Abstract
Background and Aims
In this pilot study, we assessed the safety and tolerability of combining sorafenib with 90Y radioembolization for the treatment of unresectable hepatocellular carcinoma (hcc).
Methods
The study, conducted prospectively during 2009–2012, included eligible patients with unresectable hcc and a life expectancy of at least 12 weeks. Each patient received sorafenib (400 mg twice daily) for 6–8 weeks before 90Y treatment. Safety and tolerability were assessed.
Results
Of the 40 patients enrolled, 29 completed treatment (combined therapy). In the initial cohort, the most common cause of hcc was hepatitis C (32.5%), and most patients were staged Child A (82.5%). The 29 patients who completed the study had similar baseline characteristics. Grades 1 and 2 toxicities accounted for 77.8% of all adverse events reported. The most common toxicities reported were fatigue (19.0%), alteration in liver function (7.9%), and diarrhea (6.3%). There were 12 grade 3 and 2 grade 4 toxicity events reported. One patient died of liver failure within 30 days after treatment. During the study, the sorafenib dose was reduced in 6 patients (20.7%), and sorafenib had to be interrupted in 4 patients (13.8%) and discontinued in 4 patients (13.8%). The disease control rate was 72.4% per the modified Response Evaluation Criteria in Solid Tumors, and tumour necrosis was observed in 82.8% of patients. Overall survival in patients undergoing combined therapy was 12.4 months.
Conclusions
Preliminary results demonstrate the safety and tolerability of combining 90Y radioembolization and sorafenib for advanced hcc. A larger prospective study is needed to determine the extent of the survival benefit.
Keywords: Hepatocellular carcinoma, radioembolization, sorafenib, unresectability
INTRODUCTION
Hepatocellular carcinoma (hcc) is the most common form of primary liver cancer, the 5th most common cancer in adult men worldwide, and the 3rd leading cause of cancer-related death globally1–4. The incidence of primary liver cancer in the Western world is increasing. In the United States, a 25% increase in hcc cases was observed between 1994 and 20025. Moreover, despite a general decline in the cancer mortality rate for most sites, mortality for hcc is increasing, and hcc is the fastest-growing cause of cancer-related death in men6. Curative treatments, including liver transplantation and liver resection, carry a 5-year survival of 50%–75%, but can be used in only 30%–40% of the cases in Europe and United States7,8. In patients for whom resection or transplantation is not readily feasible, locoregional treatments are offered: radiofrequency, cryo-, percutaneous alcohol injection, or microwave ablation9,10 and bland embolization, radioembolization, or transarterial chemoembolization, which might be conventional or might use drug-eluting beads11,12. Designing new treatments has been a challenge, given that most patients present with cirrhosis and associated limitations in the synthetic hepatic function. In patients with more advanced stages of hcc, best supportive care remained the only option6,7,13 until 2008, when the sharp and Asia–Pacific randomized clinical trials14,15 revealed improved overall survival with sorafenib.
Sorafenib Therapy
In 2008, the multicentre phase iii double-blind randomized placebo-controlled sharp clinical trial14, conducted in patients with advanced hcc, demonstrated that, compared with placebo, sorafenib conferred a survival advantage of 3 months (hazard ratio: 0.69; p = 0.00058). Median overall survival was 10.7 months in the sorafenib arm compared with 7.9 months with placebo. Time to tumour progression was also increased by 2.7 months (range: 2.8–5.5 months) with sorafenib. That same year, sorafenib became the first approved systemic treatment for patients with advanced hcc.
Sorafenib is an oral multi-kinase inhibitor with demonstrated antiangiogenic and antiproliferative effects. It acts by inhibiting the serine/threonine kinases Raf-1 and B-Raf and the receptor tyrosine kinase activity of vascular endothelial growth factor receptors 1, 2, and 3, and platelet-derived growth factor receptor16—pathways that have both been implicated in the pathogenesis of hcc16,17. Sorafenib is now the only systemic treatment shown to improve outcomes in advanced hcc.
90Y Therapy
Since its first use in 1969, 90Y therapy has evolved into two commercially available radioembolization devices: glass microspheres called TheraSphere (BTG International, London, U.K.) and resin microspheres called SIR-Spheres (Sirtex Medical, Sydney, Australia). TheraSphere microspheres18 are intra-arterial, minimally embolic, 20-μm to 30-μm insoluble glass particles loaded with 90Y. Compared with the resin microspheres, they have higher specific activity (2500 Bq) and a lower number of spheres per dose (1.2 million/3 GBq)19, allowing for delivery of the radiotherapeutic agent with less secondary embolization effect.
Unlike external-beam radiation, radioembolization beads are introduced through a selected hepatic artery and are consequently trapped in the microvasculature because of the size differential between the beads and the arteriolar–capillary diameter20. Unlike a healthy liver, which relies on the portal vein, intrahepatic malignancies rely on the hepatic artery for their blood supply21–23, thereby providing an effective and relatively safe avenue for delivering high doses of radioembolization agent while avoiding radiation-induced liver disease. Microspheres with 90Y exert their local radiotherapeutic effect primarily from beta-particle emission, which has an average therapeutic range of 2.5 mm in tissue (0.94 MeV per beta particle), with a maximal range of less than 1 cm. The isotope has a physical half-life of 64.2 hours and decays to stable 90Zr18,24,25.
