Abstract
Background
The overall survival (os) analysis of the icon7 trial demonstrated that frontline ovarian cancer patients with a high risk of progression (stage iii suboptimally debulked, and stage iii or iv with unresectable disease) benefited from the addition of bevacizumab to standard chemotherapy compared with standard chemotherapy alone. The objective of the present study was to investigate the cost-effectiveness, from a Canadian publicly funded perspective, of adding bevacizumab to frontline treatment of ovarian cancer at high risk of progression.
Methods
An area-under-the-curve, Markov-structured model was used to estimate the cost-effectiveness of the treatments. Long-term progression-free survival (pfs) and os were extracted from the icon7 trial (subgroup at high risk of relapse) and extrapolated by parametric time-to-event functions over a time horizon of 10 years. Canadian pfs health state utility values were obtained from the EQ-5D (EuroQoL Group, Rotterdam, Netherlands) questionnaires in the icon7 high-risk patient population. Canadian post-progression utility values were consistent with those for other gynecologic cancers. Cost inputs were informed by public sources. An annual 5% efficacy and cost discount rate was applied. A probabilistic sensitivity analysis and one-way sensitivity analyses were conducted.
Results
Ovarian cancer patients at high risk of progression receiving bevacizumab plus standard chemotherapy experienced a mean incremental quality-adjusted life year (qaly) gain of 0.374 years. At an additional cost of $35,901.54, the incremental cost-effectiveness ratio (icer) for the addition of bevacizumab to standard chemotherapy, relative to standard chemotherapy alone, was $95,942 per qaly.
Conclusions
No formal health technology assessment willingness-to-pay threshold exists in Canada. However, at a threshold of $100,000 per qaly, bevacizumab in addition to chemotherapy is a cost-effective alternative for ovarian cancer patients who are at high risk of progression (stage iii suboptimally debulked, and stage iii or iv with unresectable disease). Using the $100,000 per qaly threshold in a probabilistic sensitivity analysis, it was determined that, compared with standard chemotherapy, the addition of bevacizumab to chemotherapy is cost-effective in 56% of tested scenarios.
Keywords: Ovarian cancer, bevacizumab, cost-effectiveness, decision-making, health economics, Canada, high-risk disease, health technology assessments
INTRODUCTION
Ovarian cancer is a leading cause of cancer-related death in Canadian women. Approximately 2700 new ovarian cancer cases are diagnosed and 1750 ovarian cancer–related deaths occur each year1. Of all ovarian cancers, 75% are advanced (stages iii and iv), with a 5-year survival of 20%–40%2. Worldwide each year, 224,747 new ovarian cancers are diagnosed, and 140,163 women die from the disease3. Of the cancers unique to women, ovarian cancer has the 3rd-highest mortality3. Incidence rates vary worldwide, with the highest occurrence in Europe and the United States, and the lowest occurrence in Africa and developing countries4. Approximately 43,000 cases of ovarian cancer occur each year in Europe and 22,000 in the United States5.
Management options for advanced ovarian cancer include primary cytoreduction, followed by adjuvant chemotherapy or neoadjuvant chemotherapy, with subsequent cytoreduction and adjuvant chemotherapy. Surgical cytoreduction followed by cytotoxic chemotherapy has long been recognized as the standard-of-care management strategy in women presenting with advanced ovarian cancer in Canada. Survival depends not only on stage of the disease, but also on outcome of surgery as measured by residual disease6,7. The goal of surgery is maximal removal of tumour tissue to non-visible residual disease, because evidence has shown that survival is substantially significantly better in patients receiving debulking to microscopic disease than in those receiving debulking to macroscopic residual disease. Additionally, a survival advantage is evident when residual disease is less than 1 cm compared with more than 1 cm.
Until recently, a combination of surgery and standard chemotherapy has been the mainstay of treatment for advanced ovarian cancer when the time lag between surgery and systemic treatment is 4–6 weeks (leaving enough time for recovery after the debulking surgery). More recently, significant interest has emerged for modulation of vascular endothelial growth factor as a potential additional component of treatment.
