Abstract
Background
Phase angle could be an alternative to subjective global assessment for the assessment of nutrition status in patients with head-and-neck cancer.
Methods
We prospectively evaluated a cohort of 75 stage iiib and iv head-and-neck patients treated at the Otolaryngology Department, Head and Neck Surgery, Medical University of Lublin, Poland. Bioelectrical impedance analysis was performed in all patients using an analyzer that operated at 50 kHz. The phase angle was calculated as reactance divided by resistance (Xc/R) and expressed in degrees. The Kaplan–Meier method was used to calculate survival.
Results
Median overall survival in the cohort was 32.0 months. At the time of analysis, 47 deaths had been recorded in the cohort (62.7%). The risk of shortened overall survival was significantly higher in patients whose phase angle was less than 4.733 degrees than in the remaining patients (19.6 months vs. 45 months, p = 0.0489; chi-square: 3.88; hazard ratio: 1.8856; 95% confidence interval: 1.0031 to 3.5446).
Conclusions
Phase angle might be prognostic of survival in patients with advanced head-and-neck cancer. Further investigation in a larger population is required to confirm our results.
Keywords: Head-and-neck cancer, phase angle, bioelectrical impedance analysis, survival, prognostic factors
INTRODUCTION
“Head-and-neck cancer” (hnc) refers to malignant tumours involving the skin, soft tissues, or bones of the head and neck (excluding tumours of the brain and related tissues, and malignant melanomas)1. In 2005, hnc accounted for 3.2% (n = 39,750) of all new cancers and 2.2% (n = 12,460) of all cancer deaths in the United States2. Mucosal squamous cell carcinoma is the most frequent malignancy of the head and neck, accounting for approximately 85% of cases. Squamous cell carcinomas include cancers of the oral cavity, oropharynx, larynx, hypopharynx, paranasal sinuses, and nasopharynx (International Classification of Diseases, revision 10, codes C07–C14 and C32–C33)1.
Progressive weight loss and malnutrition are commonly found in cancer patients (not only when in hospital), and especially in those with hnc, but also in those with lung and gastrointestinal cancers3,4. Weight loss during treatment for hnc is a major concern. The obstacles to weight maintenance result not only from the cancer itself but also from its therapy: surgery, radiotherapy, and chemotherapy, which also lead to additional complications (for example, in oral intake)2,5. The incidence of malnutrition in cancer patients ranges between 40% and 80%, and the prevalence ranges from 50% to 80% depending on tumour type, tumour location, stage of disease, treatment received, and the nutrition assessment method used6. Malnutrition and nutrition deficits all have a significant effect on mortality, morbidity, and quality of life in patients with hnc7–10. Timely identification of problems with a patient’s nutrition could improve prognosis, increase response to therapy, and reduce the rate and severity of complications.
There are many methods for the subjective and objective assessment of patient’s nutrition status, including anthropometric measurements (weight change, arm muscle circumference, triceps skin-fold thickness) and biochemical parameters (albumin, transferrin). Subjective global assessment (sga)11 and patient-generated sga (pg-sga) are designed especially for cancer patients and are commonly used for patients with hnc12. The European Society for Clinical Nutrition and Metabolism also recommends other tools such as nutrition risk screening (nrs-2002 for hospital admission), the Malnutrition Universal Screening Tool, and the Mini Nutritional Assessment13. Recently introduced and relatively new tools such as phase angle (pa) as determined by bioelectrical impedance analysis (bia) allow for an objective determination of prognosis14. As an objective evaluation of body composition and nutrition status, bia has been established as a valuable tool for assessing the condition of many types of patients, including those with cancer15–18. The utility of these many nutrition screening tools has been evaluated by their ability to predict relevant clinical outcomes such as complications, treatment response, survival, and quality of life14,15. Various methods can be used to assess prognosis and predict clinical outcome, but just a few can assess the effect of nutrition on overall survival14.
No objective tool or standard of care has consistently been used for the diagnosis of nutrition status in oncology settings. Currently, most nutrition screening in oncology settings is completed by doctors or nursing professionals using the sga, which is a subjective tool, but still valid for predicting decline in a patient’s quality of life19–21. Subjective global assessment has been found to have a high degree of inter-rater reliability. It has also been found to have predictive and convergent validity, correlating well with measures of morbidity (incidence of infection, use of antibiotics, length of stay), a number of traditional objective parameters (anthropometric, biochemical, functional, and immunologic), and quality of life22.