In recent years, 90Y radioembolization has been investigated in numerous cancer types and has shown promising results in patients with hcc26–36. The 2008 meta-analysis of 90Y studies by Vente et al.37 showed a 78% response from treatment with glass microspheres in patients with hcc. Ongoing research is encouraging, showing a partial response in up to 55%–79% of patients30,33, with significant delays in tumour progression and vascular invasion, low risk for microembolic effect, and effectiveness in patients with limited hepatic reserve17.
Rationale for Combining Sorafenib and 90Y Therapy
Above and beyond sorafenib’s ability to increase time to progression and overall survival in patients with very advanced hcc, and 90Y therapy’s ability to increase treatment response rates in these patients, there is theoretical rationale for combining the two treatments—specifically using 90Y. Encouraging results from radioembolization and sorafenib studies also support a rationale for the combination. Tumour vasculature is tortuous and leaky38,39, and although it can deliver oxygen and nutrients to the core of the tumour, it is thought to be inefficient compared with normal vessels. Antiangiogenic agents have been shown to normalize the vessels and allow them to deliver oxygen more efficiently to the core of hcc tumours40,41, which in fact has been associated with enhanced radiation-induced tumour regression because sensitivity to radiation is increased38. The synergy between intra-arterial radioembolization and sorafenib therapy has also been investigated in an in vitro study, which showed an advantage of combining the two treatment modalities in human and murine hepatoma cell lines42. The same observations were also made in studies using in vivo models43–45.
We hypothesized that treating patients with sorafenib before radioembolization could increase tumour sensitivity to radiation by normalizing the tumour vasculature, thus increasing oxygenation of the tumour. The aim of the present pilot study was to evaluate the safety and tolerability of combining sorafenib with 90Y radioembolization in the treatment of unresectable hcc and to evaluate disease progression and overall survival.
METHODS
Patients
The target population consisted of consecutive eligible patients more than 18 years of age with advanced hcc as defined by guidelines for cirrhotic subjects from the American Association for the Study of Liver Diseases41.
Eligible patients required an ecog (Eastern Cooperative Oncology Group) performance status of 2 or less, a Child–Pugh liver function score of A–B, and a life expectancy of 12 weeks or more. Adequate renal (serum creatinine < 1.5 the upper limit of normal), hematologic (in particular, platelets > 50×109/L), and cardiac function were also required; a dedicated cardiac work-up, including echocardiography was ordered only if patients had a history of cardiovascular disease or congestive heart failure. Patients who had previously received locoregional, surgical, or systemic cancer treatment were not excluded. Consecutive eligible patients attending the McGill University Health Centre hcc clinic were enrolled from April 2009 to August 2012. Patients were excluded if they were scored Child–Pugh C; had an ecog status of 3–4; had extensive extrahepatic disease or presented with liver tumours representing more than 50% of liver volume; had significant pulmonary disease (chronic obstructive pulmonary disease), cardiac disease (New York Heart Association class i, active coronary artery disease, and uncontrolled hypertension), active infection or hiv infection, recent gastrointestinal bleeding (<30 days), organ allograft, or major surgery within 4 weeks of entry; or had received biologic response modifiers such as granulocyte colony–stimulating factor 3 weeks before entry or had undergone autologous bone-marrow transplant. All patients provided informed consent, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by our institution’s human research committee (protocols SDR-08-041 and BAY-14270).
All patients underwent a pre-treatment evaluation that included medical history, ecog evaluation, laboratory work-up [alpha-fetoprotein (afp), virology, renal and liver function tests] and imaging (Figure 1). Patients were classified according to the TNM system for hcc and the Barcelona Clinic Liver Cancer algorithm. Per standard protocol, all patients underwent combined diagnostic computed tomography (ct)–magnetic resonance imaging, hepatic arterial ct angiography, and mesenteric angiography before 90Y treatment.
FIGURE 1.
Timeline and study design. CT = computed tomography.
Treatment
All patients began oral sorafenib [initial dose: 400 mg twice daily (200-mg tablets)] and then underwent weekly liver function testing, hematology, standard biochemistry, and afp measurement, as well as biweekly medical consultation for a period of at least 6–8 weeks before 90Y treatment. During that period, patients also underwent planning for 90Y radioembolization, which included mesenteric angiography to map the hepatic vascular anatomy and arterial supply to the tumour. Coil embolization of non-target vessels was performed to allow for safe infusion of 90Y microspheres at the time of the actual treatment. To detect significant shunting that would cause delivery of the microspheres to the lungs and gastrointestinal tract, 99mTc macro-aggregated albumin was administered intra-arterially to measure shunt fraction.
After the 6-to 8-week sorafenib regimen, standard-technique 90Y radioembolization was performed as a 1-day outpatient procedure under conscious sedation in the interventional radiology suite of the Royal Victoria Hospital (Figure 1). Sorafenib was not interrupted on the day of the 90Y treatment. The 90Y-containing microspheres were delivered to the tumour by catheterization of the artery supplying the tumour. Radiation dose was calculated according to the manufacturer’s guidelines, based on volume data from ct imaging of the liver. If a patient had bilobar disease, a second 90Y radioembolization to treat the other side of the liver was performed approximately 3 weeks after the first treatment.