The effectiveness of vascular endothelial growth factor modulation has been demonstrated in two large landmark phase iii randomized trials. The addition of the anti–vascular endothelial growth factor monoclonal antibody bevacizumab to conventionally administered carboplatin and paclitaxel chemotherapy significantly improved progression-free survival (pfs) in treated patients8,9. The Gynecologic Oncology Group 218 trial, a phase iii randomized double-blind placebo-controlled trial, examined the efficacy of the addition of bevacizumab (15 mg/kg) to standard chemotherapy treatment in women with newly diagnosed advanced ovarian, primary peritoneal, or fallopian cancer. That trial enrolled 1873 high-risk patients with International Federation of Gynecology and Obstetrics stages iii and iv ovarian cancer and macroscopic residual disease after primary surgery; it demonstrated a significant improvement in pfs with the addition of bevacizumab (hazard ratio: 0.72; 95% confidence interval: 0.63 to 0.82; p < 0.001)5.
The International Collaborative Ovarian Neoplasm 7 (icon7) trial, a randomized open-label phase iii trial, was designed to evaluate the safety and efficacy of adding bevacizumab (7.5 mg/kg) to standard chemotherapy in patients with advanced epithelial ovarian or primary peritoneal cancer. Results from icon7 showed improved clinical benefit with the addition of bevacizumab in a broader population that included a high-risk, poor-prognosis patient group in addition to patients with early-stage disease and with optimally or suboptimally debulked advanced disease. Significant improvement in pfs (hazard ratio: 0.81; 95% confidence interval: 0.60 to 0.93; p = 0.02) was shown in all bevacizumab-treated patients after 42 months of follow-up7. Although overall os was not significantly improved in the icon7 trial, a preplanned analysis in women at high risk of disease progression showed a statistically significant improvement in os for patients randomized to the bevacizumab arm (hazard ratio: 0.78; 95% confidence interval: 0.63 to 0.97; p = 0.03)10, suggesting that the addition of bevacizumab to standard chemotherapy for patients at high risk of disease progression is an effective treatment option that can significantly improve pfs and os.
At the time of submission of this manuscript, only two cost-effectiveness analyses comparing the combination of bevacizumab (7.5 mg/kg) and standard chemotherapy with chemotherapy alone in a high-risk patient population as defined by the icon7 trial had been published. One analysis took the perspective of the National Health Service in the United Kingdom11. The other was a U.S. analysis conducted from the perspective of the Medicare system12. Neither analysis was representative of the Canadian public health care system, and the generalizability of cost-effectiveness measures from other health care systems to the Canadian context is limited. Here, we present the first cost-effectiveness analysis in Canada and discuss its adoption by the Canadian public health care system.
METHODS
Model Structure
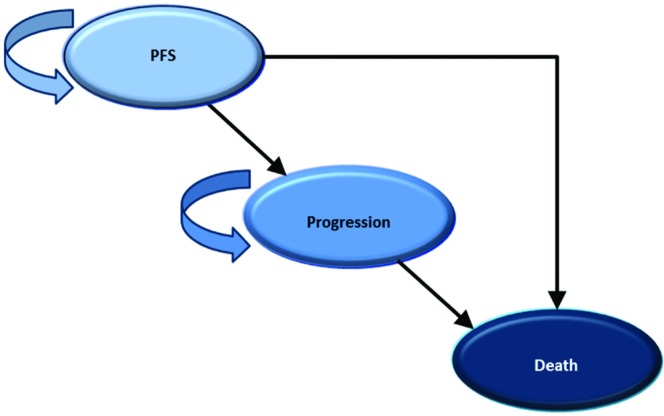
A Markov-structured area-under-the-curve model was developed in Excel (Microsoft, Redmond, WA, U.S.A.) to estimate the cost-effectiveness in Canada of combined bevacizumab and standard chemotherapy relative to standard chemotherapy alone for the treatment of advanced ovarian cancer at high risk of relapse. The model included 3 mutually exclusive health states that characterize the typical progression of oncologic diseases: PFS, Progression, and Death (Figure 1). The analysis was conducted from the perspective of the publicly funded Canadian health care system. Costs are reported in 2014 Canadian dollars, and health outcomes are assessed as quality-adjusted life years (qalys). For the base–case analysis, the time horizon was 10 years, and after the first year, all costs and outcomes were discounted by 5% annually.
FIGURE 1.
Schematic of the area-under-the-curve Markov-structured model.