Bioelectrical impedance evaluates bioelectrical properties of the body such as resistance (R) and reactance (Xc) by recording voltage drop in applied current23. The raw bia parameters derived from Xc and R are the fat mass, fat-free mass, total body water, extracellular water content, intracellular water content, and pa. The pa reflects the relative contributions of fluid (R) and cellular membranes (Xc) in the human body24. Resistance is restriction to the flow of electrical current, primarily related to the amount of water present in the tissues. Reactance is the resistive effect produced by the tissue interfaces and cell membranes25. Thanks to its potential to detect changes in tissue electrical properties, pa has been found to be an indicator for cell membrane integrity, distribution of water between the intracellular and extracellular spaces, and prediction of body cell mass in various disease conditions26–34.
The sga has been extensively validated as a nutrition assessment technique in oncology patients, and it is the method most frequently used35. The impact of nutrition on survival has been reported in single articles that also evaluated the association of pa with sga in patients with pancreatic cancer30 or advanced colorectal cancer31–36. Here, however, we report such an assessment in hnc patients for the first time.
Our prospective study was conducted to investigate the association of pa with patient survival and to identify the prognostic utility of nutrition assessment tools in adult patients with advanced hnc.
METHODS
A prospective cohort study was planned in a population of 75 presurgical, treatment-naïve patients with histologically confirmed primary squamous cell carcinoma of the head and neck treated between October 2009 and October 2012 at the Otolaryngology Department, Head and Neck Surgery, Medical University of Lublin, Poland. Exclusion criteria included a previous or coexisting cancer or the presence of a disease that affects nutrition status (ulcerative colitis, Crohn’s disease, chronic obstructive pulmonary disease of grade 2 or greater, congestive heart failure, insulin-dependent diabetes mellitus).
Informed consent was obtained from all study participants. Because the research involved human participants, it was conducted according to the guidelines laid down in the Declaration of Helsinki. The study was approved by the Ethics Committee of the Medical University of Lublin, Poland (consent no. KE-0254/170/2009).
Outcome Measures
All patients underwent a baseline assessment, which included demographics (age, sex), tumour-related details (type, grade, stage, size, site), clinical assessment (symptoms, comorbidities, metastases), and nutrition and laboratory measurements (total protein, serum albumin, transferrin). The sga was assessed by a medical doctor at the beginning of the hospitalization (during the physical exam), and bia was performed. The sga evaluation included weight, dietary intake, gastrointestinal symptoms, and changes in functional capacity and physical examination (levels of subcutaneous fat and muscle mass, ankle or sacral edema, and ascites). Those four features were noted as either normal (0), mild (1+), moderate (2+), or severe (3+)11. On the basis of the history and physical examination, the patient’s nutrition status was defined as well-nourished (sga-a), moderately malnourished (sga-b), or severely malnourished (sga-c).
All patients participating in the study were scheduled for a consultation with a medical doctor. During the consultation, the physician reviewed the sga instrument with the patient to obtain answers to all the questions (pg-sga) and also completed a physical exam to assess loss of subcutaneous fat, muscle wasting, presence of ankle and sacral edema, and ascites. The bia was performed using an SFB7 BioImp v1.55 analyzer (ImpediMed, Brisbane, Australia) while the patient was lying supine on a bed, legs apart and arms away from the torso. All evaluations were conducted by attaching 4 standard surface electrodes (tetrapolar technique) to the patient’s hand and foot on the right side. The R and Xc were measured directly in ohms at 50 kHz. The measurements were repeated 3 times in each patient, and the mean of those 3 measurements were used. The pa was obtained from the arc-tangent ratio Xc:R. To transform the result from radians to degrees, the resulting ratio was multiplied by 180 degrees divided by pi. The pa value was an absolute automatically obtained from the bioelectrical impedance analyzer.
Statistical Analysis
The statistical analyses were performed using the Statistica (version 8.0: StatSoft, Tulsa, OK, U.S.A.) and MedCalc 10 (MedCalc Software, Ostend, Belgium) software applications. Overall survival was defined as the time from the date of the first patient visit to the hospital after diagnosis (before treatment) to the date of death from any cause or to the date of last contact or the patient last being known to be alive. The Kaplan–Meier method was used to calculate survival. The log-rank test was used to evaluate survival distribution by patient stratification. Differences were considered to be statistically significant at p ≤ 0.05. For the purpose of the Kaplan–Meier survival analysis, pa measurements were categorized into two equal and mutually exclusive groups at a median pa score of 4.733 based on the receiver operating characteristic curve analysis from our previous study37. Correlations between pa and traditional measures of malnutrition were calculated using the Pearson coefficient for normally distributed variables and the Spearman rho for non-normally distributed variables.
The pa at 50 kHz was the main measure used for determining the sample size. On the basis of pilot study results, the sample size in the control and study groups was estimated by Altman nomogram (n = 31 in each group). Assuming a test power of at least 80% and two independent and equal groups for obtaining a standardized difference in the pa of 0.45 at the 5% level of statistical significance, the required size of the sample was estimated to be 75 patients.