Sorafenib treatment interruptions and dose reductions for drug-related adverse effects followed the product monograph for sorafenib and the procedures in the sharp trial14. After 90Y radioembolization, treatment with oral sorafenib (400 mg twice daily) continued for as long as patients found it tolerable, as assessed by the treating physician using the Common Terminology Criteria for Adverse Events, version 4.0.
After 90Y radioembolization, patients underwent biweekly liver function testing, hematology, standard biochemistry, and afp measurement, as well as monthly medical consultation. After 3 months of follow-up and based on health status, the frequency of the tests was decreased to monthly blood work (liver function testing, hematology, biochemistry, and afp measurement) and medical consultation. Follow-up ct imaging was obtained 3 weeks after 90Y radioembolization and then every 3 months.
Outcomes and Assessments
The primary outcome was safety and tolerability. Adverse events were recorded at each clinic visit, and patients were encouraged to report any change in health and symptoms. Secondary outcomes were tumour response, disease control rate, and overall survival. Tumour response was measured by imaging (ct or magnetic resonance imaging) according to the Response Evaluation Criteria in Solid Tumors, version 1.146, before therapy start and after radioembolization; “disease control rate” consisted of stable disease and partial and complete responses. Overall survival was measured from the date of sorafenib treatment initiation to the date of death from any cause.
Statistical Analysis
In the statistical analyses, the Fisher exact test, chi-square test, and Mann–Whitney U-test were used, as appropriate. For continuous variables, means (averages) ± standard deviation were used for normally distributed data, and medians with interquartile range (iqr) were used for non-normally distributed data. Overall survival was assessed by Kaplan–Meier analysis. Any difference was considered significant when the p value was 0.05 or less. Statistical analyses were performed using the software applications JMP (version 8.0: SAS Institute, Cary, NC, U.S.A.) and GraphPad Prism (version 6.0: GraphPad Software, San Diego, CA, U.S.A.).
RESULTS
Patients and Exclusions
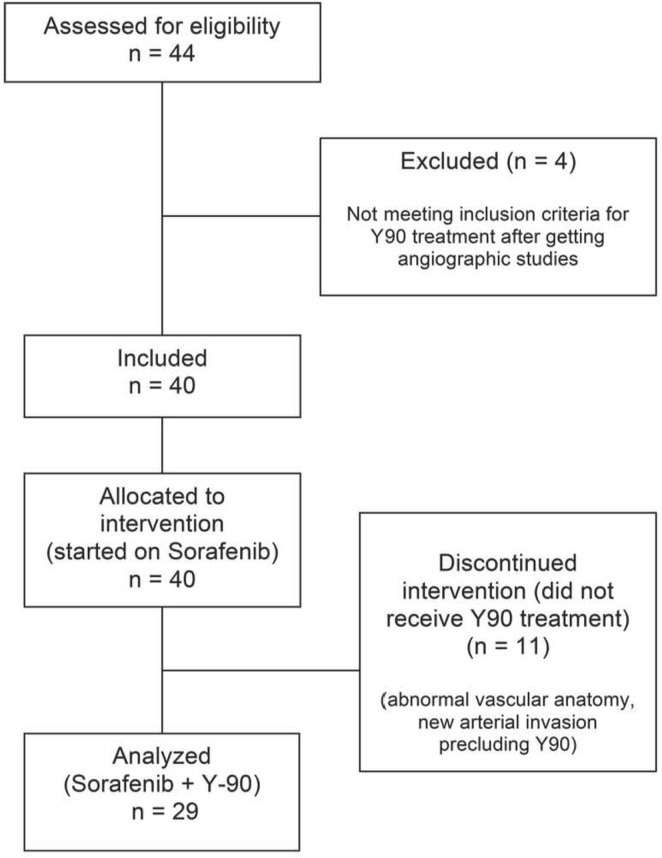
The study initially enrolled 40 patients, and 29 completed the treatment plan of combined sorafenib and 90Y radio-embolization (Figure 2). In the initial cohort of 40 patients, mean age was 62 ± 11.6 years, and 37 patients were men (92.5%). The most common cause of liver disease was hepatitis C infection (32.5%, n = 13), and almost all patients had an ecog status of 0 or 1. The Barcelona Clinic Liver Cancer classification was B for 12 patients (30.0%) and C for 21 patients (52.5%); and 12 patients had previously received locoregional treatments (Table i).
FIGURE 2.
Patient accrual and progress through the study, 2009–2012.
TABLE I.