Patient Population
The analysis presented here applies to the subpopulation of patients in the icon7 trial who were at high risk of progression—that is, those defined as having suboptimally debulked (>1 cm) stage iii disease or unresectable stage iii or iv disease (International Federation of Gynecology and Obstetrics staging). That subpopulation was represented by 502 (33%) of the 1528 patients enrolled in the icon7 trial7.
Treatment Strategies
The treatment strategies evaluated here replicate the treatment arms of the icon7 trial. In the new-treatment arm, patients received 6 three-weekly cycles of standard chemotherapy together with concurrent intravenous bevacizumab (7.5 mg/kg body weight), starting at cycle 2 (per protocol for the high-risk patient population). Additionally, patients received another 12 three-weekly cycles of bevacizumab (or until disease progression or treatment discontinuation). In the comparator-treatment arm, patients received 6 three-weekly cycles of standard chemotherapy without bevacizumab. Standard chemotherapy consisted of 6 three-weekly cycles of carboplatin (area under the curve 5 or 6) and paclitaxel (175 mg/m2 body surface area).
Clinical Effectiveness
In an area-under-the-curve partitioned-survival model, health state transitions are determined by the proportion of patients in pfs and os, and the progressed state is assumed to be the difference between the os and pfs states. Parametric extrapolation of patient-level data abstracted from the icon7 trial was used to estimate the pfs and os of patients for each treatment arm separately, with independent shape parameters7. The parametric functions were assessed for goodness of fit using the Akaike information criteria, as well as visual inspection, log logistic, Weibull, log normal, gamma, and exponential analyses. The parametric function with the best overall fit to the patient-level data was used in the base-case analysis, and the other functions were tested in sensitivity analyses. To avoid mathematical anomalies, the parametric curves were assumed to converge once they intersected, resulting in equivalent transition probabilities for both pfs and os for both treatment arms after convergence at 15 years.
Adverse Events and Toxicities
Adverse events (aes) can significantly affect the results of a pharmacoeconomic analysis with respect to increased costs for the payer and to decreased quality of life for patients. Grades 1–2 aes are considered mild to moderate in severity and require limited or no intervention; they would not affect an economic analysis. The severity of grades 3 and greater aes is much higher; such events require significant interventions that are associated with increased costs and clinical impact. The aes and toxicities included in the present analysis were modelled from the icon7 trial; aes of grade 3 and greater, observed within 28 days after discontinuation of the clinical trial treatment, are included. The ae costs were calculated as “per episode” costs, which included an assessment of the resources used to manage the ae (informed by expert opinion), combined with the estimated costs of each resource (informed by publicly available Ontario health care resource costs)13–15. Included in the model were the aes for which the episodic costs were high or the difference between the treatment arms was large. The cost of treating patients with aes was extrapolated beyond the trial follow-up by applying the average weekly ae cost by arm (total ae costs divided by the total ae follow-up in patient–weeks). The total patient–weeks of ae follow-up for each treatment arm were calculated as the number of weeks from the first dose of the study treatment until the lesser of the last dose plus 28 days or the last survival follow-up date for a patient.
Quality of Life
Health-related quality of life was incorporated into the model using utility index values derived from the EQ-5D health state questionnaire used in icon7, which was administered at the start of every treatment cycle and every 6 weeks until the end of year 1, followed by an assessment every 3 months until disease progression up to 2 years after randomization. Utility values for the pfs state were calculated by applying a Canadian time trade-off preference algorithm to the individual EQ-5D responses from the icon7 clinical trial, by cycle. The average pfs utility value by cycle was applied at the start of each 3-week period, with the follow-up utility value applied beyond the 18th week in both treatment arms (Table i). A progression health state utility of 0.680 was assigned based on a published Canadian ovarian cancer utility value16, less 1 standard deviation to account for the Progression health state.
TABLE I.