RESULTS
Patients (67 men, 8 women) with a histologically confirmed diagnosis of hnc (28 laryngeal, 29 pharyngeal, 18 oral cavity) who were treated at the Otolaryngology Department, Head and Neck Surgery, Medical University of Lublin, Poland, between October 2009 and October 2012 were included in the study. All tumours were squamous cell carcinomas. Median age of the patients was 56 years. Table i presents baseline patient characteristics and the evaluated parameters for the study group.
TABLE I.
Characteristics of the study patients
| Variable | Value |
|---|---|
| Patients (n) | 75 |
| Sex [n (%)] | |
| Men | 67 (89.3) |
| Women | 8 (10.7) |
| Tumour stage at diagnosis [n (%)] | |
| III | 27 (36) |
| IV | 48 (64) |
| SGA [n (%)] | |
| Well-nourished (SGA-A) | 45 (60) |
| Moderately malnourished (SGA-B) | 24 (32) |
| Severely malnourished (SGA-C) | 6 (8) |
| Age at diagnosis (years) | |
| Mean | 56.88±8.21 |
| Range | 37–80 |
| Phase angle at 50 kHz | |
| Overall | |
| Mean | 5.04±0.88 |
| Range | 3.27–8 |
| SGA A | |
| Mean | 5.25±0.76 |
| Range | 3.53–7.23 |
| SGA B and C | Z = 3.33 0.0009 |
| Mean | 4.73±0.96 |
| Range | 3.27–8.00 |
The pa distribution (<4.733 or ≥4.733) had no association with demographic and clinical factors such as sex, tumour location, or disease stage; however, it was significantly associated with age. Values of pa less than 4.733 were significantly more frequently recorded in younger patients (<55 years). Table ii shows the distribution of pa by demographic and clinical factors.
TABLE II.
Distribution of phase angle value by demographic and clinical factors
| Variable | Phase angle | p Valuea | Chi-square | |
|---|---|---|---|---|
|
| ||||
| <4.733 | ≥4.733 | |||
| Patients [n (%)] | 26 (34.7) | 49 (65.3) | ||
| Sex [n (%)] | ||||
| Men | 24 (35.3) | 44 (64.7) | 0.9512 | 0.004 |
| Women | 2 (28.6) | 5 (71.4) | ||
| Age [n (%)] | ||||
| <55 Years | 8 (21.6) | 29 (78.4) | 0.0358 | 4.409 |
| ≥55 Years | 18 (47.4) | 20 (52.6) | ||
| Location of tumour [n (%)] | ||||
| Upperb | 9 (33.3) | 18 (66.7) | 0.8703 | 0.027 |
| Lowerc | 8 (40) | 12 (60) | ||
| Stage of disease [n (%)] | ||||
| IIIB | 8 (34.8) | 15 (65.2) | 0.8256 | 0.049 |
| IV | 18 (35.3) | 33 (64.7) | ||
Significant value shown in boldface type.
Mouth, tongue, jaw, tonsil, nose, centre throat, maxillary sinus.
Larynx, glottis, lower part of the throat.
In the present study, pa was significantly negatively correlated only with sga (r = −0.35, p = 0.0022). Correlations between the pa and other studied factors (albumin, transferrin, total protein) were statistically insignificant (Table iii).
TABLE III.
Correlations between phase angle and indicators of malnutrition
| Indicator vs. phase angle | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Albumin | Transferrin | Total protein | SGA | ||||
|
|
|
|
|
||||
| r Value | p Value | r Value | p Value | r Value | p Value | r Value | p Value |
| 0.06 | 0.6071 | 0.08 | 0.4729 | 0.11 | 0.3454 | −0.35 | 0.0022 |
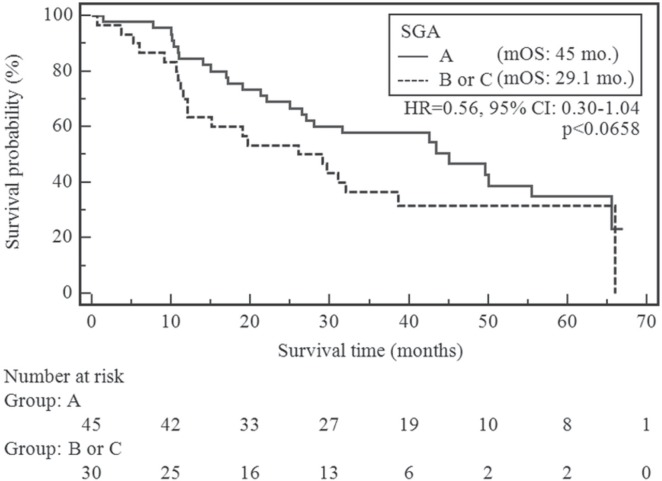
Median overall survival in our cohort was 32.0 months. At the time of analysis (observation from October 2009 to May 2015), 47 patients had died (62.7%) and 28 had been censored (having reached the end of follow-up without experiencing death). The variables of interest were rank-ordered based on the strength of their statistical association with survival. Patients without suspected or moderate malnutrition or cachexia (sga-a) had an insignificantly lower risk of shortened overall survival compared with the remaining patients [sga-b or -c (p < 0.0658; chi-square: 3.38; hazard ratio: 0.56; 95% confidence interval: 0.30 to 1.04)]. Figure 1 presents the survival curves, stratified by sga.
