Abstract
BACKGROUND
A dose-dense weekly schedule of paclitaxel (resulting in a greater frequency of drug delivery) plus carboplatin every 3 weeks or the addition of bevacizumab to paclitaxel and carboplatin administered every 3 weeks has shown efficacy in ovarian cancer. We proposed to determine whether dose-dense weekly paclitaxel and carboplatin would prolong progression-free survival as compared with paclitaxel and carboplatin administered every 3 weeks among patients receiving and those not receiving bevacizumab.
METHODS
We prospectively stratified patients according to whether they elected to receive bevacizumab and then randomly assigned them to receive either paclitaxel, administered intravenously at a dose of 175 mg per square meter of body-surface area every 3 weeks, plus carboplatin (dose equivalent to an area under the curve [AUC] of 6) for six cycles or paclitaxel, administered weekly at a dose of 80 mg per square meter, plus carboplatin (AUC, 6) for six cycles. The primary end point was progression-free survival.
RESULTS
A total of 692 patients were enrolled, 84% of whom opted to receive bevacizumab. In the intention-to-treat analysis, weekly paclitaxel was not associated with longer progression-free survival than paclitaxel administered every 3 weeks (14.7 months and 14.0 months, respectively; hazard ratio for disease progression or death, 0.89; 95% confidence interval [CI], 0.74 to 1.06; P = 0.18). Among patients who did not receive bevacizumab, weekly paclitaxel was associated with progression-free survival that was 3.9 months longer than that observed with paclitaxel administered every 3 weeks (14.2 vs. 10.3 months; hazard ratio, 0.62; 95% CI, 0.40 to 0.95; P = 0.03). However, among patients who received bevacizumab, weekly paclitaxel did not significantly prolong progression-free survival, as compared with paclitaxel administered every 3 weeks (14.9 months and 14.7 months, respectively; hazard ratio, 0.99; 95% CI, 0.83 to 1.20; P = 0.60). A test for interaction that assessed homogeneity of the treatment effect showed a significant difference between treatment with bevacizumab and without bevacizumab (P = 0.047). Patients who received weekly paclitaxel had a higher rate of grade 3 or 4 anemia than did those who received paclitaxel every 3 weeks (36% vs. 16%), as well as a higher rate of grade 2 to 4 sensory neuropathy (26% vs. 18%); however, they had a lower rate of grade 3 or 4 neutropenia (72% vs. 83%).
CONCLUSIONS
Overall, weekly paclitaxel, as compared with paclitaxel administered every 3 weeks, did not prolong progression-free survival among patients with ovarian cancer. (Funded by the National Cancer Institute and Genentech; GOG-0262 ClinicalTrials.gov number, NCT01167712.)
Ovarian cancer, the most lethal gynecologic cancer, is responsible for approximately 14,000 deaths in the United States annually.1 The incorporation of bevacizumab, a monoclonal antibody against vascular endothelial growth factor, in the treatment regimen prolongs progression-free survival but not overall survival.2–5 A dose-dense regimen of paclitaxel involving greater frequency of drug delivery may enhance its antineoplastic effect by eliciting antiangiogenic and proapoptotic properties.6–9
Weekly paclitaxel therapy prolonged survival among patients with early-stage breast cancer and those with metastatic breast cancer.10,11 In a study involving patients with ovarian cancer, Japanese investigators found that dose-dense weekly paclitaxel prolonged progression-free survival and overall survival, as compared with treatment administered every 3 weeks, which is the conventional regimen.12,13 In a Multicenter Italian Trials in Ovarian Cancer study of weekly paclitaxel combined with weekly carboplatin, the investigators did not find a benefit of weekly therapy over treatment administered every 3 weeks; however, paclitaxel was not administered in a dose-dense method.14
The encouraging clinical-trial results regarding treatment with dose-dense paclitaxel and bevacizumab in patients with ovarian and other cancers led to the design of the current study. We aimed to determine whether in the primary treatment of ovarian cancer, dose-dense weekly paclitaxel combined with carboplatin would prolong progression-free survival, as compared with paclitaxel and carboplatin administered every 3 weeks, among patients receiving and those not receiving bevacizumab.
METHODS
PATIENTS
Eligibility criteria included newly diagnosed, untreated, incompletely resected stage III or any stage IV epithelial ovarian, fallopian tube, or primary peritoneal cancer. After the closure of a competing trial, Gynecologic Oncology Group (GOG)–0252 (ClinicalTrials.gov number, NCT00951496), patients with stage II or stage III disease with no residual lesions that were larger than 1 cm in the greatest dimension were also included. In addition, patients who desired to undergo neoadjuvant therapy were permitted to be included in the trial. The histologic findings were reviewed centrally by the GOG pathology committee. All the patients had a GOG performance-status score of 0 (fully active) to 2 (ambulatory and capable of self-care but unable to work; up and about >50% of waking hours). All the patients provided written informed consent.
