Abstract
Aims
Statins have modest adverse effects on glycaemic control. Alirocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor, lowers low-density lipoprotein cholesterol. This study assessed the effects of alirocumab on new-onset diabetes and pre-diabetes incidence in individuals without diabetes at baseline.
Methods and results
Pooled analysis of 10 ODYSSEY Phase 3 trials (n = 4974) of 24–104 weeks duration. Six trials (n = 4211) were ≥52 weeks in length. Most patients received background maximally tolerated statin. Alirocumab effect on the rate of diabetes-related treatment-emergent adverse events (TEAEs), and/or fasting plasma glucose (FPG) and glycated haemoglobin A1C (HbA1C) was measured at baseline and every 12–24 weeks. Transition to diabetes analysis combined TEAE and FPG/HbA1C laboratory data. At baseline, 30.7% of individuals had diabetes and were excluded from the current analysis. The remaining 3448 individuals without diabetes had pre-diabetes (39.6%) or were normoglycaemic (29.7%). The hazard ratio (HR; 95% confidence interval) for diabetes-related TEAEs in alirocumab was 0.64 (0.36–1.14) vs. placebo and 0.55 (0.22–1.41) vs. ezetimibe. The HR associated for transition from pre-diabetes to new-onset diabetes for alirocumab was 0.90 (0.63–1.29) vs. placebo and 1.10 (0.57–2.12) vs. ezetimibe. Mean change in FPG/HbA1C over time showed no difference between treatment groups in patients without diabetes.
Conclusions
There was no evidence of an effect of alirocumab on transition to new-onset diabetes in 3448 individuals without diabetes at baseline with a follow-up period of 6–18 months, compared to either placebo or ezetimibe. Longer follow-up with larger number of individuals is needed to conclusively rule out an effect.
Keywords: Glycaemia, Diabetes, PCSK9 inhibitor, Low-density lipoprotein cholesterol, HbA1C, Fasting plasma glucose
See page 2990 for the editorial comment on this article (doi:10.1093/eurheartj/ehw346)
Introduction
Recent evidence suggests that statin therapy increases the rate of transition to diabetes.1–5 In those receiving rosuvastatin 20 mg in the JUPITER (Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) study,3,4 there was a 28% excess risk of diabetes (hazard ratio [HR] 1.28, 95% confidence interval [CI] 1.07–1.54, P= 0.01) compared with placebo. The excess risk of diabetes occurred only in those with one or more risk factors for developing diabetes; no increase in diabetes was observed in those individuals without a major diabetes risk factor (HR 0.99, 95% CI 0.45–2.21, P= 0.99).4 Since the initial JUPITER report, several meta-analyses reported a small increase in the risk of statin-associated diabetes.1,5 Although most reports conclude that the cardiovascular (CV) and mortality benefits from statin therapy outweigh the diabetes hazard, the US Food and Drug Administration (FDA) implemented safety label changes stating increase in glycated haemoglobin A1C (HbA1C) and fasting plasma glucose (FPG) levels with statin use.6 Meanwhile, the exact mechanism of the statin-associated glycaemic effect is not understood raising the question of whether such an effect will pertain for other low-density lipoprotein cholesterol (LDL-C) lowering drugs.7–10
Alirocumab is a fully human proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibody that lowers LDL-C levels by increasing the number of low-density lipoprotein receptors (LDLRs).11 Alirocumab reduces LDL-C by up to 61% as monotherapy or in addition to statins, with or without other lipid-lowering therapy (LLT) in Phase 3 studies and is now approved by the FDA and European Medicines Agency (EMA) for use in hypercholesterolemia patients who require additional lowering of LDL-C in adjunct to diet and maximally tolerated statin therapy12–18 In the US, alirocumab is indicated additionally in patients with clinical atherosclerotic CV disease, while the EMA has approved the use of alirocumab in patients with dyslipidaemia. Given that statin therapy for LDL-C reduction has been shown to affect glycaemic control, it is important to assess effects of alirocumab on the development of new-onset diabetes with regard to its safety profile as well as to yield mechanistic insight on statin-induced diabetes.
Methods
Study design
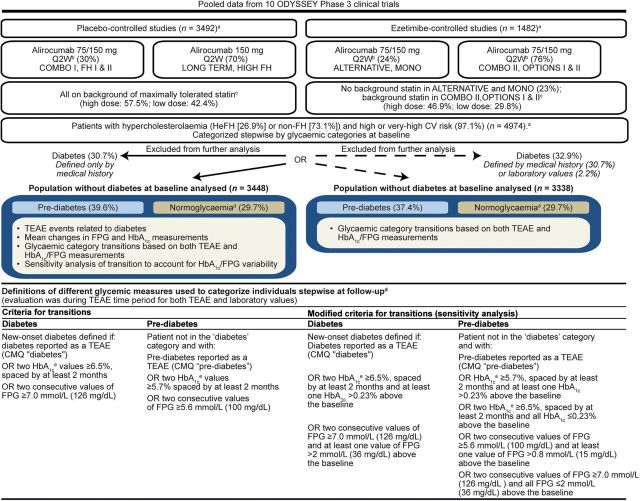
The pooled analysis included 10 main ODYSSEY Phase 3 randomized, double-blind, controlled trials (n = 4974) with subcutaneous alirocumab dosing every 2 weeks (Q2W) (24–102 weeks' duration). Patients were randomized to alirocumab, or to either placebo (LONG TERM [NCT01507831], FH I [NCT01623115], FH II [NCT01709500], HIGH FH [NCT01617655], COMBO I [NCT01644175]) or to ezetimibe (COMBO II [NCT01644188], MONO [NCT01644474], OPTIONS I [NCT01730040], OPTIONS II [NCT01730053], ALTERNATIVE [NCT01709513]) as comparators (Supplementary material online, Table S1). LONG TERM and HIGH FH utilized 150 mg Q2W from the outset (Figure 1) and the other studies used alirocumab 75 mg Q2W with uptitration to 150 mg Q2W at Week 12 if LDL-C goals were not achieved at Week 8 depending on CV risk. Treatment allocation was in addition to maximally tolerated statin therapy in ODYSSEY LONG TERM, FH I, FH II, COMBO I, COMBO II, and HIGH FH; in addition to commonly used statin doses which could be up-titrated in the OPTIONS I and II studies; and there was no background statin in the MONO and ALTERNATIVE studies. Intensity of statin doses across the pooled studies is provided in Figure 1.