Demographics and baseline clinical characteristics of the eligible and treated patient cohorts
| Variable | Cohort | |
|---|---|---|
|
| ||
| Eligible | Treated with sorafenib and 90Y | |
| Patients (n) | 40 | 29 |
| Mean age (years) | 62±11.6 | 63±12.5 |
| Male sex [n (%)] | 37 (92.5) | 26 (89.7) |
| Cause of liver disease [n (%)] | ||
| Hepatitis C virus | 13 (32.5) | 9 (31.0) |
| Hepatitis B virus | 9 (22.5) | 6 (20.7) |
| Alcoholic steatohepatitis | 9 (22.5) | 6 (20.7) |
| Non-alcoholic steatohepatitis | 3 (7.5) | 2 (6.9) |
| Cryptogenic or other | 5 (12.5) | 6 (20.7) |
| ECOG performance status [n (%)] | ||
| 0 | 18 (45.0) | 11 (37.9) |
| 1 | 17 (42.5) | 13 (44.8) |
| 2 | 5 (12.5) | 5 (17.2) |
| Child–Pugh score [n (%)] | ||
| A | 32 (80.0) | 23 (79.3) |
| B | 8 (20.0) | 6 (20.7) |
| BCLC classification [n (%)] | ||
| A | 7 (17.5) | 5 (17.2) |
| B | 12 (30.0) | 9 (31.0) |
| C | 21 (52.5) | 15 (51.7) |
| Portal vein thrombosis [n (%)] | 14 (35.0) | 10 (34.5) |
| Extrahepatic disease [n (%)] | ||
| Lymph nodes | 9 (22.5) | 7 (24.1) |
| Lungs | 1 (2.5) | 0 |
| Other | 1 (2.5) | 1 (3.4) |
| Previous locoregional treatment [n (%)] | ||
| TACEa or bland embolization | 9 (22.5) | 2 (6.9)b |
| Radiofrequency or microwave ablation | 3 (7.5) | 2 (6.9)b |
| Initial biochemistry [median (IQR)] | ||
| Bilirubin (μmol/L) | 18 (132–9.8) | 17 (122–8.1) |
| Albumin (g/L) | 33 (303–7) | 33 (303–9) |
| Alpha-fetoprotein (μg/L) | 421 (362–305) | 310 (249–58) |
| Treatment with 90Y [n (%)] | ||
| 1 | — | 21 (72.4) |
| 2 | — | 8 (27.6) |
| Bilobar disease | — | 6 (20.7) |
| Unilobar disease | — | 2 (6.9) |
Includes doxorubicin beads.
Before sorafenib therapy.
ECOG = Eastern Cooperative Oncology Group; BCLC = Barcelona Clinic Liver Cancer; TACE = transarterial chemoembolization; IQR = interquartile range.
Of the 29 patients who completed the full study, 89.7% (n = 26) were men, and mean age in the group was 63.1 ± 12.5 years (Table i). The Child–Pugh classification was A in 23 patients (79.3%), and B in the remaining 6 patients (20.7%). The ecog status was 0 in 11 patients (37.9%), 1 in 13 patients (44.8%), and 2 in 5 patients (17.2%). Extra hepatic disease, mainly limited to lymph nodes, was present in 7 patients (24.1%), and 4 patients (13.8%) had received prior locoregional treatment (transarterial chemoembolization, radiofrequency ablation, or microwave ablation). Before 90Y radioembolization, patients took sorafenib for a median of 73 days (iqr: 56–128 days). Median pre-treatment afp was 310.0 μg/L (iqr: 24.0–957.8 μg/L). The 29 analyzed patients received a total of 37 90Y treatments, with 8 patients each receiving 2 treatments (6 to treat the contralateral lobe, 2 to treat the same lobe). The median administered dose of 90Y was 137.4 Gy (iqr: 131.1–143.0 Gy).
The 11 patients who did not receive 90Y radioembolization were ineligible because of large lung or bowel shunts, or variant vascular anatomy (5 patients); worsening ecog or patient status as assessed by the treating physician (4 patients); new portal vein thrombosis (1 patient) and new hepatic arterial thrombus (1 patient); and refusal to proceed with 90Y treatment (1 patient). Of the excluded patients, 9 (81.8%) were classified Child–Pugh A, and 2 (18.2%) were classified Child-Pugh B.
Safety and Toxicity
Sorafenib discontinuation was required in 4 patients (13.8%), and sorafenib interruption in 4 patients, including 2 patients who required interruption before 90Y treatment (Table ii). The sorafenib dose had to be adjusted or reduced (to 200 mg twice daily) in 6 patients (20.7%, Table ii). If additional sorafenib dose reductions were required, patients took 200 mg every second day. The most common toxicities reported were fatigue (19.0%); alteration in liver function (7.9%, most commonly increased ascites); and diarrhea (6.3%). Most toxicities (63.3%, corresponding to 19 patients) were of grade 2 or less. Grade 3 toxicities (19.0% of all reported events) included abdominal pain (4.8%), alteration in liver function (4.8%), hand–foot syndrome (3.2%), diarrhea (1.6%), fatigue (1.6%), bleeding (1.6%), and alteration in coagulation (increased international normalized ratio, 1.6%). Most toxicities were reported before 90Y radioembolization and were generally self-limiting or responded to dose adjustment. Grade 4 toxicities occurred in 2 patients (pulmonary embolism and progression of cirrhosis with liver failure). The 12 grade 3 toxicities were reported from 12 different patients, and 2 of those 12 patients were classified Child–Pugh B, the rest being classified Child–Pugh A. The grade 4 toxicities were reported from 2 other patients.
TABLE II.