FIGURE 1.
Comparison of overall survival by subjective global assessment (SGA) status. mOS = median overall survival; HR = hazard ratio; CI = confidence interval.
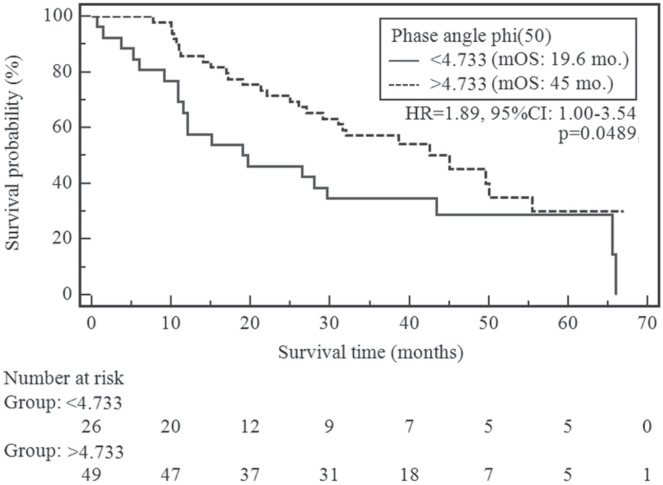
In patients whose pa was less than 4.733, the risk of a shortened overall survival was significantly higher than it was in the other patients (19.6 months vs. 45 months, p = 0.0489; chi-square: 3.88; hazard ratio: 1.8856; 95% confidence interval: 1.0031 to 3.5446). Figure 2 presents the Kaplan–Meier curves stratified by pa.
FIGURE 2.
Comparison of overall survival change by phase angle value. mOS = median overall survival; HR = hazard ratio; CI = confidence interval.
The overall survival duration had no statistically significant associations with the other demographic and clinical factors (univariate analysis in Table iv).
TABLE IV.
Univariate Kaplan–Meier survival analysis
| Variable | Comparator | Statistical significance | HR | 95% CI | |
|---|---|---|---|---|---|
|
| |||||
| p Value | Chi-square | ||||
| Sex (men) | Women | 0.8327 | 0.0446 | 1.1120 | 0.4153 to 2.9770 |
| Age ≥55 years | <55 Years | 0.5476 | 0.3616 | 0.8386 | 0.4724 to 1.4885 |
| Well-nourished by SGA | Suspected or moderate malnutrition, cachexia | 0.0658 | 3.3838 | 0.5587 | 0.3005 to 1.0389 |
| Phase angle <4.733 | ≥4.733 | 0.0489 | 3.8794 | 1.8856 | 1.0031 to 3.5446 |
| Low transferrin (mg/dL) (men < 215, women < 250) | Normal transferrin | 0.1497 | 2.0751 | 1.7869 | 0.8111 to 3.9368 |
| Low albumin (<3.2 g/dL) | Normal albumin | 0.0825 | 2.8310 | 8.23211 | 0.7064 to 95.9373 |
HR = hazard ratio; CI = confidence interval.
Cox multivariate logistic regression demonstrated that none of studied factors was significantly associated with shortened overall survival in the study group (chi-square for overall model fit: 5.25; p = 0.2621; Table v).
TABLE V.
Multivariate Cox proportional hazards modela
| Variable | β | p Value | HR | 95% CI |
|---|---|---|---|---|
| Male sex | 0.5358 | 0.9287 | 1.0491 | 0.3690 to 2.9824 |
| Age < 55 years | 0.3196 | 0.9445 | 1.0225 | 0.5483 to 1.9068 |
| Suspected or moderate malnutrition, cachexia by SGA | 0.3236 | 0.2038 | 1.5087 | 0.8027 to 2.8354 |
| Phase angle < 4.733 | 0.3417 | 0.1948 | 0.6421 | 0.3297 to 1.2503 |
| Low transferrin (mg/dL) (men < 215, women < 250) | 0.6028 | 0.2036 | 1.8271 | 0.7248 to 4.6062 |
| Low albumin (<3.2 g/dL) | 0.4040 | 0.5990 | 1.4977 | 0.3348 to 6.6979 |
Overall fit: chi-square: 11.035; p < 0.0262.