Figure 1.
Schematic of population characteristics and study design. an values represent number of individuals in the safety analysis. bDose adjustment to 150 mg Q2W at Week 12 if LDL-C was not at goal by Week 8. cBackground statin was maximally tolerated dose in COMBO II, atorvastatin 20–40 mg in OPTIONS I, and rosuvastatin 10–20 mg in OPTIONS II and no statin in MONO and ALTERNATIVE. In addition, patients were allowed to receive other LLT (except for ezetimibe in ezetimibe-controlled studies). Maximally tolerated statin was defined as high-dose statin (atorvastatin 40–80 mg, rosuvastatin 10–20 mg, or simvastatin 80 mg daily), or lower doses with an investigator-documented reason, e.g. intolerance. dAn individual not in diabetes or pre-diabetes category as defined above was considered normoglycaemic. Categorization of individuals at both baseline and follow-up was performed stepwise. eHbA1C was measured once during baseline. CMQ, Custom MedDRA Query; CV, cardiovascular; FH, familial hypercholesterolaemia; FPG, fasting plasma glucose; HbA1C, glycated haemoglobin A1C; HeFH, heterozygous familial hypercholesterolemia; LLT, lipid-lowering therapy; Q2W, every 2 weeks; TEAE, treatment-emergent adverse event.
Patients
All patients provided written informed consent and were aged ≥18 years. The MONO study included only patients with moderate CV risk, based on European guidelines.19,20 The ALTERNATIVE study included statin intolerant patients with moderate, high, or very high CV risk and the remaining studies were in those at high or very high CV risk (Figure 1; Supplementary material online, Table S1). Inclusion criteria for baseline LDL-C were ≥1.8 mmol/L for those who had experienced prior CV events or ≥2.6 mmol/L for those who had experienced no prior experience of CV events with the exception of the HIGH FH and LONG TERM study in which LDL-C had to be ≥4.14 mmol/L and ≥1.8 mmol/L, respectively, for all individuals (Supplementary material online, Table S1) at baseline.
Categorization of individuals into diabetes, pre-diabetes, or normoglycaemia
Individuals were categorized as having diabetes, pre-diabetes, or being normoglycaemic at baseline and follow-up. Four sources of data were used: medical history data at baseline; treatment-emergent adverse events (TEAEs); HbA1C, measured once during baseline and then measured at Week 12, 24, 52, and 78; and FPG measured twice during the baseline phase and at Week 12, 24, 36, 52, 64, and 78. The term pre-diabetes (as per American Diabetes Association [ADA], guidelines)21 was used instead of impaired glucose tolerance since evaluation of individuals for diabetes and pre-diabetes status was performed using FPG/HbA1C measurements and not oral glucose tolerance test measurements.
For the main analyses, diabetes at baseline was considered present if a Custom Medical Dictionary for Regulatory Activities (MedDRA) Query (CMQ) of the medical history reported ‘diabetes’. We considered this a more specific definition than additionally including the baseline phase FPG (≥7.0 mmol/L) or a single HbA1C (≥6.5%) measure to define baseline diabetes but we also report sensitivity analyses using these laboratory measurements. As per the ADA 2015 Standards of Care, two different tests above diagnostic threshold (such as HbA1C ≥6.5% and FPG ≥7.0 mmol/L) confirms diagnosis of diabetes.22 The latter increased the number of patients with diabetes at baseline from 1526 to 1636 (+7%: Supplementary material online, Table S2). Pre-diabetes was defined as those assigned CMQ code ‘pre-diabetes’ recorded in the medical history OR baseline HbA1C ≥5.7%, OR two values of FPG (at screening and randomization) ≥5.6 mmol/L.
Definition of transition to diabetes or to pre-diabetes during follow-up
Figure 1 details the definition but, in brief, transition to new-onset diabetes during follow-up in individuals without diabetes at baseline was explored in two ways. The first analysis was of TEAEs based on the CMQ ‘diabetes mellitus or diabetic complications’ which included MedRA terms that refer to ‘high glucose or glycaemia’. New anti-diabetes drug use was requested to be reported in TEAEs. Second, in order to maximize the sensitivity for detecting transition to diabetes, additional analyses were conducted for transition to diabetes based on two consecutive values of FPG ≥7.0 mmol/L or two values of HbA1C ≥6.5% at least 2 months apart consistent with diabetes or a CMQ code for ‘diabetes’ but excluding MedRA terms that refer to ‘high glucose or glycaemia’ (Figure 1). Thirty-eight patients reported new diabetes drug use; all were captured as transition to diabetes or pre-diabetes except three: two patients belonging to alirocumab and one to the placebo group.
In the absence of transition to diabetes, transition to pre-diabetes during follow-up in individuals with baseline normoglycaemia was defined as having a TEAE report with CMQ code ‘pre-diabetes’ or two consecutive values of FPG ≥5.6 mmol/L or two values of HbA1C ≥5.7% at least 2 months apart. Regression to normoglycaemia among those with pre-diabetes at baseline was also quantified.
Additional sensitivity analysis of transition to new-onset diabetes during follow-up
To account for HbA1C/FPG variability, a sensitivity analysis was performed in which FPG and HbA1C were further required to have increased by a specified amount from baseline (Figure 1; Supplementary material online, Methods). Post hoc we considered whether analyses allowing two consecutive diagnostic values, one of FPG and one of HbA1C to qualify as diabetes were warranted. However, this would have increased the number of transitions very little; five individuals in the placebo-controlled pool (two in placebo, three in alirocumab) and one patient in the ezetimibe-controlled pool (one in alirocumab) and so was not reported further.