STUDY OVERSIGHT
The NRG Oncology–Gynecologic Oncology Group and GOG designed and conducted the trial. The study was approved by the research ethics board at each participating center or by a central institutional review board. The data were collected, held, and analyzed by the NRG Oncology–Gynecologic Oncology Group and GOG, with reviews by the data and safety monitoring committee. The first author (study chair) vouches for the integrity of the data and analyses reported and for the fidelity of the trial to the protocol, available with the full text of this article at NEJM.org. The first author wrote the manuscript with input from all the coauthors.
The National Cancer Institute distributed bevacizumab to the GOG under a cooperative research and development agreement. Genentech provided supplemental support to the GOG. Representatives from the sponsors (the Cancer Therapy Evaluation Program of the National Cancer Institute and Genentech) reviewed the protocol and the manuscript, but the final content was determined by the authors. The protocol amendments and statistical analysis plan are available with the protocol at NEJM.org.
STUDY DESIGN
GOG-0262 was an open-label, randomized phase 3 trial that was designed to compare two regimens. Regimen 1 was paclitaxel, administered intravenously at a dose of 175 mg per square meter of body-surface area over a period of 3 hours on day 1 of a 21-day cycle, plus a carboplatin dose in milligrams (in a dose equivalent to an area under the curve [AUC] of 6), also administered intravenously on day 1 of the cycle, for six cycles. Regimen 2 was paclitaxel, administered intravenously at a dose of 80 mg per square meter over a period of 1 hour on days 1, 8, and 15 of a 21-day cycle, plus carboplatin (AUC, 6), administered intravenously on day 1 of the cycle, for six cycles.
During the design of the current study in 2010, the GOG completed protocol 218, which showed that the addition of bevacizumab to chemotherapy significantly prolonged progression-free survival in a similar population of patients with ovarian cancer. Therefore, the current study provided bevacizumab to each patient who chose to receive it. The patients were prospectively stratified according to whether they elected to receive bevacizumab; they were then randomly assigned to receive weekly paclitaxel or paclitaxel administered every 3 weeks.
In the case of patients who elected to receive bevacizumab, the drug was administered at a dose of 15 mg per kilogram of body weight every 21 days beginning with the second cycle of therapy and continuing until the onset of disease progression or until an adverse event precluded further therapy. Patients undergoing neoadjuvant chemotherapy with interval cytoreductive surgery after core-needle biopsy were to receive three cycles of neoadjuvant chemotherapy, followed by interval cytoreductive surgery between cycles 3 and 4, and then three additional cycles of chemotherapy. In this case, if bevacizumab was chosen, it was to be administered during cycles 2, 5, and 6 but omitted during cycles 1, 3, and 4.
Before the initiation of study treatment, disease was assessed by means of computed tomography or magnetic resonance imaging, measurement of the serum cancer antigen 125 (CA-125) level, and physical examination.15 Imaging was repeated after chemotherapy cycles 3 and 6. Serum CA-125 levels were measured and physical examinations were performed at the beginning of each chemotherapy cycle. After the completion of chemotherapy, imaging, serum CA-125 measurements, and physical examination were repeated every 3 months for 2 years, then every 6 months for 3 years, and then annually.
The Trial Outcome Index of the Functional Assessment of Cancer Therapy (FACT)–Ovary (FACT-O TOI) was used to capture data on patient-reported assessments (on a scale from 0 to 4, with higher scores indicating better health-related quality of life; see the Supplementary Appendix).16,17 Patient-reported outcomes were analyzed for patients who underwent primary cytoreductive surgery followed by chemotherapy. The neurotoxicity subscale short form (FACT/GOG-NTX) and the abdominal-discomfort subscale short form (FACT-GOG-AD) were used to capture patients’ self-reported chemotherapy-induced neuropathy and abdominal discomfort, respectively. Patients are asked to answer each question, as it applied during the previous 7 days, with the use of a 5-point scale that ranged from “not at all” (0) to “a little bit” (1) to “somewhat” (2) to “quite a bit” (3) to “very much” (4). The FACT-O TOI scores were assessed by means of a linear mixed-effects model. The minimally important difference in FACT-O TOI scores is between 5.2 and 7.8 units (see the Supplementary Appendix).16
Safety and toxic effects were closely monitored during every cycle. The use of myeloid growth factor was allowed in order to treat grade 4 neutropenia (absolute neutrophil count, <500 per cubic millimeter) that persisted for 7 days or more or febrile neutropenia or as subsequent prophylaxis. In patients with hypersensitivity or dose-limiting peripheral neuropathy that was due to paclitaxel, the drug was replaced with docetaxel (at a dose of 75 mg per square meter). Delay or discontinuation of bevacizumab was allowed if the patient had uncontrolled hypertension, proteinuria, wound or bowel-wall disruption, intestinal obstruction, or vascular disorders.