DISCUSSION
Bioelectrical impedance analysis is a relatively new tool for the assessment of nutrition status; it is useful as diagnostic method both in health and in chronic disease (amyotrophic lateral sclerosis18, cirrhosis25, hemodialysis27, hiv28, cancer). An alternative method, bioelectrical impedance spectroscopy, is a technique that measures impedance using 50 or more frequencies; the resulting values are then used to calculate the resistance of the extracellular and intracellular water. In view of the scarcity of equations or references for comparison in the literature, the latter technique is generally recommended for healthy individuals with no structural or hydration abnormalities38.
Phase angle is relatively independent from confounders such as body mass index and age. However, pa values vary with a patient’s condition or stage of disease36,37,39,40. Because of limitations in the bia method and also in the pa (adjusted or unadjusted), the value of those measures as prognostic factors for clinical decision-making in day-to-day practice in advanced cancers is still questioned. The main issue still under investigation is their effect on survival prediction, which is crucial for guiding clinical decisions.
Nutrition status can be assessed using a variety of tools. Some are relevant and already have confirmed significance as prognostic indicators: the Palliative Prognostic Index, serum albumin, lean body mass. In the present study, only unadjusted pa values were used as described by Hui et al.36, who confirmed that the unadjusted pa and the sga are both significantly associated with survival and correlate with each other. Their final recommendation was that the unadjusted pa provides valuable information and can be used as prognostic factor.
In many studies conducted in various cancers, a lower (under some threshold) pa was significantly associated with a lower survival rate14,31,40–42. Studies using other diagnostic tools (anthropometric measures, computed tomography, and magnetic resonance imaging42) have been conducted, but could not be applied in all cancers; they had to be assessed separately depending on the type and stage of the cancer.
In a systematic review, Lis et al.6 found an association between weight loss and swelling. In patients with advanced hnc who undergo surgery, lymph node stage, non-radical resection margins, and occurrence of major postoperative complications have been demonstrated to affect disease-specific survival in men and women alike. The combination of male sex, preoperative weight loss, and major postoperative complications has been associated with earlier death8. Nutrition status as measured by bia or sga has been tested as a prognostic factor in a few studies.
The study by Hui et al.36 confirmed that pa is a significant predictor of poor survival, independent of established prognostic factors in an advanced cancer setting. The median survivals for patients with pas of 2–2.9, 3–3.9, 4–4.9, 5–5.9, and 6 or more were 35 days, 54 days, 112 days, 134 days, and 220 days respectively. In that study, the results applied to a general population with advanced cancer (breast, gastrointestinal, genitourinary gynecologic, head-and-neck, hematologic, lung, and others). Those authors and the authors of other publications43 reported about various types of cancer; only a few studies have reported detailed data for single cancer types30–33,37,43: breast cancer, advanced non-small-cell lung cancer, colorectal cancer, hepatocellular carcinoma, and finally, in our study, hnc. In the present prospective study of a homogeneous population with advanced hnc, pa was observed to be as significant a reference test as the sga with respect to overall survival.
Earlier studies of bia in patients with cancer have mostly had a retrospective design30–33 and have enrolled patients with mixed cancers or varying stages of disease14,36,40,44,45. The key issues in the prognosis of hnc patients are disease stage and tumour grade, but an effect of prior treatment was avoided because our study enrolled only treatment-naïve hospitalized patients with stage iii or iv disease. One of the key findings by Hui et al.36, confirmed by Davis et al.44, was that a low pa discriminates patients with a short life expectancy and a higher pa is correlated with improved survival. Our study also confirms the status of pa as an independent prognostic factor in the context of many other known prognostic factors (albumin, transferrin). Some other prognostic factors (Palliative Prognostic Index, Karnofsky performance status) were not assessed. Additionally, as in other studies30–33, hydration status before pa measurement was not assessed (an exception is the study by Hui et al.36).
Because it reflects cell function, muscle mass, and nutrition status, pa could be used to assess the risk of bacteremia, surgical complications46, or other risks connected with cancer or its treatment. Being an objective and noninvasive measure, pa can, because of low cost and simple operation of a bioelectrical impedance analyzer, easily be repeatedly measured in the daily clinical routine (5–10 minutes).