Statistical methods
The safety population was defined as all randomized individuals who received at least one full or partial dose of study drug. Data were pooled into four groups: alirocumab and placebo from the placebo-controlled trials; and alirocumab and ezetimibe from the ezetimibe-controlled trials. The evaluation period was from the first dose of study treatment up to the last injection plus 70 days (termed the TEAE period). Follow-up time was up to transition to diabetes or, if diabetes did not occur, on transition to pre-diabetes or else at the end of the TEAE period. Occurrence of events of interest was summarized using incidence rate, event rate per 100 patient-years, with 95% CI. The mid-P method was used to determine these CIs due to the discrete distribution of the endpoint and the low number of events.23 The HR was calculated using a Cox proportional hazards model stratified on the study in order to compare treatment groups. Effects on HbA1C and FPG measurements are presented using descriptive statistics and graphs during the treatment period, i.e. up to 21 days after the last injection. These data focus on the placebo-controlled rather than ezetimibe-controlled studies as these had the longer follow-up (median exposure of 78 weeks vs. 24–38 weeks in the placebo-controlled vs. the ezetimibe-controlled studies, respectively). These analyses were not right-censored for new diabetes drugs initiation. SAS version 9.2 was used for the statistical analyses.
Results
Baseline categorization of individuals related to their glycaemic status
The prevalence of diabetes, pre-diabetes, and normoglycaemia was comparable between treatment groups in both placebo-controlled and ezetimibe-controlled pools (Table 1). Overall, across the studies, 30.7% had diabetes, 39.6% had pre-diabetes, and 29.7% were normoglycaemic. Demographic and baseline characteristics of individuals without diabetes at baseline for the randomized populations of the 10 studies were similar within the pooled treatment groups (Supplementary material online, Table S3). Median duration of alirocumab exposure of individuals without diabetes at baseline in the placebo-controlled pool was ∼78 weeks. In the ezetimibe-controlled pool, median duration of exposure to study drug ranged from 24 to 38 weeks (Supplementary material online, Table S4). Overall, in individuals without diabetes at baseline, 63% received alirocumab 150 mg Q2W (mostly derived from LONG TERM where individuals were treated with a fixed dose of 150 mg Q2W) and 37% were treated with alirocumab 75 mg Q2W. Cumulative exposure for alirocumab vs. placebo was 2128.7 and 1078.2 patient-years, respectively; for alirocumab vs. ezetimibe, it was 474.6 and 292 patient-years, respectively (Supplementary material online, Table S4) among this subset of patients without diabetes at baseline. There was no difference between the treatment arms in the dosage category or frequency of background statin usage during the TEAE period (Supplementary material online, Figure S1).
Table 1.
Glycaemic categories at baseline in the pool of 10 Phase 3 studies
| n (%) | Placebo-controlled pool |
Ezetimibe-controlled pool |
||
|---|---|---|---|---|
| Placebo (n = 1174) | Alirocumab (n = 2318) | Ezetimibe (n = 618) | Alirocumab (n = 864) | |
| Diabetes as per medical history (excluded from further analysis)a | 356 (30.3) | 698 (30.1) | 190 (30.7) | 282 (32.6) |
| Pre-diabetesb | 453 (38.6) | 903 (39.0) | 254 (41.1) | 359 (41.6) |
| As per medical history | 28 (2.4) | 52 (2.2) | 20 (3.2) | 33 (3.8) |
| As per laboratory data only | 425 (36.2) | 851 (36.7) | 234 (37.9) | 326 (37.7) |
| As per HbA1C only | 214 (18.2) | 452 (19.5) | 118 (19.1) | 138 (16.0) |
| As per FPG only | 64 (5.5) | 141 (6.1) | 47 (7.6) | 57 (6.6) |
| Normoglycaemiac | 365 (31.1) | 717 (30.9) | 174 (28.2) | 223 (25.8) |
aDiabetes at baseline is defined as those assigned CMQ code ‘diabetes’ recorded in the medical history.
bPre-diabetes defined as those assigned CMQ code ‘pre-diabetes’ recorded in the medical history OR baseline HbA1C ≥5.7%, OR two values of FPG (at screening and randomization) ≥5.6 mmol/L. HbA1C was measured once during baseline.
cAn individual not in diabetes or pre-diabetes category as defined above was considered normoglycaemic. Categorization of individuals at baseline was performed stepwise.
CMQ, Custom MedDRA Query; FPG, fasting plasma glucose; HbA1C, glycated haemoglobin A1C.
Transition to diabetes based on treatment-emergent adverse event data alone
The transition to diabetes based solely on the TEAE terms among those without diabetes at baseline is shown in Table 2. Overall, there were 66 such transitions across the studies. There was no increase in the incidence of diabetes, thus defined, in the alirocumab group compared with controls with HR (95% CI) = 0.64 (0.36–1.14) in the placebo-controlled pool and 0.55 (0.22–1.41) in the ezetimibe-controlled pool. The combined estimate across the two pools was HR = 0.62 (0.38–1.00).
Table 2.