STATISTICAL ANALYSIS
At the time of randomization, a minimization procedure was performed to ensure that nearly equal numbers of patients received each study treatment on the basis of the following factors: GOG performance-status score, disease stage, status with respect to cytoreduction procedure (yes vs. no), and the decisions to use or not use bevacizumab or neoadjuvant therapy.18 The primary study end point was progression-free survival. The onset of clinical progression was defined as either radiographic evidence of increasing disease on the basis of the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, version 1.1,19 or an overall deterioration in health or a rise in the CA-125 level as assessed with the use of the Gynecologic Cancer Inter-Group criteria.20
Data on progression-free survival were censored at the date of last contact for those patients who remained alive and free from disease progression.18 Equivalence of the hazard of disease progression or death between the two treatment groups was assessed by means of a stratified log-rank test.21 The Common Terminology Criteria for Adverse Events, version 4.0, of the National Cancer Institute were used to grade the adverse events.
The stratification factors for the log-rank procedure were the initial performance-status score (0 vs. 1 vs. 2), stage of disease according to the International Federation of Gynecology and Obstetrics staging system (II vs. III vs. IV), option to receive bevacizumab (yes vs. no), and option to undergo neoadjuvant treatment (yes vs. no). The final analysis was to occur when at least 414 events of disease progression or death had been reported. We calculated that if weekly paclitaxel is truly associated with a rate of disease progression or death that is 25% less than that with paclitaxel administered every 3 weeks, this number of events would provide the study with approximately 90% power to detect this difference while limiting the overall one-sided type I error to 2.5%. A proportional-hazards model was used to estimate the treatment hazard ratio and its corresponding confidence interval, as well as to perform interaction tests between the randomly assigned treatment and the four design stratification factors (initial performance-status score, size of residual disease, option to receive bevacizumab, and option to undergo neoadjuvant treatment). A linear mixed-effects model that included the covariates of baseline score, age, stage of disease, and bevacizumab option was used to assess the hypothesis that the mean patient-reported outcome scores for the study treatments would be equivalent. All the reported P values are two-sided.
RESULTS
STUDY PATIENTS
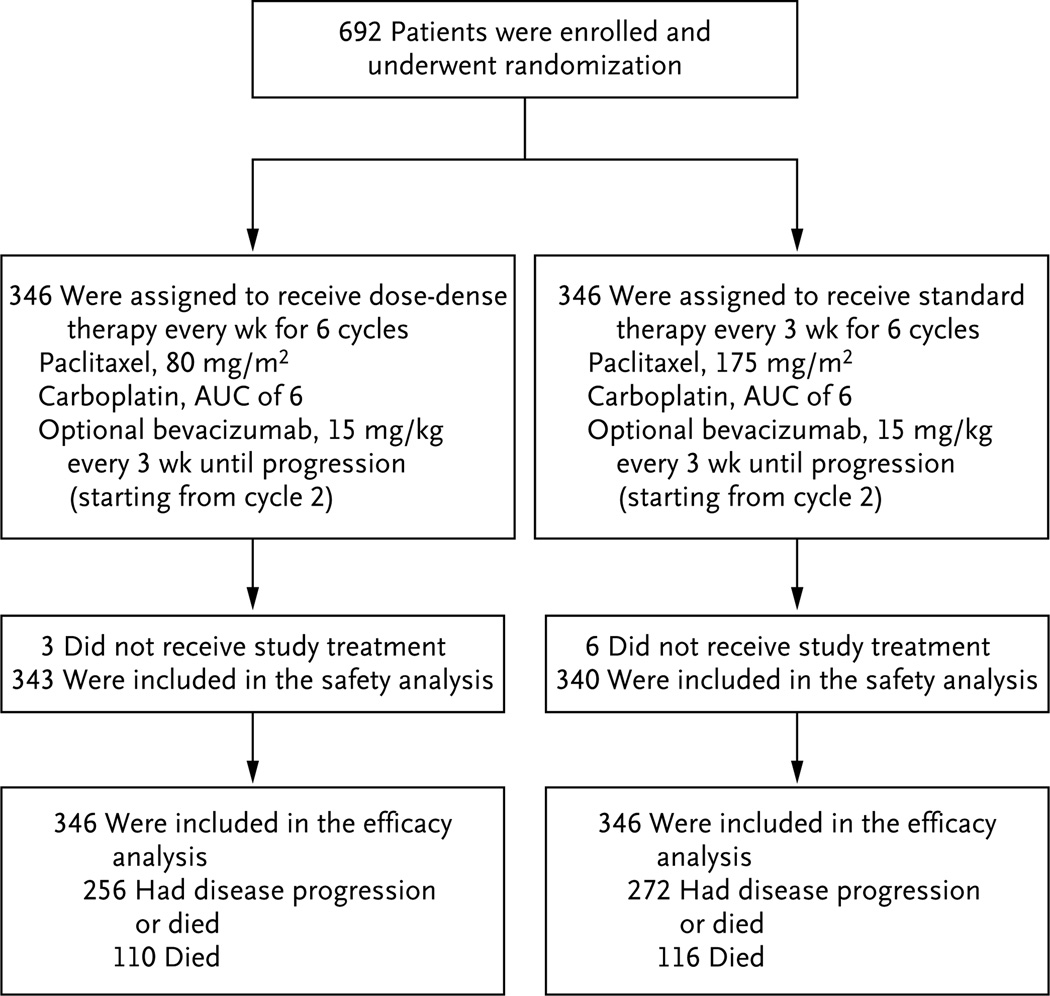
A total of 692 patients with newly diagnosed, previously untreated ovarian cancer were enrolled from September 2010 through February 2012 at more than 209 clinics in the United States, Canada, and South Korea. The prespecified number of events that was required for the analysis of progression-free survival occurred in June 2013, and the survival data were updated for this report in August 2015. The enrollment, randomization, and follow-up of the study patients are shown in Figure 1. Of the 692 patients, 85% were non-Hispanic whites. A total of 3% of the patients had stage II disease, 67% had stage III disease, and 30% had stage IV disease. Most of the patients (611 patients [88%]) had serous histologic findings, and 63% (438) had gross residual disease. A total of 84% of the patients (580 patients) opted to receive bevacizumab, and 13% (88) had neoadjuvant chemotherapy followed by interval cytoreductive surgery (Table 1). After central eligibility review, 32 patients (4.6%; 16 patients in each treatment group) were deemed to be ineligible or had incompletely documentation of eligibility.