The present study was undertaken to investigate whether bia—and especially pa—could be used as an indicator of nutrition status, and like the sga, could predict survival in advanced hnc. In Hui et al.36, median survival was assessed depending on a range of pa values: 2–3, 4–5, and 6 or more, associated with median survivals of less than 3 months, 3–6 months, and more than 6 months respectively. In studies involving specific cancers, thresholds or cut-points were also presented26,28,30–33,41, with results being similar in studies involving pancreatic cancer, advanced lung cancer, and colorectal cancer. The mean pa stratification threshold was 4.58 for advanced lung cancer47, 5.08 for advanced pancreatic cancer30, and 5.58 for advanced colorectal cancer. In other conditions, such as hiv29, dialysis28, or cirrhosis26, different pa cut-points were indicated, but the marker remained prognostic for survival or progression of disease. The selected cut-point has major practical implications and could be used in making decisions about further treatment in specific cancers.
Our study has several limitations. First, we enrolled only small population of hospitalized patients with advanced hnc in a single specialized centre. Further research is necessary to determine whether the study findings also apply to patients in outpatient care with advanced disease or to patients with advanced hnc generally. We did not collect data about other factors (C-reactive protein, the Palliative Prognostic Index, Karnofsky performance status) that are known to be prognostic and that have been reported to be associated with pa and serum albumin or transferrin35.
The extrapolation of the results reported here to a general population depends on age, sex, and body mass characteristics as assessed by bia48, but also on methodology issues connected to the size of the population and the standardization of bia measurement from one study to another. The results of the present study could be treated as pilot result in the clarification of the role and place of pa in clinical practice (alone or in combination with other prognostic tools) and of bia technique in advanced hnc.
CONCLUSIONS
We found that pa might be a predictor of survival in patients with advanced hnc. Further investigation in a larger population must be conducted to confirm our results, alone or in comparison with other prognostic factors, to assess their accuracy in advanced hnc.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Deschler DG, Moore MG, Smith RV. Quick Reference Guide to TNM Staging of Head and Neck Cancer and Neck Dissection Classification. 4th ed. Alexandria, VA: American Academy of Otolaryngology–Head and Neck Surgery Foundation; 2014. [Available online at: http://www.entnet.org/sites/default/files/NeckDissection_QuickRefGuide_highresFINAL.pdf; cited 18 August 2016] [Google Scholar]
- 2.Döbróssy L. Epidemiology of head and neck cancer: magnitude of the problem. Cancer Metastasis Rev. 2005;24:9–17. doi: 10.1007/s10555-005-5044-4. [DOI] [PubMed] [Google Scholar]
- 3.Segura A, Pardo J, Jara C, et al. An epidemiological evaluation of the prevalence of malnutrition in Spanish patients with locally advanced or metastatic cancer. Clin Nutr. 2005;24:801–14. doi: 10.1016/j.clnu.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Peris P, Paron L, Velasco C, et al. Long-term prevalence of oropharyngeal dysphagia in head and neck cancer patients: impact on quality of life. Clin Nutr. 2007;26:710–17. doi: 10.1016/j.clnu.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Nugent B, Lewis S, O’Sullivan JM. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev. 2013:CD007904. doi: 10.1002/14651858.CD007904.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lis CG, Gupta D, Lammersfeld CA, Markman M, Vashi PG. Role of nutritional status in predicting quality of life outcomes in cancer—a systematic review of the epidemiological literature. Nutr J. 2012;11:27. doi: 10.1186/1475-2891-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langius JA, Zandbergen MC, Eerenstein SE, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr. 2013;32:671–8. doi: 10.1016/j.clnu.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 8.van Bokhorst–de van der Schuer, van Leeuwen PA, Kuik DJ, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. 1999;6:519–27. doi: 10.1002/(SICI)1097-0142(19990801)86:3<519::AID-CNCR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Naber TH, Schermer T, de Bree A, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997;66:1232–9. doi: 10.1093/ajcn/66.5.1232. [DOI] [PubMed] [Google Scholar]