Transition to diabetes based on treatment-emergent adverse event data alone by treatment group
| Placebo-controlled pool |
Ezetimibe-controlled pool |
|||
|---|---|---|---|---|
| Placebo (n = 818) | Alirocumab (n = 1620) | Ezetimibe (n = 428) | Alirocumab (n = 582) | |
| Incident diabetes mellitus or diabetic complications TEAE | ||||
| n (%,95% mid-P CI) | 21 (2.6, 1.6–3.8) | 27 (1.7, 1.1–2.4) | 9 (2.1, 1.0–3.8) | 9 (1.5, 0.8–2.8) |
| Number of patients with an event per 100 patient-yeara (95% CI) | 1.8 (1.1–2.8) | 1.2 (0.8–1.7) | 2.8 (1.3–5.4) | 1.8 (0.8–3.4) |
| HR vs. control (95% CI)b | 0.64 (0.36–1.14) | 0.55 (0.22–1.41) | ||
| Diabetes mellitus or diabetic complications TEAE, n (%) | ||||
| Diabetes mellitus or diabetic complications (CMQ) | 21 (2.6) | 27 (1.7) | 9 (2.1) | 9 (1.5) |
| Type 2 diabetes mellitus | 13 (1.6) | 21 (1.3) | 2 (0.5) | 5 (0.9) |
| Diabetes mellitus | 4 (0.5) | 4 (0.2) | 2 (0.5) | 2 (0.3) |
| Diabetic neuropathy | 0 | 1 (<0.1) | 0 | 0 |
| Diabetic retinopathy | 0 | 1 (<0.1) | 0 | 0 |
| Glucose tolerance decreased | 0 | 1 (<0.1) | 0 | 0 |
| Blood glucose increased | 1 (0.1) | 0 | 3 (0.7) | 1 (0.2) |
| Hyperglycaemia | 3 (0.4) | 0 | 2 (0.5) | 1 (0.2) |
MedDRA 17.1. The selection of preferred terms (PTs) is based on the CMQ ‘diabetes mellitus or diabetic complications’.
n (%) = number and percentage of individuals with at least one event. Table sorted by decreasing incidence of PT in the alirocumab group of placebo-controlled pool. Individuals without diabetes at baseline defined as those not assigned CMQ code ‘diabetes’ recorded in medical history.
aCalculated as number of individuals with an event divided by total patient years.
bCalculated using a Cox model stratified on the study.
CI, confidence interval; CMQ, Custom Medical Query; HR, hazards ratio; MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse event.
Changes in mean fasting plasma glucose and HbA1C measurements during follow-up by treatment allocation
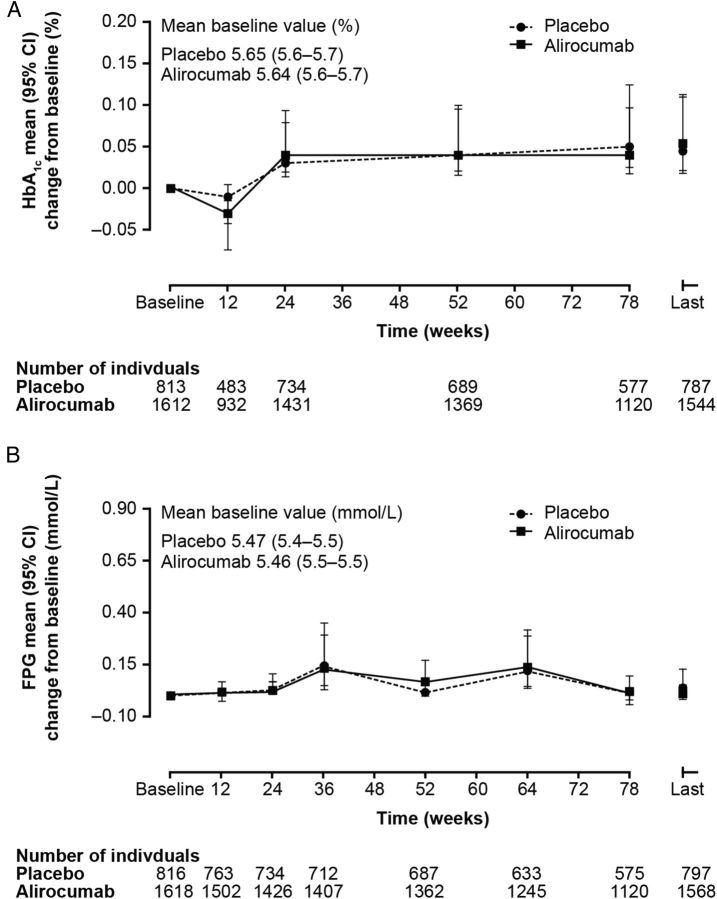
Mean HbA1C (Figure 2A) and FPG (Figure 2B) change from baseline measures between different treatment groups compared with placebo control showed no meaningful difference in individuals without diabetes at baseline. As shown in Figure 2, both HbA1C and FPG varied slightly in both alirocumab and placebo groups over the course of the studies. These variations seen in mean HbA1C and FPG change from baseline were of similar magnitude in both the alirocumab and placebo groups (0.04% and 0.05% for HbA1C and −0.02 mmol/dL and −0.02 mmol/dL for FPG at the Week 78 visit, in alirocumab and placebo group, respectively). Minor variations in biochemical parameters can occur during trials and emphasize the need for appropriate comparator groups. Such individuals in the placebo-controlled pool were on background maximally tolerated statin therapy (Figure 1). As shown in Supplementary material online, Figure S2, there is no evidence of any significant adverse effect of alirocumab compared with ezetimibe in the very small number of participants not on background statins, in the ezetimibe-controlled studies. Due to the small sample size, the power to detect effects from these data is very limited.
Figure 2.
HbA1C (%) and fasting plasma glucose (mmol/L) trajectories (change from baseline) in those without diabetes at baseline: alirocumab vs. placebo. Placebo-controlled studies: Phase 3 (LONG TERM, FH I, FH II, HIGH FH, COMBO I). At Week 12, HbA1C was measured only in the LONG TERM. The last on-treatment value is defined as the last value collected up to 21 days after the last double-blind IMP injection. Patients who had that parameter assessed at baseline and/or at follow-up are included. Individuals without diabetes at baseline defined as those not assigned CMQ code ‘diabetes’ recorded in medical history. CMQ, Custom MedDRA Query; FPG, fasting plasma glucose; HbA1C, glycated haemoglobin A1C; IMP, Investigational Medicinal Product; CI, confidence interval.