Health state utilities
| Cycle | Observations (n) | Mean utility | Standard error |
|---|---|---|---|
| 1 | 340 | 0.7252 | 0.0081 |
| 2 | 383 | 0.767 | 0.0074 |
| 3 | 380 | 0.7798 | 0.0074 |
| 4 | 365 | 0.7971 | 0.0069 |
| 5 | 367 | 0.7968 | 0.0077 |
| 6 | 360 | 0.7835 | 0.0081 |
| 8 | 308 | 0.7969 | 0.0092 |
| 10 | 299 | 0.8059 | 0.0092 |
| 12 | 287 | 0.804 | 0.0095 |
| 14 | 226 | 0.8136 | 0.011 |
| 16 | 206 | 0.7985 | 0.0109 |
| 18 | 181 | 0.815 | 0.0119 |
| Follow-up | 395 | 0.8438 | 0.0078 |
Treatment-Related Costs
To avoid delayed wound healing after surgery in the high-risk population, all patients initiated carboplatin and paclitaxel chemotherapy at cycle 1 and bevacizumab at cycle 2 per the icon7 trial protocol. The costs of the three treatments (carboplatin plus paclitaxel, carboplatin plus paclitaxel, and bevacizumab) are calculated and applied every 3 weeks, because all regimens had a treatment cycle of 3 weeks. Because bevacizumab is available in vials, the model includes bevacizumab drug wastage in the calculation of the number of vials used, and no wastage was applied to the carboplatin and paclitaxel.
Drug administration costs, including clinical consultation, pharmacy, and chair time, were applied every 3rd week (starting at the first administration) per the cycle length of all drugs8. The cost of drug administration was derived from the resources used for administration of chemotherapy (pre-medications; pharmacist and nurse time; hospital overhead cost, including chair time). Chair time (4 hours total) for carboplatin and paclitaxel administration was based on the Cancer Care Ontario formulary specification for carboplatin plus paclitaxel in the frontline treatment of ovarian cancer17. Chair time for bevacizumab (30 minutes) was obtained from clinician interviews. Costs for pre-medications and hourly costs for overhead and nursing time were taken from Canadian sources, inflated to 2014 dollars using the Consumer Price Index9,18.
The supportive care costs for patients in the PFS health state included physician visits after administration, cancer antigen 125 immunohistochemistry tests, and computed tomography imaging examinations (applied every time an immunohistochemistry test is positive, which is assumed to be 80% of the time). The frequency of physician visits and cancer antigen 125 tests during the PFS phase (after the drug administration phase) was based on expert opinion and was applied for every 2nd drug administration cycle (that is, 6 weeks) for the 1st year after administration during the PFS phase, every 12 weeks during year 2, and every 24 weeks in subsequent years. On average, it was estimated that the patient would see the physician every 15 weeks after first-line treatment. The supportive care costs for patients in the Progression health state included 2nd and subsequent treatments as observed in the icon7 trial, immunohistochemistry cancer antigen 125 tests, and computed tomography imaging exams (80% of the time). The subsequent treatment, test, and administration costs were applied to all patients transitioning from the PFS health state at the time of the transition. Progression-state supportive care costs were applied to all patients with progressive disease until death.
Table ii summarizes all treatment-related costs used in the analysis.
TABLE II.
Base-case model parameters
| Parameter | Value | Data source |
|---|---|---|
| Discounting for costs and QALYs (%) | 5 | CADTH |
| Mean body weight of cohort (kg) | 64.86 | ICON7 trial7 |
| Mean height of cohort (cm) | 162.78 | ICON7 trial7 |
| Transition probabilities | ||
| PFS to Progressed | Figure 2 | ICON7 trial7, log logistic parametric function |
| PFS to Death | ||
| Progressed to Death | ||
| Drug acquisition costs ($ per cycle) | ||
| Bevacizumab + CTx regimen | 2,653.48 | Hoffmann–La Roche |
| CTx regimen | 153.48 | Ontario Drug Benefit (ODB) formulary14 |
| Administration costs ($ per cycle) | ||
| Bevacizumab + CTx regimen | Intravenous administration (chair time): Cancer Care Ontario17; | |
| Cycles 2–6 | 599.573 | premedications: ODB formulary14; |
| Cycles 7–18 | 103.69 | clinical fees: Ontario Schedule of Benefits13 |
| CTx regimen | ||
| Cycles 1–6 | 533.54 | |
| Supportive care costs ($ weekly) | ||
| PFS state | 8.06 | Ontario Schedule of Benefits13 |
| Progression state | 17.26 | |
| Adverse event costs ($) | ||
| Bevacizumab + CTx | 1,798.72 | Adverse event rate: ICON7 trial7; |
| CTx | 1,454.75 | Adverse event cost: Ontario Case Costing Initiative15, |
| Health state utilities | Ontario Schedule of Benefits13 | |
| PFS | EQ-5Da by treatment cycle | ICON7 trial7 |
| Progression | 0.64 | Naik et al., 201416 |
EuroQoL Group, Rotterdam, Netherlands.