Treatment safety and tolerability, and reported adverse events, by grade
| Adverse event | Instances reported [n (%a)] | ||
|---|---|---|---|
|
| |||
| Grades 1–2 | Grade 3 | Grade 4 | |
| Constitutional | |||
| Fatigue | 12 (19.0) | 1 (1.6) | 0 |
| Dermatologic | |||
| Hand–foot syndrome | 3 (4.8) | 2 (3.2) | 0 |
| Pruritus | 2 (3.2) | 0 | 0 |
| Rash | 2 (3.2) | 0 | 0 |
| Alopecia | 2 (3.2) | 0 | 0 |
| Other | 1 (1.6) | 0 | 0 |
| Gastrointestinal | |||
| Diarrhea | 4 (6.3) | 1 (1.6) | 0 |
| Constipation | 3 (4.8) | 0 | 0 |
| Abdominal pain or cramps | 3 (4.8) | 3 (4.8) | 0 |
| Other | 4 (6.3) | 0 | 0 |
| Hepatobiliary | |||
| Worsening of cirrhosis or liver dysfunctionb | 5 (7.9) | 3 (4.8) | 1 (1.6) |
| Hematologic events | |||
| Bleedingc | 3 (4.8) | 1 (1.6) | 0 |
| Decreased platelets | 1 (1.6) | 0 | 0 |
| Increased international normalized ratio | 0 | 1 (1.6) | 0 |
| Thromboembolic eventd | 0 | 0 | 1 (1.6) |
| Other | |||
| Leg edema | 3 (4.8) | 0 | 0 |
| Hypertension | 1 (1.6) | 0 | 0 |
| TOTAL | 49 | 12 | 2 |
| Sorafenib adjustments |
|
||
| [n (%) of treated patients] | |||
|
| |||
| Dose reductionse | 6 (20.7) | ||
| Drug interruptions | 4 (13.8) | ||
| Drug discontinuations | 4 (13.8) | ||
Based on total reported adverse events, all grades.
Includes ascites and encephalopathy.
Includes bleeding from gums, nose, and other sites.
Includes pulmonary embolism.
Twice-daily oral sorafenib dose decreased to 200 mg from 400 mg.
The 30-day mortality (after 90Y radioembolization) was 3.4%, corresponding to 1 patient who died of liver failure, possibly related to treatment. When looking at the 6 patients classified Child–Pugh B who received combination therapy, grade 2 and 3 toxicities occurred in 3 and 2 patients respectively. Similar proportions were observed in the 23 Child–Pugh A patients who received combined therapy (grade 2 and 3 toxicities in 16 and 10 patients respectively). No mortality was observed in the 6 patients classified Child–Pugh B who received both radioembolization and sorafenib, and only 1 patient of those patients had received a score of 9, the remainder having been scored 7 or 8.
Tumour Response
Of 22 patients with complete afp measures, 12 (54.5%) experienced a decrease in afp after 90Y treatment (median absolute decrease: 530.5 μg/L; iqr: 107.0–1426.0 μg/L; p = 0.0010). The remaining 10 patients experienced an afp increase (median absolute increase: 194.5 μg/L; iqr: 4.3–2053.0 μg/L; p = 0.0020).
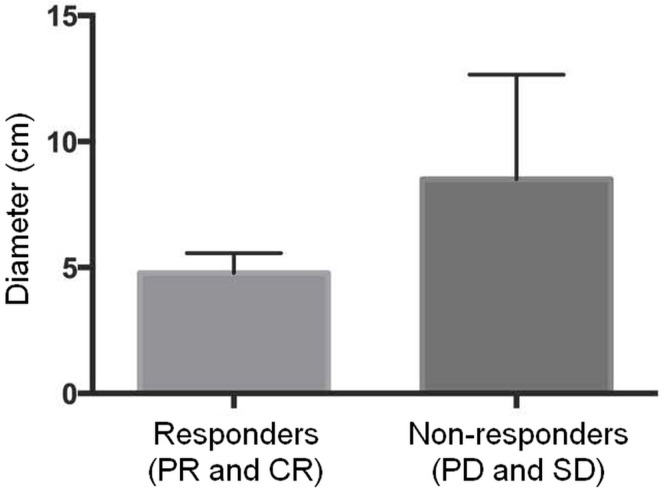
Evaluation of tumour response based on the modified Response Evaluation Criteria in Solid Tumors47 resulted in a disease control rate of 72.4%: 12 patients (41.4%) had stable disease, 8 patients (27.6%) had a partial response, and 1 patient (3.4%) had a complete response (Table iii). Compared with patients who were not responders (as defined by stable or progressive disease), those who experienced a disease response (complete or partial) after combined treatment trended toward having a smaller disease burden (total sum of lesion diameters) at baseline before therapy [median: 5.0 cm (iqr: 4.4–6.0 cm) vs. 9.3 cm (iqr: 5.2–11.9 cm); Figure 3], although the difference did not reach statistical significance (p = 0.1350). Most of the 90Y treatments (82.8%) resulted in necrosis or reduced tumour enhancement.
TABLE III.
Radiologic response after treatment with sorafenib and 90Y, by RECIST criteria
| Variable | Value |
|---|---|
| Interval between CT imaging tests (days)a | |
| Median | 148 |
| IQR | 90–206 |
| Lesions at start of treatment (n) | |
| Median | 3 |
| IQR | 1–5 |
| Change in size of target lesion (%)b | |
| Median | −0.35 |
| IQR | −22.3 to 15.8 |
| Modified RECIST classificationc [n (%)] | |
| Complete response (CR) | 1 (3.5) |
| Partial response (PR) | 83 (27.6) |
| Stable disease (SD) | 129 (41.4) |
| Progressive disease (PD) | 86 (27.6) |
| Disease control rated [n (%)] | 213 (72.4) |
| Presence of necrosis [n (%)] | 24 (82.8) |
Interval in days between CT before sorafenib treatment and CT after 90Y treatment.