Transition to diabetes based on combined treatment-emergent adverse event and fasting plasma glucose/HbA1C laboratory data
Transition to new-onset diabetes and pre-diabetes using both the TEAE data and the FPG and HbA1C data are shown in Table 3 and further detailed in Supplementary material online, Table S5. Among those with pre-diabetes at baseline, the majority remained in this category during follow-up. Overall, 8.7% of these individuals transitioned to new-onset diabetes and most progressions were based on laboratory parameters rather than clinical TEAE reports (Supplementary material online, Table S5). The HR (95% CI) for transition associated with alirocumab was not significant in either placebo (0.90 [0.63–1.29]) or ezetimibe (1.10 [0.57–2.12]) controlled pools (Table 3). Among those with pre-diabetes at baseline, regression to normoglycaemia was common but there was no difference between alirocumab and control groups on this regression.
Table 3.
Transition between glycaemic categories based on the treatment-emergent adverse event or laboratory data by treatment groupa
| Placebo-controlled pool |
Ezetimibe-controlled pool |
|||
|---|---|---|---|---|
| Placebo (n = 818) | Alirocumab (n = 1620) | Ezetimibe (n = 428) | Alirocumab (n = 582) | |
| Pre-diabetes at baseline, n/N1 (%) | ||||
| Developed diabetesa | 47/453 (10.4) | 84/903 (9.3) | 14/254 (5.5) | 26/359 (7.2) |
| Remained with pre-diabetesa | 342/453 (75.5) | 663/903 (73.4) | 162/254 (63.8) | 237/359 (66.0) |
| Regressed to normoglycaemia | 64/453 (14.1) | 156/903 (17.3) | 78/254 (30.7) | 96/359 (26.7) |
| Analysis of transition from baseline pre-diabetes to new-onset diabetes (by TEAE or laboratory measurements) | ||||
| n (%,95% mid-P CI) | 47 (10.4, 7.8–13.4) | 84 (9.3, 7.5–11.3) | 14 (5.5, 3.2–8.9) | 26 (7.2, 4.9–10.3) |
| Number of patients with an event/100 patient-yearb (95% CI) | 7.7 (5.6–10.2) | 6.9 (5.5–8.5) | 7.2 (3.9–12.1) | 8.4 (5.5–12.3) |
| HR vs. control (95% CI)c | 0.90 (0.63–1.29) | 1.10 (0.57–2.12) | ||
| Normoglycaemic at baseline, n/N1 (%) | ||||
| Developed diabetesa | 1/365 (0.3) | 1/717 (0.1) | 0/174 | 1/223 (0.4) |
| Developed pre-diabetesa | 115/365 (31.5) | 261/717 (36.4) | 42/174 (24.1) | 59/223 (26.5) |
| Remained normoglycaemic | 249/365 (68.2) | 455/717 (63.5) | 132/174 (75.9) | 163/223 (73.1) |
| Analysis of transition from baseline normoglycaemic to pre-diabetes (by TEAE or laboratory measurements) | ||||
| n (%,95% mid-P CI) | 115 (31.5, 26.9–36.4) | 261 (36.4, 32.9–40.0) | 42 (24.1, 18.2–30.9) | 59 (26.5, 21.0–32.5) |
| Number of patients with an event/100 patient-yearb 95% CI) | 28.8 (23.8–34.6) | 35.1 (31.0–39.7) | 45.2 (32.6–61.1) | 42.9 (32.6–55.3) |
| HR vs. control (95% CI)c | 1.20 (0.96–1.49) | 0.88 (0.59–1.32) | ||
The number (n) represents the subset of the total number of individuals who met the criterion at least once during the TEAE period. The denominator (N1) for each parameter within a treatment group is the number of individuals who had that parameter assessed at follow-up (not missing) during the TEAE period, by baseline glycaemic category. Diabetes at baseline is defined using medical history. Pre-diabetes and diabetes at follow-up are defined using specific TEAE terms reported, raw values in HbA1C, and FPG (Figure 1). MedDRA terms for high glucose or glycaemia were not included here since FPG and HbA1C are being directly queried.
aBreakdown of the basis of transition classification by TEAE or laboratory values in Supplementary material online, Table S5.
bCalculated as number of individuals with an event divided by total patient years.
cCalculated using a Cox model stratified on the study.
CI, confidence interval; FPG, fasting plasma glucose; HbA1C, glycated haemoglobin A1C; HR, hazards ratio; MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment emergent adverse event.
Among those categorized normoglycaemic at baseline, there were no transitions to diabetes or pre-diabetes based on TEAEs and just three transitions to new-onset diabetes all based on FPG measurements (Supplementary material online, Table S5). Transition to pre-diabetes based on lab parameters was common across all treatment groups - about one-third of all individuals in the placebo-controlled pool and about one-quarter in the ezetimibe-controlled pool (Supplementary material online, Table S5). The HR (95% CI) for transition associated with alirocumab was not significant for either the placebo (1.20 [0.96–1.49]) or ezetimibe (0.88 [0.59–1.32]) controlled pools (Table 3).
The sensitivity analysis, assessing the variability of HbA1C/FPG, did not alter the conclusions. Similar and non-significant HRs for transition to new-onset diabetes or pre-diabetes in both treatment groups was observed as for the main analyses (Supplementary material online, Table S6).
A further sensitivity analysis of the transition to new-onset diabetes further excluding those with baseline diabetes based also on laboratory baseline parameters rather than medical history alone did not alter the conclusions (Supplementary material online, Table S7). When analyses were restricted to the LONG TERM study in which the higher fixed dose of alirocumab 150 mg Q2W was used, the conclusions were unchanged. Our analysis includes all those who received at least one dose of drug. Discontinuation rates were low and were balanced between the treatment arms. In the placebo pool, the treatment discontinuation rate at 24 weeks was 7.7% in both the alirocumab- and placebo-treated groups. In the ezetimibe pool, the treatment discontinuation rate is 13.5% in the alirocumab-treated group and 15.7% in the ezetimibe-treated group.