QALY = quality-adjusted life-year; CADTH = Canadian Agency for Drugs and Technologies in Health; PFS = progression-free survival; CTx = chemotherapy.
RESULTS
In the base-case analysis, the addition of bevacizumab to standard chemotherapy for the treatment of high-risk-of-relapse advanced ovarian cancer yielded 2.661 qalys at a total cost of $54,396. That result compares favourably with treatment using standard chemotherapy alone, which yielded 2.287 qalys at a total cost of $18,495. The mean incremental cost-effectiveness ratio (icer) for standard chemotherapy treatment plus bevacizumab compared with standard chemotherapy alone was therefore $95,942 per qaly. In 1-way sensitivity analyses, the model parameters that resulted in the highest degree of variability in the outcome of the analyses included the time horizon, the health state utilities, and the parametric function used to extrapolate os. The sensitivity analyses resulted in icers ranging from $89,364 to $110,340 per qaly (Table iii).
TABLE III.
Variables and results of one-way sensitivity analyses
| Variable | Value | ICER ($/QALY) | |
|---|---|---|---|
|
| |||
| Base case | Sensitivity analysis | ||
| Base case | 95,942.18 | ||
| PFS health state utility value | EQ–5Da | +10% | 89,363.82 |
| by treatment cycle | −10% | 103,566.00 | |
| Progression health state utility value | 0.64 | +10% | 93,475.65 |
| −10% | 98,542.39 | ||
| Weekly supportive care costs | |||
| PFS | $8.06 | −10% | 95,918.27 |
| +10% | 95,966.08 | ||
| Progression | $17.26 | −10% | 95,905.16 |
| +10% | 95,979.19 | ||
| Dosage and wastage | Actual dose, including bevacizumab wastage | Actual dose, excluding wastage | 94,541.25 |
| Planned dose, including wastage | 95,947.56 | ||
| Planned dose, excluding wastage | 93,497.75 | ||
| Cost discount | 5% | 0% | 96,819.59 |
| 3% | 96,272.46 | ||
| Time horizon | 10 Years | 14 Years | 92,858.17 |
| 6 Years | 110,339.92 | ||
| Adverse events cost | $1,798.72 (bevacizumab) | +10% | 96,034.10 |
| $1454.75 (carboplatin + paclitaxel) | −10% | 95,850.25 | |
EuroQoL Group, Rotterdam, Netherlands.
ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year; PFS = progression-free survival.
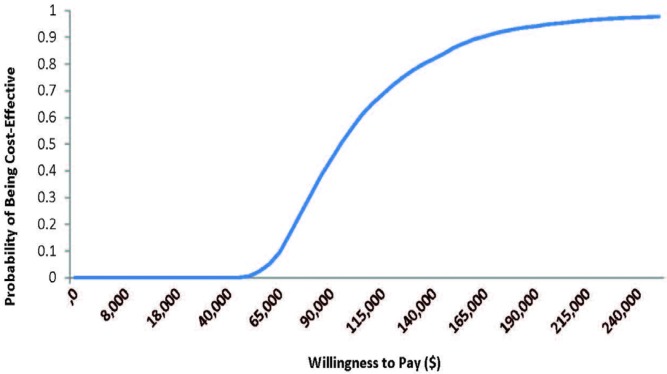
After a probabilistic analysis of 5000 iterations, the mean icer was $93,142 per qaly. At a willingness-to-pay threshold of $100,000 per qaly, 56% of the tested scenarios were considered cost-effective (Figure 2).
FIGURE 2.
Cost-effectiveness acceptability curve for the probabilistic sensitivity analysis. At a willingness to pay threshold of $100,000 per QALY, the addition of bevacizumab to standard chemotherapy is considered a cost-effective treatment alternative to standard chemotherapy alone in ovarian cancer patients who are at high risk of relapse.
DISCUSSION
Recent results of two large-scale phase iii randomized trials demonstrated that the addition of bevacizumab to standard chemotherapy significantly improves pfs and os in ovarian cancer patients who are at high risk of disease progression5,7. Those results provide a clinical framework for the appropriate use of bevacizumab in the management of advanced ovarian cancer. The objective of the present analysis was to examine the cost-effectiveness of that clinical framework and to discuss its adoption for the Canadian health care system.