Figure 1. Enrollment, Randomization, and Follow-up of the Study Patients.
Patients who were randomly assigned to dose-dense therapy received paclitaxel at a dose of 80 mg per square meter of body-surface area on days 1, 8, and 15 of a 21-day cycle, plus a carboplatin dose in milligrams (dose equivalent to an area under the curve [AUC] of 6) on day 1 of the cycle, for six cycles; patients who were assigned to the conventional regimen received paclitaxel at a dose of 175 mg per square meter on day 1 of a 21-day cycle, plus carboplatin (AUC, 6) on day 1 of the cycle, for six cycles. Patients in either group could opt to receive bevacizumab, starting from cycle 2, at a dose of 15 mg per kilogram of body weight, every 3 weeks until disease progression occurred.
Table 1.
Demographic and Clinicopathological Characteristics of the Patients, According to Treatment Group.*
| Characteristic | Total (N = 692) |
Weekly Paclitaxel (N = 346) |
Every-3-Wk Paclitaxel (N = 346) |
|---|---|---|---|
| number (percent) | |||
| Age <60 yr | 315 (46) | 156 (45) | 159 (46) |
| Stage of disease | |||
| II | 18 (3) | 8 (2) | 10 (3) |
| III | 464 (67) | 241 (70) | 223 (64) |
| IV | 210 (30) | 97 (28) | 113 (33) |
| Site of origin | |||
| Ovary | 550 (79) | 269 (78) | 281 (81) |
| Fallopian tube | 68 (10) | 36 (10) | 32 (9) |
| Peritoneum | 74 (11) | 41 (12) | 33 (10) |
| Size of residual disease | |||
| Microscopic | 166 (24) | 84 (24) | 82 (24) |
| Gross | 438 (63) | 218 (63) | 220 (64) |
| Not assessed† | 88 (13) | 44 (13) | 44 (13) |
| Histologic features | |||
| Serous | 611 (88) | 302 (87) | 309 (89) |
| Endometrioid | 16 (2) | 8 (2) | 8 (2) |
| Clear cell | 18 (3) | 11 (3) | 7 (2) |
| Mucinous | 7 (1) | 3 (1) | 4 (1) |
| Other | 40 (6) | 22 (6) | 18 (5) |
| Opted to receive bevacizumab | |||
| Yes | 580 (84) | 291 (84) | 289 (84) |
| No | 112 (16) | 55 (16) | 57 (16) |
| Opted to receive neoadjuvant therapy | |||
| Yes | 88 (13) | 44 (13) | 44 (13) |
| No | 604 (87) | 302 (87) | 302 (87) |
Percentages may not sum to 100 because of rounding. There were no significant differences between the groups in the characteristics at baseline. For details on race, performance-status scores, and histologic grade, see Table S1 in the Supplementary Appendix.
The size of residual disease was not assessed in patients who underwent neoadjuvant therapy.