Regarding general safety across the ODYSSEY Phase 3 programme in all 10 studies including individuals with and without diabetes, the percentage of individuals who experienced at least one TEAE, at least one treatment-emergent serious adverse event, and any TEAE leading to permanent treatment discontinuation was similar between the alirocumab and control groups. Injection-site reactions, influenza, and pruritus occurred more frequently in individuals receiving alirocumab than control. These events were transient and mild in intensity and did not necessitate treatment discontinuation.
Discussion
In this pooled analysis of 10 Phase 3 studies from the ODYSSEY programme, we found no evidence that alirocumab affects the incidence of new-onset diabetes in 3448 individuals without diabetes at baseline with a follow-up period of 6–18 months. Our study comprises all the available data to-date on transition to diabetes in the ODYSSEY programme for the PCSK9 inhibitor alirocumab. Further data and longer follow-up are needed to conclusively rule out any effect. However, so far there was no evidence of an effect of alirocumab on transition to diabetes based on TEAEs related to diabetes and diabetic complications (Table 2), or on a composite measure combining TEAEs with threshold values for HbA1C or FPG (Table 3). Neither was there evidence of any effect on transition from normoglycaemia to pre-diabetes. Transitions in both directions between the normoglycaemic and pre-diabetes states were common. The percent of those patients transiting to pre-diabetes from normoglycaemia and the percent of patients regressing from pre-diabetes to normoglycaemia were both higher for alirocumab than control-treated pools but with no significant effects in either direction being found. In contrast, a 9% increased risk for new-onset diabetes (odds ratio [OR] 1.09; 95% CI 1.02–1.17) was associated with statin use in a meta-analyses with 91 140 participants followed for an average of 4 years.5 In a subsequent analysis of five statin trials (n = 32 752) with a weighted mean (standard deviation [SD]) follow-up of 4.9 (1.9) years, intensive-statin therapy was associated with an increased risk of new-onset diabetes compared with moderate-statin therapy (OR 1.12, 95% CI 1.04–1.22).1 In the JUPITER trial where a similar 50% reduction in LDL-C to that reported in the placebo-controlled studies here was seen, the HR for physician-reported diabetes was 1.25 overall. While our CI for the equivalent diabetes incidence TEAEs are wide, they do exclude this estimate.
Few of the previous studies on statins analyzing the risk of new-onset diabetes have measured HbA1C or FPG serially during the treatment period. We found no meaningful difference in effect of alirocumab vs. comparator on changes in FPG or HbA1C when serially measured in these studies of up to 78 weeks follow-up. In contrast, a small but significant difference in HbA1C values was previously reported in a comparatively larger rosuvastatin trial of >15 000 individuals at 24 months exposure.3,4 In the Collaborative Atorvastatin in Diabetes (CARDS) trial, among individuals with diabetes at baseline, atorvastatin 10 mg daily increased HbA1C by only 0.14% (95% CI 0.08–0.21) (1.5 mmol/mol [95% CI 0.8–2.3]) an effect that was seen within the first year of exposure in 2739 participants.24
An important aspect of our analysis is that the majority of people were already on background statin therapy. Around 54% of the individuals in our pooled analysis (n = 4974) were receiving high-dose background statin therapy, namely atorvastatin 40–80 mg, rosuvastatin 20–40 mg, or simvastatin 80 mg daily. There were too few persons in the trial programme not on any statin to examine incidence of diabetes in this group separately (n = 289 in the ezetimibe-controlled studies from MONO and ALTERNATIVE).
The exact molecular mechanism by which statins impact glycaemic control is not understood.10 The most recent meta-analysis of 129 170 trial participants observed the effect of statin on the incidence of diabetes and reported an association with modestly increased body weight but, unlike effects on glycaemia, was dose independent.7 In that same study, a Mendelian randomization performed with common variants of the 3-hydroxy-methylglutaryl coenzyme A reductase (HMGCR) gene indicated the association of certain single nucleotide polymorphisms of HMGCR gene with type 2 diabetes, consistent with the glycaemic effects of statins resulting from the downstream effects of HMGCR inhibition itself rather than an off-target effect.7 A 6-year observational cohort study in 8749 participants reported decreases in insulin sensitivity and insulin secretion associated in statin users.9 Others have postulated a lipotoxic effect on the pancreatic β-cell due to increased accumulation of cholesterol in the β-cell. Consistent with this, the prevalence of type 2 diabetes was reduced in the presence of LDLR mutations in HeFH individuals suggesting a causal relationship between LDLR-mediated transmembrane cholesterol transport and type 2 diabetes and inverse associations were found between type 2 diabetes incidence and LDL-C levels in the Framingham Heart Study.25,26 Given the uncertainty regarding the underlying mechanism, an important question is whether the increased risk of new-onset diabetes is an inevitable consequence of LDL-C lowering with any drug class. A recent analysis of 18 144 individuals on background statin therapy in the IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial) programme, associated ezetimibe with a net 24% reduction in LDL-C vs. placebo but found no difference in the new-onset of diabetes between groups.27 While this suggests that a glycaemic effect is not inevitable with LDL-C lowering, the question remains whether more powerful LDL-C lowering agents, or those that increase LDL receptor levels as statins and PCSK9 inhibitors do, will lead to a worsening of glycaemia.