- 10.Hammerlid E, Wirblad B, Sandin C, et al. Malnutrition and food intake in relation to quality of life in head and neck cancer patients. Head Neck. 1998;20:540–8. doi: 10.1002/(SICI)1097-0347(199809)20:6<540::AID-HED9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 12.Capuano G, Gentile PC, Bianciardi F, Tosti M, Palladino A, Di Palma M. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support Care Cancer. 2010;18:433–7. doi: 10.1007/s00520-009-0681-8. [DOI] [PubMed] [Google Scholar]
- 13.Marin Caro MM, Laviano A, Pichard C. Nutritional intervention and quality of life in adult oncology patients. Clin Nutr. 2007;26:289–301. doi: 10.1016/j.clnu.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Norman K, Stobaus N, Zocher D, et al. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr. 2010;92:612–19. doi: 10.3945/ajcn.2010.29215. [DOI] [PubMed] [Google Scholar]
- 15.United Kingdom National Health Service (nhs), nhs Digital . Percentage having pre-treatment dietetic assessment: Cohort: All England (MDT) larynx/ OC/ *pharynx/ MSG diagnoses in audit year with AHP applicable treatment (surgery/chemo/radio/chemoradiotherapy) recorded, any intent [Web resource] Leeds, U.K.: NHS Digital; 2014. [Available online at: http://www.hscic.gov.uk/catalogue/PUB14257/clin-audi-supp-prog-head-neck-dahn-12-13-rep24.pdf; cited 12 December 2015] [Google Scholar]
- 16.Pencharz PB, Azcue M. Use of bioelectrical impedance analysis measurements in the clinical management of malnutrition. Am J Clin Nutr. 1996;64(suppl):485S–8S. doi: 10.1093/ajcn/64.3.485S. [DOI] [PubMed] [Google Scholar]
- 17.Leandro-Merhi VA, Braga de Aquino JL. Comparison of nutritional diagnosis methods and prediction of clinical outcomes in patients with neoplasms and digestive tract diseases. Clin Nutr. 2015;34:647–51. doi: 10.1016/j.clnu.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Desport JC, Preux PM, Bouteloup-Demange C, et al. Validation of bioelectrical impedance analysis in patients with amyotrophic lateral sclerosis. Am J Clin Nutr. 2003;77:1179–85. doi: 10.1093/ajcn/77.5.1179. [DOI] [PubMed] [Google Scholar]
- 19.Mueller C, Compher C, Ellen D, on behalf of the American Society for Parenteral and Enteral Nutrition (aspen) Board of Directors aspen clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16–24. doi: 10.1177/0148607110389335. [DOI] [PubMed] [Google Scholar]
- 20.Cederholm T, Bosæus I, Barazzoni R, et al. Diagnostic criteria for malnutrition—an espen consensus statement. Clin Nutr. 2015;34:335–40. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Couch ME, Dittus K, Toth MJ, et al. Cancer cachexia update in head and neck cancer: definitions and diagnostic features. Head Neck. 2015;37:594–604. doi: 10.1002/hed.23599. [DOI] [PubMed] [Google Scholar]
- 22.Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27:921–33. doi: 10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]
- 23.Zarowitz BJ, Pilla AM. Bioelectrical impedance in clinical practice. DICP. 1989;23:548–55. doi: 10.1177/1060028089023007-803. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner RN, Chumlea WC, Roche AF. Bioelectric impedance phase angle and body composition. Am J Clin Nutr. 1988;48:16–23. doi: 10.1093/ajcn/48.1.16. [DOI] [PubMed] [Google Scholar]
- 25.Barbosa-Silva MC, Barros AJ, Post CL, Waitzberg DL, Heymsfield SB. Can bioelectrical impedance analysis identify malnutrition in preoperative nutrition assessment? Nutrition. 2003;19:422–6. doi: 10.1016/S0899-9007(02)00932-2. [DOI] [PubMed] [Google Scholar]
- 26.Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86:509–16. doi: 10.1007/s00421-001-0570-4. [DOI] [PubMed] [Google Scholar]
- 27.Faisy C, Rabbat A, Kouchakji B, Laaban JP. Bioelectrical impedance analysis in estimating nutritional status and outcome of patients with chronic obstructive pulmonary disease and acute respiratory failure. Intensive Care Med. 2000;26:518–25. doi: 10.1007/s001340051198. [DOI] [PubMed] [Google Scholar]
- 28.Maggiore Q, Nigrelli S, Ciccarelli C, Grimaldi C, Rossi GA, Michelassi C. Nutritional and prognostic correlates of bioimpedance indexes in hemodialysis patients. Kidney Int. 1996;50:2103–8. doi: 10.1038/ki.1996.535. [DOI] [PubMed] [Google Scholar]
- 29.Schwenk A, Beisenherz A, Romer K, Kremer G, Salzberger B, Elia M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in hiv-infected patients in the era of highly active antiretroviral treatment. Am J Clin Nutr. 2000;72:496–501. doi: 10.1093/ajcn/72.2.496. [DOI] [PubMed] [Google Scholar]