EFFICACY
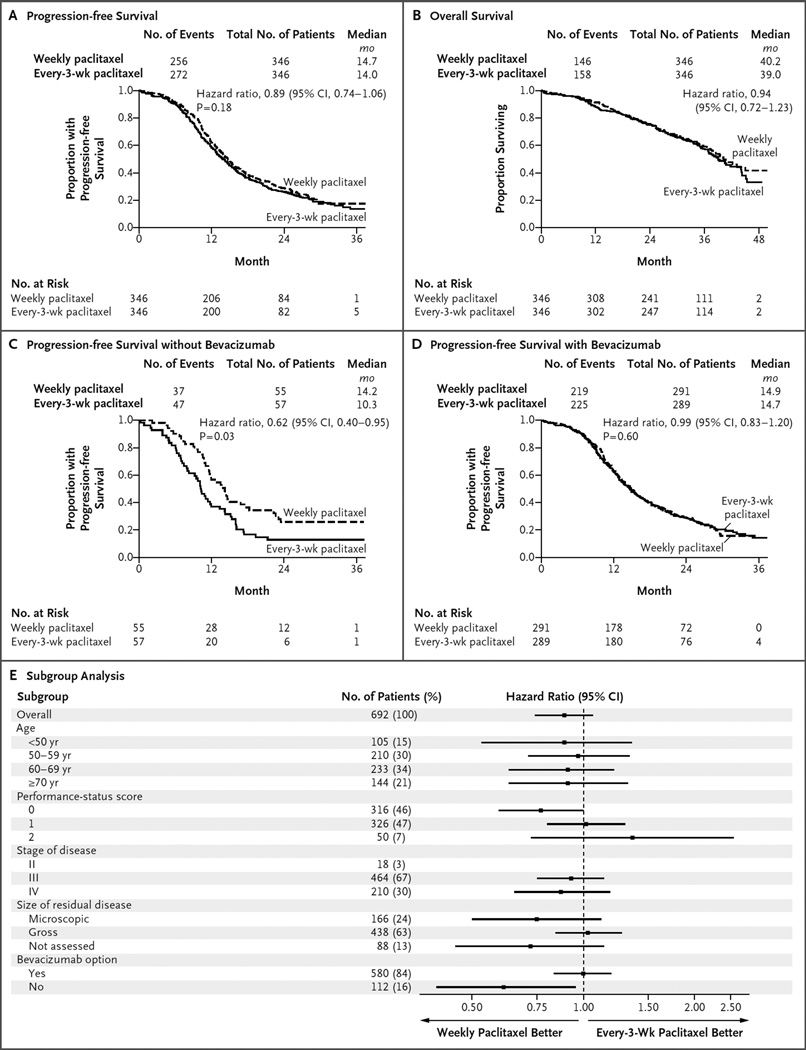
After a median follow-up of 28 months, 67% of the patients were alive. In the overall intention-to-treat analysis, weekly paclitaxel did not appreciably prolong progression-free survival, as compared with paclitaxel administered every 3 weeks (14.7 months and 14.0 months, respectively; hazard ratio for disease progression or death, 0.89; 95% confidence interval [CI], 0.74 to 1.06; P = 0.18) (Fig. 2A). Sensitivity analyses that included only eligible patients were consistent with the intention-to-treat analyses. A Kaplan–Meier plot of overall survival, with data updated in August 2015, is shown in Figure 2B (hazard ratio, 0.94; 95% CI, 0.72 to 1.23).
Figure 2. Primary and Subgroup Analyses of Progression-free Survival, According to Treatment Group.
In the overall intention-to-treat analysis, dose-dense weekly therapy with paclitaxel did not prolong progression-free survival, as compared with paclitaxel administered every 3 weeks (14.7 months and 14.0 months, respectively) (Panel A). In the case of progression-free survival, the hazard ratio is for disease progression or death. In the overall intention-to-treat analysis, weekly paclitaxel did not prolong overall survival, as compared with paclitaxel administered every 3 weeks (Panel B). In the case of overall survival, the hazard ratio is for death. No P value is available for the analysis of overall survival because the prespecified number of events has not occurred yet for this analysis. In the analysis of progression-free survival among patients who opted not to receive bevacizumab, weekly paclitaxel was associated with progression-free survival that was 3.9 months longer than that observed with paclitaxel administered every 3 weeks (14.2 vs. 10.3 months; hazard ratio for disease progression or death, 0.62; 95% CI, 0.40 to 0.95; P = 0.03) (Panel C). In the analysis of progression-free survival among patients who opted to receive bevacizumab, weekly paclitaxel did not prolong progression-free survival, as compared with paclitaxel administered every 3 weeks (14.9 months and 14.7 months, respectively; hazard ratio, 0.99; 95% CI, 0.83 to 1.20; P = 0.60) (Panel D). A forest plot of progression-free survival, according to randomized treatment, in subgroups defined according to prognostic factors is also shown (Panel E). Performance-status scores ranged from 0 (fully active) to 2 (ambulatory and capable of self-care but unable to work; up and about >50% of waking hours). In the subgroup of patients with stage II disease, there were too few patients to reliably estimate the treatment hazard ratio. The size of residual disease was not assessed in patients who underwent neoadjuvant therapy.
We performed an interaction test between randomly assigned treatments and each stratification factor (size of residual disease, option to receive neoadjuvant treatment, and option to receive bevacizumab) across strata. The effect of the paclitaxel regimen on progression-free survival did not differ significantly between patients who were left with microscopic residual disease and those who were left with macroscopic residual disease, nor did it differ significantly between those who had neoadjuvant chemotherapy followed by interval cytoreduction and those who received primary cytoreduction.
However, the effect was dissimilar among the group of patients who opted to receive bevacizumab versus the group of those who opted not to receive bevacizumab (P = 0.047 for interaction) (Fig. 2E) Among patients who opted not to receive bevacizumab, weekly paclitaxel was associated with a median progression-free survival that was 3.9 months longer than that observed with paclitaxel administered every 3 weeks (14.2 vs. 10.3 months; hazard ratio, 0.62; 95% CI, 0.40 to 0.95; P = 0.03), after adjustment for performance-status score, disease stage, and residual disease status (Fig. 2C). However, among patients who opted to receive bevacizumab, progression-free survival was similar in the group that received weekly paclitaxel and the group that received paclitaxel every 3 weeks (14.9 months and 14.7 months, respectively; hazard ratio, 0.99; 95% CI, 0.83 to 1.20; P = 0.60) (Fig. 2D).