Accordingly, our data on the alirocumab trials to date are important in suggesting that PCSK9 inhibition may not lead to hyperglycaemia. For evolocumab, another drug in the PCSK9 inhibitor class, Blom et al. reported no significant effects on glycaemia in a smaller dataset of 901 participants in the DESCARTES (Durable Effect of PCSK9 Antibody CompARed wiTh placEbo Study) trial followed for 52 weeks28 and Henry et al. reported no effect on glycaemia in 1104 participants over 52 weeks in the OSLER trial.29 A recent random-effects meta-analysis of randomized clinical trials comparing the efficacy of evolocumab, placebo, and ezetimibe30 also found no evidence of evolocumab effect on glycaemic measures.30 Other sources of data on the effect of PCSK9 inhibition on glycaemia have been inconclusive. In a recent Mendelian randomization analysis, Bonnefond et al. reported that the R46L loss-of-function variant in the PCSK9 gene was associated with a −0.393 mmol/L lower LDL-C but was not associated with any altered markers of glucose homeostasis or type 2 diabetes risk.31 Another study reported a difference of 10% vs. 6% in the combined frequency of pre-diabetes and diabetes between carriers and non-carriers of the PCSK9 InsLeu loss-of-function variant (P= 0.04).32 Studies conducted in PCSK9-null mice investigating the role of the protein on glycaemic control have also thus far yielded contradictory results.33,34
In summary, pooled analysis from the Phase 3 ODYSSEY trial programme for the PCSK9 inhibitor alirocumab shows no evidence of an effect on diabetes incidence at this point despite the very substantial 40–60% LDL-C lowering achieved.12–18 As effects of statin on glycaemic control were not suggested until data from larger outcomes studies were available, longer follow-up with a larger number of individuals will be important to determine definitively the effect of PCSK9 inhibitors on the development of diabetes. The ongoing ODYSSEY OUTCOMES trial, randomizing ∼18 000 individuals for 2–5 years follow-up capturing diabetes events, serial HbA1C, and FPG measurements, will provide further data to establish whether alirocumab has any effect on glycaemic measures.
Authors’ contributions
L.M. performed statistical analysis. All authors were involved in data analysis and interpretation. H.G., J.R., L.L., and B.C. were involved in data acquisition. H.C., H.G., J.R., M.B.D., and R.P were involved in the conception and design of the research. H.C., M.B.D., and L.M. were involved in drafting the manuscript. All authors made critical revision of the manuscript for key intellectual content.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Conflict of interest: H.M.C. reports grants, personal fees, and non-financial support from Sanofi and Regeneron Pharmaceuticals, Inc., during the conduct of the study; grants, personal fees, and non-financial support from Eli Lilly & Company, grants and other from Roche Pharmaceuticals, grants from Pfizer Inc., grants from Boehringer Ingelheim, grants from AstraZeneca LP, and shareholding in Bayer and Roche, outside the submitted work. H.N.G. reports grants and personal fees from Sanofi, grants from Regeneron, and personal fees from Amgen and Pfizer, outside the submitted work. J.G.R. reports grants from Amarin, Amgen, Astra-Zeneca, Eli Lilly, Esai, Glaxo-Smith Kline, Merck, Pfizer, Regeneron/Sanofi, Takeda, and personal fees from Akcea/Ionis, Amgen, Eli Lilly, Esperion, Merck, Pfizer, Regeneron/Sanofi, outside the submitted work. L.A.L. reports personal fees from Aegerion, grants and personal fees from Amgen, grants and personal fees from Astra-Zeneca, grants and personal fees from Eli Lilly, grants and personal fees from Merck, grants from Pfizer, grants and personal fees from Regeneron, grants and personal fees from Sanofi, outside the submitted work. D.M.-W. reports speakers bureau and consultant/advisory board fees from Boehringer Ingelheim, Novartis, Novo-Nordisk, and speakerśs bureau, consultant/advisory board as well as research funding from Amgen, MSD (Merck), and Sanofi. R.R.H. reports personal fees from: Sanofi-Aventis during the conduct of the study; grants from Hitachi, Viacyte, Fuiji chemicals, and Remd Bio therapeutics and personal fees from Alere, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Elcelyx, Intarcia, Ionis, Janssen/Johnson and Johnson, Novo-Nordisk, Sanofi-Aventis outside the submitted work. B.C. reports personal fees from Sanofi, during the conduct of the study; personal fees from Amgen, Lilly, Genfit, MSD, Novo-Nordisk, Pierre Fabre, Astra-Zeneca, and Sanofi-Regeneron, outside the submitted work. M.T.B.-D. and L.M. are employees and shareholders of Sanofi. R.P. reports that he is an employee and stock holder of Regeneron Pharmaceuticals, Inc. R.H.E. reports personal fees from Regeneron/Sanofi, outside the submitted work.
Supplementary Material
Acknowledgements
Sanofi and Regeneron Pharmaceuticals, Inc. in conjunction with the steering committee, were involved in the study design, and collection, management, and analysis of the data. H.M.C. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors would like to thank the following persons from the sponsors for their contributions to data collection and analysis, assistance with statistical analysis, or critical review of the manuscript: Regeneron: Carol Hudson, BPharm.; Veronica Lee, MD; William J. Sasiela, PhD; Sanofi: Laurence Bessac, MD; Maja Bujas-Bobanovic, MD; Jay Edelberg, MD, PhD, and Michael Howard, MBA. Medical writing support was provided by Aparna Shetty, PhD, of Prime Medica Ltd, Knutsford, Cheshire, UK, funded by Sanofi and Regeneron Pharmaceuticals, Inc. Responsibility for all opinions, conclusions, and data interpretation lies with the authors.
References
- 1. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. J Am Med Assoc 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 2. Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care 2009;32:1924–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 6. FDA. Important Safety Label Changes to Cholesterol Lowering Statin Drugs: FDA Drug Safety Communication http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm (21 June 2016).