- 30.Gupta D, Lis CG, Dahlk SL, Vashi PG, Grutsch JF, Lammersfeld CA. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr. 2004;92:957–62. doi: 10.1079/BJN20041292. [DOI] [PubMed] [Google Scholar]
- 31.Gupta D, Lammersfeld CA, Burrows JL, et al. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am J Clin Nutr. 2004;80:1634–8. doi: 10.1093/ajcn/80.6.1634. [DOI] [PubMed] [Google Scholar]
- 32.Gupta D, Lammersfeld CA, Vashi PG, et al. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer. 2008;8:249. doi: 10.1186/1471-2407-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta D, Lammersfeld CA, Vashi PG, et al. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in stage iiib and iv non-small cell lung cancer. BMC Cancer. 2009;9:37. doi: 10.1186/1471-2407-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isenring E, Bauer J, Capra S, Davies PS. Evaluation of foot-to-foot bioelectrical impedance analysis for the prediction of total body water in oncology outpatients receiving radiotherapy. Eur J Clin Nutr. 2004;58:46–51. doi: 10.1038/sj.ejcn.1601744. [DOI] [PubMed] [Google Scholar]
- 35.Simons JP, Schols AM, Westerterp KR, ten Velde GP, Wouters EF. The use of bioelectrical impedance analysis to predict total body water in patients with cancer cachexia. Am J Clin Nutr. 1995;61:741–5. doi: 10.1093/ajcn/61.4.741. [DOI] [PubMed] [Google Scholar]
- 36.Hui D, Bansal S, Morgado M, Dev R, Chisholm G, Bruera E. Phase angle for prognostication of survival in patients with advanced cancer: preliminary findings. Cancer. 2014;120:2207–14. doi: 10.1002/cncr.28624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Małecka-Massalska T, Mlak R, Smolen A, Morshed K. Bioelectrical impedance phase angle and subjective global assessment in detecting malnutrition among newly diagnosed head and neck cancer patients. Eur Arch Otorhinolaryngol. 2016;273:1299–305. doi: 10.1007/s00405-015-3626-5. [DOI] [PubMed] [Google Scholar]
- 38.Haverkort EB, Reijven PL, Binnekade JM, et al. Bioelectrical impedance analysis to estimate body composition in surgical and oncological patients: a systematic review. Eur J Clin Nutr. 2015;69:3–13. doi: 10.1038/ejcn.2014.203. [DOI] [PubMed] [Google Scholar]
- 39.Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN., Jr Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49–52. doi: 10.1093/ajcn.82.1.49. [DOI] [PubMed] [Google Scholar]
- 40.Santarpia L, Marra M, Montagnese C, Alfonsi L, Pasanisi F, Contaldo F. Prognostic significance of bioelectrical impedance phase angle in advanced cancer: preliminary observations. Nutrition. 2009;25:930–1. doi: 10.1016/j.nut.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients—a comprehensive review. Eur J Clin Nutr. 2015;69:1290–7. doi: 10.1038/ejcn.2015.126. [DOI] [PubMed] [Google Scholar]
- 42.Lee SY, Lee YJ, Yang JH, Kim CM, Choi WS. The association between phase angle of bioelectrical impedance analysis and survival time in advanced cancer patients: preliminary study. Korean J Fam Med. 2014;35:251–6. doi: 10.4082/kjfm.2014.35.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schutte K, Tippelt B, Schulz C, et al. Malnutrition is a prognostic factor in patients with hepatocellular carcinoma (hcc) Clin Nutr. 2015;34:1122–7. doi: 10.1016/j.clnu.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Davis MP, Yavuzsen T, Khoshknabi D, et al. Bioelectrical impedance phase angle changes during hydration and prognosis in advanced cancer. Am J Hosp Palliat Care. 2009;26:180–7. doi: 10.1177/1049909108330028. [DOI] [PubMed] [Google Scholar]
- 45.Paiva SI, Borges LR, Halpern-Silveira D, Assunção MC, Barros AJ, Gonzalez MC. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support Care Cancer. 2010;19:187–92. doi: 10.1007/s00520-009-0798-9. [DOI] [PubMed] [Google Scholar]
- 46.Barbosa-Silva MC, Barros AJ. Bioelectric impedance and individual characteristics as prognostic factors for post-operative complications. Clin Nutr. 2005;24:830–8. doi: 10.1016/j.clnu.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Gupta D, Vashi PG, Lammersfeld CA, Braun DP. Role of nutritional status in predicting the length of stay in cancer: a systematic review of the epidemiological literature. Ann Nutr Metab. 2011;59:96–106. doi: 10.1159/000332914. [DOI] [PubMed] [Google Scholar]
- 48.Toso S, Piccoli A, Gusella M, et al. Altered tissue electric properties in lung cancer patients as detected by bioelectric impedance vector analysis. Nutrition. 2000;16:120–4. doi: 10.1016/S0899-9007(99)00230-0. [DOI] [PubMed] [Google Scholar]