QUALITY OF LIFE
A total of 560 patients (277 patients receiving weekly paclitaxel and 283 receiving paclitaxel every 3 weeks) could be evaluated for patient-reported outcomes. After adjustment for baseline scores, age, disease stage, and option to receive bevacizumab, patients who received weekly paclitaxel reported lower scores on the FACT-O TOI (reflecting lower quality of life) during the assessment period than did those who received paclitaxel every 3 weeks. The maximum decrease in the FACT-O TOI score was 2.7 points (97.5% CI, −5.44 to 0.02; P = 0.02) after completion of six cycles of chemotherapy, but this difference was not clinically significant.17 Although the incidence of patient-reported neuropathy was similar in the two treatment groups, the patient-reported severity of the neuropathy was greater among those receiving weekly paclitaxel than among those receiving paclitaxel every 3 weeks, and that finding persisted throughout the study period (Fig. S2 in the Supplementary Appendix). The health-related quality-of-life scores among the 16% of patients who did not receive bevacizumab did not differ significantly between the two study groups.
SAFETY
In the overall study population, the most common adverse events of grade 3 or higher were neutropenia (in 78% of the patients), gastrointestinal disorders (in 21%, including 2% who had gastrointestinal-wall disruption, such as perforation, fistula, or necrosis), thrombocytopenia (in 18%), infection (in 4%), and anemia (in 26%) (Table 2). Anemia of grade 3 or higher was reported in 36% (124 of 340 patients) of the patients who received weekly paclitaxel, as compared with 16% of those (54 of 343) treated with paclitaxel every 3 weeks (P<0.001). However, neutropenia of grade 3 or higher occurred less often in the group that received weekly paclitaxel than in the group that received paclitaxel every 3 weeks (72% [246 of 340 patients] vs. 83% [286 of 343], P<0.001).
Table 2.
Adverse Events According to Treatment Group and Grade of Event.*
| Event According to System Organ Class | Weekly Paclitaxel (N = 340) |
Every-3-Wk Paclitaxel (N = 343) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Event |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
No Event |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|
| number of events | ||||||||||||
| Blood or lymphatic system | ||||||||||||
| Anemia† | 4 | 27 | 185 | 117 | 7 | 0 | 19 | 109 | 161 | 47 | 7 | 0 |
| Febrile neutropenia | 327 | 0 | 0 | 12 | 1 | 0 | 327 | 0 | 0 | 14 | 2 | 0 |
| Neutropenia‡ | 9 | 19 | 66 | 157 | 89 | 0 | 18 | 15 | 24 | 84 | 202 | 0 |
| Thrombocytopenia | 66 | 139 | 68 | 51 | 16 | 0 | 86 | 146 | 57 | 39 | 15 | 0 |
| Cardiovascular system | ||||||||||||
| Hypertension | 218 | 22 | 56 | 44 | 0 | 0 | 205 | 27 | 60 | 47 | 4 | 0 |
| Venous or arterial thromboembolism | 311 | 1 | 14 | 9 | 5 | 0 | 319 | 2 | 15 | 6 | 1 | 0 |
| Gastrointestinal system | ||||||||||||
| Nausea | 124 | 132 | 64 | 20 | 0 | 0 | 104 | 144 | 84 | 11 | 0 | 0 |
| Vomiting | 224 | 63 | 34 | 18 | 1 | 0 | 209 | 78 | 41 | 14 | 1 | 0 |
| Diarrhea | 186 | 94 | 37 | 23 | 0 | 0 | 194 | 110 | 28 | 11 | 0 | 0 |
| Gastrointestinal-wall disruption | 326 | 0 | 3 | 7 | 4 | 0 | 336 | 0 | 3 | 3 | 1 | 0 |
| Genitourinary system | ||||||||||||
| Proteinuria | 285 | 25 | 23 | 7 | 0 | 0 | 296 | 16 | 18 | 13 | 0 | 0 |
| Neurologic system | ||||||||||||
| Peripheral motor neuropathy | 320 | 11 | 6 | 3 | 0 | 0 | 324 | 12 | 4 | 3 | 0 | 0 |
| Peripheral sensory neuropathy§ | 107 | 145 | 79 | 9 | 0 | 0 | 99 | 183 | 54 | 7 | 0 | 0 |
The numbers and percentages of patients who discontinued therapy early owing to adverse events (patient’s decision to withdraw) were as follows: 35 of 291 patients (12%) in the group that received weekly paclitaxel and opted to receive bevacizumab, 6 of 55 (11%) in the group that received weekly paclitaxel and opted not to receive bevacizumab, 8 of 289 (3%) in the group that received paclitaxel every 3 weeks and opted to receive bevacizumab, and 4 of 57 (7%) in the group that received paclitaxel every 3 weeks and opted not to receive bevacizumab. A total of 14 deaths (6 in the group that received weekly paclitaxel and 8 in the group that received paclitaxel every 3 weeks) were considered by the investigators to be at least possibly related to the study intervention. Of the 6 patients randomly assigned to receive weekly paclitaxel, 3 died from sepsis, 1 from gastrointestinal hemorrhage, 1 from ventricular fibrillation, and 1 from a nonspecific cause. Of the 8 patients randomly assigned to receive paclitaxel every 3 weeks, 3 died from sepsis, 1 from respiratory failure, 1 from myocardial infarction, 1 from stroke, 2 from nonspecific causes.