- 7. Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, Sofat R, Stender S, Johnson PC, Scott RA, Leusink M, Verweij N, Sharp SJ, Guo Y, Giambartolomei C, Chung C, Peasey A, Amuzu A, Li K, Palmen J, Howard P, Cooper JA, Drenos F, Li YR, Lowe G, Gallacher J, Stewart MC, Tzoulaki I, Buxbaum SG, van der AD, Forouhi NG, Onland-Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor-Madry R, Stepaniak U, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Veglia F, Ford I, Jukema JW, Westendorp RG, de Borst GJ, de Jong PA, Algra A, Spiering W, Maitland-van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Eaton CB, Robinson JG, Duggan D, Kjekshus J, Downs JR, Gotto AM, Keech AC, Marchioli R, Tognoni G, Sever PS, Poulter NR, Waters DD, Pedersen TR, Amarenco P, Nakamura H, McMurray JJ, Lewsey JD, Chasman DI, Ridker PM, Maggioni AP, Tavazzi L, Ray KK, Seshasai SR, Manson JE, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben-Shlomo Y, Schreiner PJ, Fornage M, Siscovick DS, Cushman M, Kumari M, Wareham NJ, Verschuren WM, Redline S, Patel SR, Whittaker JC, Hamsten A, Delaney JA, Dale C, Gaunt TR, Wong A, Kuh D, Hardy R, Kathiresan S, Castillo BA, van der Harst P, Brunner EJ, Tybjaerg-Hansen A, Marmot MG, Krauss RM, Tsai M, Coresh J, Hoogeveen RC, Psaty BM, Lange LA, Hakonarson H, Dudbridge F, Humphries SE, Talmud PJ, Kivimaki M, Timpson NJ, Langenberg C, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Reiner AP, Keating BJ, Hingorani AD, Sattar N. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 2015;385:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol 2010;55:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cederberg H, Stancakova A, Yaluri N, Modi S, Kuusisto J, Laakso M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Diabetologia 2015;58:1109–1117. [DOI] [PubMed] [Google Scholar]
- 10. Brault M, Ray J, Gomez YH, Mantzoros CS, Daskalopoulou SS. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism 2014;63:735–745. [DOI] [PubMed] [Google Scholar]
- 11. Urban D, Poss J, Bohm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol 2013;62:1401–1408. [DOI] [PubMed] [Google Scholar]
- 12. Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, Merlet L, Pordy R, Baccara-Dinet MT. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol 2014;176:55–61. [DOI] [PubMed] [Google Scholar]
- 13. Roth EM, McKenney JM. ODYSSEY MONO: effect of alirocumab 75 mg subcutaneously every 2 weeks as monotherapy versus ezetimibe over 24 weeks. Future Cardiol 2015;11:27–37. [DOI] [PubMed] [Google Scholar]
- 14. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 15. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, Bruckert E, Jacobson TA, Kopecky SL, Baccara-Dinet MT, Du Y, Pordy R, Gipe DA, ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs. ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol 2015;9:758–769. [DOI] [PubMed] [Google Scholar]
- 16. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J 2015;169:906–915. [DOI] [PubMed] [Google Scholar]
- 17. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, Zhao J, Pordy R, Baccara-Dinet MT, Gipe DA, Geiger MJ, Farnier M. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015;36:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J 2015;36:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 20. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 21. Association AD. Standards of medical care in diabetes – 2010. Diabetes Care 2010;33(Suppl. 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Standards of Medical Care in Diabetes – 2016. Summary of revisions. Diabetes Care 2016;39(Suppl. 1):S4–S5. [DOI] [PubMed] [Google Scholar]
- 23. Berry G, Armitage P. Mid-p confidence intervals: a brief review. The Statistician 1995;44:417–423. [Google Scholar]
- 24. Livingstone SJ, Looker HC, Akbar T, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Fuller JH, Colhoun HM. Effect of atorvastatin on glycaemia progression in patients with diabetes: an analysis from the Collaborative Atorvastatin in Diabetes Trial (CARDS). Diabetologia 2016;59:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andersson C, Lyass A, Larson MG, Robins SJ, Vasan RS. Low-density-lipoprotein cholesterol concentrations and risk of incident diabetes: epidemiological and genetic insights from the Framingham Heart Study. Diabetologia 2015;58:2774–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. J Am Med Assoc 2015;313:1029–1036. [DOI] [PubMed] [Google Scholar]
- 27. Blazing MA. Incidence of new-onset diabetes in the IMPROVE-IT trial: does adding ezetimibe to simvastatin increase risk compared to simvastatin alone? FP Number: 5774 In: European Society of Cardiology Congress. London, UK, 2015. [Google Scholar]
- 28. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014;370:1809–1819. [DOI] [PubMed] [Google Scholar]
- 29. Henry RR, Holman RR, Giugliano RP, Raal FJ, Sullivan D, Honarpour N, Nelson P, Elliott M, Liu T, Wasserman SM, Koren MJ. Effects of long-term, monthly administration of the PCSK9 inhibitor evolocumab in patients with dysglycemia or metabolic syndrome. Can J Diabetes 2014;38:S18. [Google Scholar]
- 30. Sattar N, Preiss D, Robinson JG, Djedjos CS, Elliott M, Somaratne R, Wasserman SM, Raal FJ. Lipid-lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta-analysis of individual patient data. Lancet Diabetes Endocrinol 2016;4:403–410. [DOI] [PubMed] [Google Scholar]
- 31. Bonnefond A, Yengo L, Le MC, Fumeron F, Marre M, Balkau B, Charpentier G, Franc S, Froguel P, Cariou B. The loss-of-function PCSK9 p.R46L genetic variant does not alter glucose homeostasis. Diabetologia 2015;58:2051–2055. [DOI] [PubMed] [Google Scholar]
- 32. Saveedra YG, Dufour R, Baass A. Familial hypercholesterolemia: PCSK9 InsLEU genetic variant and prediabetes/diabetes risk. J Clin Lipidol 2015;9:786–793.e1. [DOI] [PubMed] [Google Scholar]
- 33. Langhi C, Le MC, Gmyr V, Vandewalle B, Kerr-Conte J, Krempf M, Pattou F, Costet P, Cariou B. PCSK9 is expressed in pancreatic delta-cells and does not alter insulin secretion. Biochem Biophys Res Commun 2009;390:1288–1293. [DOI] [PubMed] [Google Scholar]
- 34. Mbikay M, Sirois F, Mayne J, Wang GS, Chen A, Dewpura T, Prat A, Seidah NG, Chretien M, Scott FW. PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities. FEBS Lett 2010;584:701–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.