At a total cost of $54,396.36, the addition of beva-cizumab to standard chemotherapy for the treatment of ovarian cancer increased the total cost of therapy by $36,000 over the current standard-of-care chemotherapies, for an icer of $95,942 per qaly. Canada has no official cost-effectiveness threshold that determines the willingness-to-pay of the public health care system. However, many of the oncology therapies currently funded have icers well above $100,000 per qalya. At that willingness-to-pay threshold, bevacizumab plus standard chemotherapy would be considered a cost-effective therapy for patients with ovarian cancer. A probabilistic analysis examined the impact that the overall uncertainty associated with this economic analysis has on the cost-effectiveness of bevacizumab. At a willingness-to-pay threshold of $100,000 per qaly, bevacizumab has a greater probability than not of being cost-effective—that is, a 56% chance of being cost-effective. That probability increases significantly as the willingness-to-pay threshold increases, which is not uncommon for cancer therapies in Canada.
A brief review of currently funded oncology medications and their icers submitted to the pan-Canadian Oncology Drug Review would suggest that the willingness-to-pay threshold of the Canadian public health care system for oncology therapeutics is significantly higher than $100,000 per qaly19. At a threshold of $150,000 per qaly, the probability is greater than 85% that the bevacizumab-containing therapy is a cost-effective option, removing much of the uncertainty concerning the cost-effectiveness of adding bevacizumab to standard chemotherapy.
Cost-effectiveness analyses vary considerably based on the perspective from which they are conducted. Two previously conducted cost-effectiveness analyses compared standard chemotherapy alone with the addition of bevacizumab to standard chemotherapy in the high-risk population defined in the icon7 trial10,11. One analysis conducted from the perspective of the U.K. National Health Service reported an icer of £48,975 per qaly for the addition of bevacizumab. The icer estimate from that analysis was comparable to ours; however, the threshold of cost-effectiveness used by the U.K. National Health Service (approximately £30,000 per qaly) is much lower than that in Canada, and the addition of bevacizumab to standard chemotherapy was considered not cost-effective from that perspective. The other cost-effectiveness analysis took the perspective of the U.S. Medicare system and estimated an icer of approximately $170,000 per qaly. That estimate is significantly higher than ours; however, the United States has no cost-effectiveness threshold, and given that situation, the authors were unable to conclude whether the therapy would be considered cost-effective from their perspective.
As with many cost-effective analyses, noting the limitations associated with the methodology is important. The clinical parameters in the present model are based on a single cohort of patients from one prospective clinical trial, and it was impossible to obtain detailed clinical data for all possible clinical scenarios. As a result, uncertainty in the clinical outcomes must be evaluated in sensitivity analyses, and missing information must be extrapolated based on available data and knowledge. The uncertainties for both the clinical and economic parameters were evaluated in 1-way sensitivity analyses and in a probabilistic analysis to determine their effect on the overall icer estimate. Additionally, given that the cost-effectiveness of a drug is measured over the lifetime of the patient, a key limitation of our analysis is the need to extrapolate the results beyond the available data so as to estimate the lifetime cost-effectiveness of treatment for each patient. Testing the assumptions for the extrapolation of clinical benefit indicated that those assumptions had the largest effect on the icer estimate. However, the resulting icers increased less than 15% from the icer in the base–case analysis. That observation suggests that the base-case analysis is robust against the parameter assumptions in the model.
CONCLUSIONS
The present analysis provides supportive evidence to inform the potential cost-effectiveness, in the frontline setting, of the addition of bevacizumab to standard chemotherapy in ovarian cancer patients at a high risk of progression.
Footnotes
Examples can be found at the Web site of the Canadian Agency for Drugs and Technologies [Home > About CADTH > What We Do > Programs and Services > CADTH pan-Canadian Oncology Drug Review > Transparency > Find a Review (pCODR) (https://www.cadth.ca/about-cadth/what-we-do/products-and-services/pcodr/transparency/find-a-review)].
