 10.1016/j.breast.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Fischer JP, Fox JP, Nelson JA, Kovach SJ, Serletti JM. A longitudinal assessment of outcomes and healthcare resource utilization after immediate breast reconstruction-comparing implant- and autologous-based breast reconstruction. Ann Surg. 2015;262:692–9. doi: 10.1097/SLA.0000000000001457. [DOI] [PubMed] [Google Scholar]
- 49.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008;27:759–69. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 50.Zhong T, Fernandes KA, Saskin R, et al. Barriers to immediate breast reconstruction in the Canadian universal health care system. J Clin Oncol. 2014;32:2133–41. doi: 10.1200/JCO.2013.53.0774. [DOI] [PubMed] [Google Scholar]
- 51.Yang RL, Newman AS, Lin IC, et al. Trends in immediate breast reconstruction across insurance groups after enactment of breast cancer legislation. Cancer. 2013;119:2462–8. doi: 10.1002/cncr.28050. [DOI] [PubMed] [Google Scholar]
- 52.Roberts A, Baxter N, Camacho X, Lau C, Zhong T. Once is rarely enough: a population-based study of reoperations after postmastectomy breast reconstruction. Ann Surg Oncol. 2015;22:3302–7. doi: 10.1245/s10434-015-4716-8. [DOI] [PubMed] [Google Scholar]
- 53.Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46:3219–32. doi: 10.1016/j.ejca.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 54.Neuschatz AC, DiPetrillo T, Safaii H, Price LL, Schmidt-Ullrich RK, Wazer DE. Long-term follow-up of a prospective policy of margin-directed radiation dose escalation in breast-conserving therapy. Cancer. 2003;97:30–9. doi: 10.1002/cncr.10981. [DOI] [PubMed] [Google Scholar]