Anemia of grade 3 or higher occurred in 124 patients (36%) in the group that received weekly paclitaxel, as compared with 54 (16%) in the group that received paclitaxel every 3 weeks (P<0.001).
Neutropenia of grade 3 or higher occurred in 246 patients (72%) in the group that received weekly paclitaxel, as compared with 286 (83%) in the group that received paclitaxel every 3 weeks (P<0.001).
Sensory neuropathy of grade 2 or higher occurred in 88 patients (26%) in the group that received weekly paclitaxel, as compared with 61 (18%) in the group that received paclitaxel every 3 weeks (P = 0.01).
Patients who received weekly paclitaxel were more likely than those who received paclitaxel every 3 weeks to receive a cytokine (30% vs. 22%, P = 0.02) or red-cell transfusion (55% vs. 23%, P<0.001). Although there was no significant between-group difference in the incidence of sensory neuropathy of grade 3 or higher, more patients in the group that received weekly paclitaxel than in the group that received paclitaxel every 3 weeks had sensory neuropathy of grade 2 or higher (26% [88 of 340 patients] vs. 18% [61 of 343], P = 0.01). A total of 14 deaths (6 in the group that received weekly paclitaxel and 8 in the group that received paclitaxel every 3 weeks) were considered by the investigators to be at least possibly related to the study intervention. Of the 6 patients randomly assigned to receive weekly paclitaxel, 3 died from sepsis, 1 from gastrointestinal hemorrhage, 1 from ventricular fibrillation, and 1 from a nonspecific cause. Of the 8 patients randomly assigned to receive paclitaxel every 3 weeks, 3 died from sepsis, 1 from respiratory failure, 1 from myocardial infarction, 1 from stroke, 2 from nonspecific causes.
DISCUSSION
Among patients with ovarian cancer in a Japanese Gynecologic Oncologic Group (JGOG) trial, dose-dense weekly paclitaxel was associated with longer overall survival than was treatment as conventionally administered every 3 weeks.12 The greater number of infusions and longer duration of paclitaxel exposure with a dose-dense regimen enhance intratumoral drug perfusion and inhibit angiogenesis.6–9 In addition, two randomized prospective trials showed that bevacizumab, a monoclonal antibody against vascular endothelial growth factor, was associated with longer progression-free survival than was placebo.2,3 However, these two trials used a regimen of paclitaxel administered every 3 weeks rather than weekly. The current study combined dose-dense weekly paclitaxel with bevacizumab to target angiogenesis. The overall results of our study showed that a regimen of weekly paclitaxel did not prolong progression-free survival as compared with a regimen of treatment every 3 weeks. However, among patients who did not receive bevacizumab, weekly paclitaxel led to progression-free survival that was 3.9 months longer than that observed with paclitaxel administered every 3 weeks.
On the basis of results from two prior trials,2,3 we anticipated that bevacizumab would have an effect on progression-free survival. Therefore, our randomization procedure promoted a balance of bevacizumab use across the study treatments. We performed prespecified analyses that were aimed at assessing the consistency of the primary findings across various subgroups of patients; the results of these analyses suggested that weekly paclitaxel may have had a different effect in patients who did not receive bevacizumab. This difference did not appear to be due to imbalances in known prognostic factors (Table S4 in the Supplementary Appendix). Nevertheless, the nonrandomization of bevacizumab use may have allowed for unknown biases related to the characteristics of patients who opted to receive bevacizumab. It is important to note that these analyses did not arise from any pre-specified hypotheses and were based on only 16% of the overall study cohort. Although the subgroup analyses were not part of the primary analysis, these types of analyses have served to guide drug approvals in subgroups of patients within randomized trials.22
In the subgroup of patients who did not receive bevacizumab, dose-dense weekly paclitaxel was associated with longer progression-free survival than was paclitaxel administered every 3 weeks (14.2 vs. 10.3 months; hazard ratio for progression or death, 0.62). These findings were consistent with results in the group that received weekly paclitaxel in the JGOG trial (17.2 months, vs. 28.0 months in the group that received paclitaxel every 3 weeks; hazard ratio, 0.71).12 However, the aggregated analysis of our study did not show an effect of weekly paclitaxel. This result may be due to the fact that 84% of the patients in our trial elected to receive bevacizumab. Thus, it is possible that the overall difference was dominated by the addition of bevacizumab to the study treatment. However, we cannot dismiss the fact that this difference could be due to an imbalance of unknown prognostic factors.