Based on modified RECIST criteria.
CR: disappearance of any arterial enhancement of target lesions. PR: 30% decrease in the sum of the longest diameter of viable target lesions (enhancing on arterial phase). SD: Small changes that do not meet the criteria of CR, PR, or PD. PD: 20% increase in the sum of the longest diameter of viable target lesions (enhancing on arterial phase), or any change with the presence of 1 or more new lesions.
Includes SD, PR, and CR.
RECIST = Response Evaluation Criteria in Solid Tumors; CT = computed tomography; IQR = interquartile range.
FIGURE 3.
The burden of disease trended smaller in therapy responders than in non-responders, although the difference was nonsignificant. PR = partial response; CR = complete response; PD = progressive disease; SD = stable disease.
Overall Survival
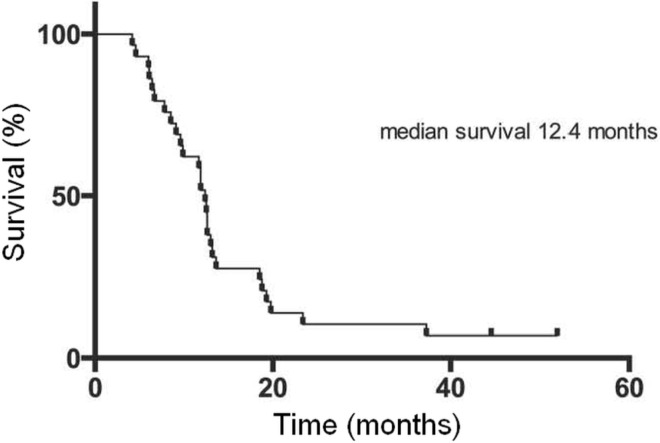
The median overall survival for the entire cohort of 40 patients was 11.9 months (iqr: 6.2–18.7 months). The 29 patients who received the sorafenib–90Y combination therapy experienced a median overall survival of 12.4 months (Figure 4). Patients who did not proceed to 90Y therapy trended toward a lower median overall survival of 5.3 months (iqr: 3.7–22.8 months), p = 0.5924.
FIGURE 4.
Kaplan–Meier survival curve for 29 patients completing the sorafenib plus 90Y regimen.
DISCUSSION
Although some retrospective data48 have suggested that the combination of radioembolization and sorafenib therapy could be beneficial for advanced hcc, we believe that ours is the first prospective study to evaluate the combination of 90Y radioembolization and sorafenib for unresectable hcc. Our preliminary results demonstrate the safety and tolerability of the two treatments in combination.
Almost three-quarters of the reported adverse events were grades 1 and 2, with 12 grade 3 events and only 2 grade 4 events reported. The reported symptoms were mostly similar to those described for the sharp trial (fatigue, hand–foot syndrome, rash and pruritus, and gastrointestinal symptoms such as diarrhea) and were thus possibly secondary to sorafenib. Worsening of liver function, observed as an increase in ascites or alterations in liver enzymes, was in contrast most likely related to the 90Y radio embolization, because those events were comparable to the ones reported in earlier hcc and radioembolization studies33,49. Within the 30 days after 90Y treatment, 1 patient died—a mortality rate similar to those reported in cohorts of hcc patients receiving radioembolization49. However, a fact worth noting is that the sorafenib dose had to be reduced for several patients (6 of 29) to 400 mg daily at maximum, and sorafenib treatment had to be interrupted at some point in the course of therapy for 8 patients. Those reductions and interruptions might suggest that the optimal dose would preferably be lower for patients undergoing this type of combined treatment, because half the patient cohort did not seem to be able to tolerate a full dose of sorafenib. That hypothesis also accords with results of a study by Kulik et al.50, which prospectively evaluated patients receiving 90Y with and without sorafenib, and which also pointed toward a sorafenib dose reduction (400 mg daily), because dose reductions were required for all patients in the combined treatment arm.
For secondary outcomes, our study set out to assess tumour response, disease control rate, and overall survival. Treatment efficacy can be demonstrated by the tumour response assessment, which found that 82.8% of patients (n = 24) demonstrated radiologic evidence of necrosis, and by the disease control rate of 72.4% (n = 21). Patients experiencing partial or complete response tended to have a smaller burden of disease at baseline. Larger studies to identify variables associated with disease response would be warranted in an attempt to stratify patients, allowing for judicious selection of patients who might benefit most from this combination therapy.
Despite the nature of the present study as a pilot with a small number of patients, the combination of 90Y and sorafenib was associated with a median survival duration of 12.4 months, which is comparable to the overall survival of 10.7 months reported in the sharp trial14. Moreover, subject selection in the sharp trial, which assessed the efficacy of sorafenib alone, favoured patients in better general condition (restricting enrollees to Child-Pugh A status, for example). The application of strict inclusion criteria was justified to avoid possible confounders with respect to causes of death, permitting the best possible assessment of the effect of sorafenib therapy alone on the tumours, and avoiding the effect on survival exerted by severe liver disease and cirrhosis. However, the indications for sorafenib do not exclude patients with more severe liver disease, and hence we opted not to exclude patients classified as Child-Pugh B and also to select patients based on functional status (ecog).