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: all authors are affiliated or have involvement with Hoffmann–La Roche Ltd.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. [Google Scholar]
- 2.Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. figo 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Ervik M, et al., editors. globocan 2012. Estimated cancer incidence, mortality and prevalence worldwide in 2012 [Web resource] Lyon, France: International Agency for Research on Cancer; 2013. [Available at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx; cited 14 April 2014] [Google Scholar]
- 4.World Health Organization International Agency for Research on Cancer (iarc) globocan 2012. Home > Online analysis > Incidence/mortality > Dual multi-bar chart: populations/sexes by cancer [Web resource] Lyon, France: IARC; 2013. [Available at: http://globocan.iarc.fr/Pages/bar_sex_site_sel.aspx; cited 29 August 2016] [Google Scholar]
- 5.Horvath G, Andersson H, Paulsson G. Characteristic odour in the blood reveals ovarian carcinoma. BMC Cancer. 2010;10:643. doi: 10.1186/1471-2407-10-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (ago-ovar) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (gineco) Cancer. 2009;115:1234–44. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 7.Stuart GC, Kitchener H, Bacon M, et al. on behalf of the participants of 4th Ovarian Cancer Consensus Conference and of the Gynecologic Cancer Intergroup 2010 Gynecologic Cancer InterGroup (gcig) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011;21:750–5. doi: 10.1097/IGC.0b013e31821b2568. [DOI] [PubMed] [Google Scholar]
- 8.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 9.Perren TJ, Swart AM, Pfisterer J, et al. on behalf of the icon7 investigators A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [Erratum in: N Engl J Med 2012;366:284] [DOI] [PubMed] [Google Scholar]
- 10.Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (icon7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–36. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinde S, Epstein D, Cook A, Embleton A, Perren T, Sculpher M. The cost-effectiveness of bevacizumab in advanced ovarian cancer using evidence from the icon7 trial. Value Health. 2016;19:431–9. doi: 10.1016/j.jval.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Chan JK, Herzog TJ, Hu L, et al. Bevacizumab in treatment of high-risk ovarian cancer—a cost-effectiveness analysis. Oncologist. 2014;19:523–7. doi: 10.1634/theoncologist.2013-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ontario Ministry of Health and Long-term Care . Schedule of Benefits. Physician Services Under the Health Insurance Act (Amended May 1, 2014) Toronto, ON: Government of Ontario; 2014. [Google Scholar]
- 14.Ontario Ministry of Health and Long-Term Care (mohltc) Ontario Drug Benefit Formulary/Comparative Drug Index. Ed. 42. Toronto, ON: MOHLTC; 2016. [Available online at: http://www.health.gov.on.ca/en/pro/programs/drugs/formulary42/edition_42.pdf; cited 1 September 2016] [Google Scholar]
- 15.Ontario Ministry of Health and Long-Term Care (mohltc), Health Data Branch . Web Portal > Ontario Case Costing Initiative > OCCI Costing Analysis Tool [online resource, available by registration only] Toronto, ON: MOHLTC; n.d. [Registration available at: https://hsimi.on.ca/hdbportal/; cited 1 September 2016] [Google Scholar]
- 16.Naik H, Qiu X, Brown MC, et al. Health utility estimates of Canadian cancer patients [abstract 5] J Popul Ther Clin Pharmacol. 2014;21:e457. [Available for download at: http://www.jptcp.com/pubmed.php?articleId=487; 27 August 2014] [Google Scholar]
- 17.Cancer Care Ontario (cco), Drug Formulary . CRBPPA-CL+BEVA Regimen (Paclitaxel-Carboplatin–Bevacizumab) [monograph] Toronto, ON: CCO; 2016. [Google Scholar]
- 18.Mittmann N, Verma S, Koo M, Alloul K, Trudeau M. Cost effectiveness of tac versus fac in adjuvant treatment of node-positive breast cancer. Curr Oncol. 2010;17:7–16. doi: 10.3747/co.v17i1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans G. Should different drugs have different thresholds? A CED Perspective [slide presentation]. Presented at Evidence, Decisions, Outcomes: Optimizing the Use of Drugs and Health Technologies. CADTH Symposium 2009; Ottawa, ON. 5–7 April 2009; [Available online at: https://www.cadth.ca/media/symp-2009/presentations/CS-14/Gerald%20Evans%20-%20Should%20Different%20Drugs%20Have%20Different%20Thresholds%20-%20A%20CED%20Perspective.pdf; cited 27 August 2014] [Google Scholar]