Although a direct comparison is difficult, our survival results also approach the neighbourhood of the survival duration data reported in 90Y series, which range between 5 months and 17 months for Barcelona Clinic Liver Cancer classes B and C respectively32,51,52, thus representing most of the subjects included in our study. Our results, derived from a cohort of patients whose disease, at baseline, was more severe than that experienced by patients in the sharp trial, still showed comparable survival results. However, those survival data contrast with data from a study by Rana et al.53, who retrospectively reviewed 10 patients receiving sorafenib followed by radioembolization. In their study, which included 7 patients classified as Child–Pugh A and 3 classified as Child–Pugh B, the side effects with combined therapy were similar to those reported here—namely, anorexia, fatigue, gastrointestinal symptoms, and also the more severe side effects related to ascites and liver enzymes—and yet their reported overall survival duration was barely greater than 7 months, which is worse than the survival durations associated with both therapies alone. Those result could potentially be explained by the inclusion of patients at a worse disease stage or by the retrospective nature of the study, which can introduce confounding factors and make interpretation difficult. More insights about the specific side effects, toxicities, and tumour control data for each individual therapy might be provided by results from the sarah trial (NCT01482442 at http://ClinicalTrials.gov), which compared the two treatments head-to-head, but which has yet to be published.
The rationale behind the combination of sorafenib and 90Y radioembolization originates from the concept of creating a vasculature normalization window38. Since the introduction of angiogenic agents such as sorafenib for advanced hcc, there has been a theory that such agents could potentially “normalize” the tumour vasculature54, which is typically more disorganized. A sufficient duration of sorafenib treatment before the application of radioembolization (6–8 weeks was used in the present study) could theoretically allow for the development of the nor-malization window, thereby favouring a more effective radiation-induced tumour response. However, finding evidence for that hypothesis was not the aim of our study, and longitudinal studies evaluating the optimal normalization window would be needed to identify the best timing for administration of TheraSphere treatment.
CONCLUSIONS
Results of the present study show the safety and tolerability of combining 90Y radioembolization with sorafenib for patient with unresectable hcc, although our results suggest that a lower dose of sorafenib might be administered when that treatment is given concurrently with radioembolization. The optimal timing of 90Y treatment relative to sorafenib is still not well defined, and further investigations to objectively measure vascular normalization and to perform radioembolization at varying time intervals after sorafenib initiation might be warranted. Nonetheless, our data concerning tumour response and overall survival seem to show promising preliminary results. Our findings, in combination with larger prospective trials, should propose a new approach for hcc patients that could eventually be integrated into treatment algorithms.
ACKNOWLEDGMENTS
Thanks go to Ms. Jeanne Bouteau, clinical research coordinator.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interest: PM’s institution received funding from Bayer and BTG International for a trial in which is principal investigator.
This work was supported by Bayer (in support of the research team) and BTG International (TheraSphere doses).
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [Erratum in: CA Cancer J Clin 2011;61:134] [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–53. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 4.Kabbach G, Assi HA, Bolotin G, Schuster M, Lee HJ, Tadros M. Hepatobiliary tumors: update on diagnosis and management. J Clin Transl Hepatol. 2015;3:169–81. doi: 10.14218/JCTH.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona–2000 easl conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/S0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Beaugrand M. Hepatocellular carcinoma: present status and future prospects. J Hepatol. 2003;38(suppl 1):S136–49. doi: 10.1016/S0168-8278(02)00432-4. [DOI] [PubMed] [Google Scholar]
- 8.Tabrizian P, Roayaie S, Schwartz ME. Current management of hepatocellular carcinoma. World J Gastroenterol. 2014;20:10223–37. doi: 10.3748/wjg.v20.i30.10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–63. doi: 10.4254/wjh.v7.i8.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Zheng Y, Li S, Li B, Zhang Y, Yuan Y. Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol. 2013;68:21–6. doi: 10.1016/j.crad.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Benson AB, 3rd, Abrams TA, Ben-Josef E, et al. nccn clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–91. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckel F, Brunner T, Jelic S, on behalf of the esmo Guidelines Working Group Biliary cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(suppl 6):vi40–4. doi: 10.1093/annonc/mdr375. [DOI] [PubMed] [Google Scholar]
- 13.Palmer DH, Hussain SA, Johnson PJ. Systemic therapies for hepatocellular carcinoma. Expert Opin Investig Drugs. 2004;13:1555–68. doi: 10.1517/13543784.13.12.1555. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia–Pacific region with advanced hepatocellular carcinoma: a phase iii randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and vegf and pdgf receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Cao Y, Chen C, et al. Sorafenib blocks the Raf/mek/erk pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 18.Carr BI. Hepatic arterial 90yttrium glass microspheres (TheraSphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10(suppl 1):S107–10. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of 90Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–63. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Lewandowski RJ, Salem R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin Intervent Radiol. 2006;23:64–72. doi: 10.1055/s-2006-939842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–77. [PMC free article] [PubMed] [Google Scholar]
- 22.Lin G, Lunderquist A, Hägerstrand I, Boijsen E. Postmortem examination of the blood supply and vascular pattern of small liver metastases in man. Surgery. 1984;96:517–26. [PubMed] [Google Scholar]
- 23.Tygstrup N, Winkler K, Mellemgaard K, Andreassen M. Determination of the hepatic arterial blood flow and oxygen supply in man by clamping the hepatic artery during surgery. J Clin Invest. 1962;41:447–54. doi: 10.1172/JCI104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murthy R, Nunez R, Szklaruk J, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radio-graphics. 2005;25(suppl 1):S41–55. doi: 10.1148/rg.25si055515. [DOI] [PubMed] [Google Scholar]
- 25.Shaheen M, Hassanain M, Aljiffry M, et al. Predictors of response to radio-embolization (TheraSphere) treatment of neuroendocrine liver metastasis. HPB (Oxford) 2012;14:60–6. doi: 10.1111/j.1477-2574.2011.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305–14. doi: 10.1002/cncr.24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau WY, Ho S, Leung TW, et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int J Radiat Oncol Biol Phys. 1998;40:583–92. doi: 10.1016/S0360-3016(97)00818-3. [DOI] [PubMed] [Google Scholar]
- 28.Cao X, He N, Sun J, et al. Hepatic radioembolization with yttrium-90 glass microspheres for treatment of primary liver cancer. Chin Med J (Engl) 1999;112:430–2. [PubMed] [Google Scholar]
- 29.Dancey JE, Shepherd FA, Paul K, et al. Treatment of non-resectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med. 2000;41:1673–81. [PubMed] [Google Scholar]
- 30.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 31.Sangro B, Bilbao JI, Boan J, et al. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:792–800. doi: 10.1016/j.ijrobp.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 32.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Salem R, Lewandowski RJ, Atassi B, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627–39. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- 34.Braat AJ, Smits ML, Braat MN, et al. 90Y Hepatic radioembolization: an update on current practice and recent developments. J Nucl Med. 2015;56:1079–87. doi: 10.2967/jnumed.115.157446. [DOI] [PubMed] [Google Scholar]
- 35.Sangro B, Inarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–73. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Mosconi C, Cappelli A, Pettinato C, Golfieri R. Radioembolization with yttrium-90 microspheres in hepatocellular carcinoma: role and perspectives. World J Hepatol. 2015;7:738–52. doi: 10.4254/wjh.v7.i5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol. 2009;19:951–9. doi: 10.1007/s00330-008-1211-7. [DOI] [PubMed] [Google Scholar]
- 38.Senan S, Smit EF. Design of clinical trials of radiation combined with antiangiogenic therapy. Oncologist. 2007;12:465–77. doi: 10.1634/theoncologist.12-4-465. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y, Liu Q. Therapeutic targets of multiple angiogenic factors for the treatment of cancer and metastasis. Adv Cancer Res. 2007;97:203–24. doi: 10.1016/S0065-230X(06)97009-2. [DOI] [PubMed] [Google Scholar]
- 40.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by vegfr2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-vegf receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pracht M, Edeline J, Lepareur N, et al. In vitro demonstration of synergy/additivity between 188rhenium and sorafenib on hepatoma lines: preliminary results. Anticancer Res. 2013;33:3871–7. [PubMed] [Google Scholar]
- 43.Suen AW, Galoforo S, Marples B, et al. Sorafenib and radiation: a promising combination in colorectal cancer. Int J Radiat Oncol Biol Phys. 2010;78:213–20. doi: 10.1016/j.ijrobp.2010.02.064. [DOI] [PubMed] [Google Scholar]
- 44.Yu W, Gu K, Yu Z, et al. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2013;329:109–17. doi: 10.1016/j.canlet.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Plastaras JP, Kim SH, Liu YY, et al. Cell cycle dependent and schedule-dependent antitumour effects of sorafenib combined with radiation. Cancer Res. 2007;67:9443–54. doi: 10.1158/0008-5472.CAN-07-1473. [DOI] [PubMed] [Google Scholar]
- 46.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised recist guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Lencioni R, Llovet JM. Modified recist (mrecist) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 48.Chaudhury PK, Hassanain M, Bouteaud JM, et al. Complete response of hepatocellular carcinoma with sorafenib and Y radioembolization. Curr Oncol. 2010;17:67–9. doi: 10.3747/co.v17i5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geschwind JF, Salem R, Carr BI, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127(suppl 1):S194–205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 50.Kulik L, Vouche M, Koppe S, et al. Prospective randomized pilot study of Y90±sorafenib as bridge to transplantation in hepatocellular carcinoma. J Hepatol. 2014;61:309–17. doi: 10.1016/j.jhep.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Kwok PC, Leung KC, Cheung MT, et al. Survival benefit of radioembolization for inoperable hepatocellular carcinoma using yttrium-90 microspheres. J Gastroenterol Hepatol. 2014;29:1897–904. doi: 10.1111/jgh.12621. [DOI] [PubMed] [Google Scholar]
- 52.Sangro B, Carpanese L, Cianni R, et al. on behalf of the European Network on Radioembolization with Yttrium-90 Resin Microspheres Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona Clinic Liver Cancer stages: a European evaluation. Hepatology. 2011;54:868–78. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 53.Rana N, Ju AW, Bazylewicz M, et al. Yttrium-90 radioembolization in patients with hepatocellular carcinoma who have previously received sorafenib. Front Oncol. 2013;3:323. doi: 10.3389/fonc.2013.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6:395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]