Weekly paclitaxel was associated with a higher rate of sensory neuropathy of grade 2 or higher, as well as higher patient-reported neurotoxicity scores, than was paclitaxel administered every 3 weeks. The JGOG investigators did not find a higher rate of neuropathy associated with weekly paclitaxel than with paclitaxel administered every 3 weeks, but this result may have been due to the higher rate of discontinuation of treatment in the weekly dosing group.12 Although weekly paclitaxel did not affect the rate of sensory neuropathy of grade 3 or higher in our study, it was associated with a lower rate of neuropathy of grade 3 or higher than was paclitaxel administered every 3 weeks in a trial involving patients with breast cancer.10
We found a significantly lower rate of neutropenia of grade 3 or higher with weekly paclitaxel than with paclitaxel administered every 3 weeks, whereas the JGOG investigators found more treatment delays due to neutropenia in the group receiving weekly paclitaxel, although the difference was not significant. Although we did not identify a difference in the rate of neutropenic fever, prior studies have shown a lower rate of neutropenic fever in groups receiving weekly paclitaxel than in those receiving paclitaxel every 3 weeks.10,23 We also found a higher rate of anemia of grade 3 or higher in the group receiving weekly paclitaxel than in the group receiving paclitaxel every 3 weeks. The rate of severe anemia in the group that received weekly paclitaxel in the JGOG study was 69%, which may be attributed to the higher dose of paclitaxel used in that study, the pharmacokinetic differences between the two patient populations, or both.12,24,25 We did not identify any toxic effects that were due to the combined use of paclitaxel and bevacizumab.
The survival among patients with ovarian cancer in Japan and Europe varies from the survival we observed in this study involving patients who were primarily from the United States.12–14 A recent population-based study with the use of data from the National Cancer Institute showed that Asian patients had a higher rate of survival than whites.26 Pharmacogenomic variations between these groups of patients with respect to treatment response and toxic effects may partially explain these differences in outcome.27,28
Among patients who received bevacizumab in our study, weekly paclitaxel did not prolong progression-free survival as compared with paclitaxel administered every 3 weeks. Similarly, in a randomized trial involving patients with metastatic breast cancer, investigators did not find a difference in response rate among patients who received treatment with paclitaxel combined with bevacizumab every week, those who received the regimen every 2 weeks, and those who received it every 3 weeks in first-line treatment.29 Furthermore, the dose of antivascular drug may influence vascular normalization.30,31 Lower doses of an antivascular drug might improve perfusion and drug delivery, whereas higher doses can lead to rapid vessel pruning that results in less drug delivery and more metastasis.32,33 The results of this study may be further validated in the International Collaborative Ovarian Neoplasm Trial protocol (ICON) 8B trial (NCT01654146).
In patients with recurrent platinum-resistant ovarian cancer, the addition of bevacizumab to chemotherapy (pegylated doxorubicin, topotecan, or weekly paclitaxel) prolonged progression-free survival as compared with chemotherapy alone.5 Moreover, there was a more pronounced effect in the subgroup of patients who received weekly paclitaxel than in the other subgroups. However, the sample size was small and there was no randomization to different chemotherapies.5 Clearly, the scheduling of taxanes and other chemotherapies combined with biologic therapy in initial treatment versus therapy in the context of recurrent disease warrants further investigation.
In addition, comparative effectiveness studies are needed with consideration of economic costs. Although paclitaxel and carboplatin administered every 3 weeks and combined with bevacizumab may be more convenient than weekly paclitaxel and carboplatin without bevacizumab, the every-3-week regimen is also associated with higher costs, with an incremental cost-effectiveness ratio as calculated by others of $401,088 versus $5,809 per progression-free life–year saved.34–36
In conclusion, our data suggest that weekly paclitaxel did not prolong progression-free survival, as compared with paclitaxel administered every 3 weeks, among patients with ovarian cancer.
Supplementary Material
Acknowledgments
Supported by the National Cancer Institute and Genentech.
We thank Drs. Robert Mannel and Michael A. Bookman for scientific advice; our research staff (especially Kathleen Cavanaugh and Lilian Hu); and Dr. John A. Kerner and the Denise and Prentis Cobb Hale Endowed Chairs for administrative support.
Appendix
The authors’ full names and academic degrees are as follows: John K. Chan, M.D., Mark F. Brady, Ph.D., Richard T. Penson, M.D., Helen Huang, M.S., Michael J. Birrer, M.D., Ph.D., Joan L. Walker, M.D., Paul A. DiSilvestro, M.D., Stephen C. Rubin, M.D., Lainie P. Martin, M.D., Susan A. Davidson, M.D., Warner K. Huh, M.D., David M. O’Malley, M.D., Matthew P. Boente, M.D., Helen Michael, M.D., and Bradley J. Monk, M.D.
The authors’ affiliations are as follows: the California Pacific–Palo Alto Medical Foundation, Sutter Cancer Research Institute, San Francisco (J.K.C.); NRG Oncology–Gynecologic Oncology Group Statistics and Data Center, Roswell Park Cancer Institute, Buffalo, NY (M.F.B., H.H.); Massachusetts General Hospital, Boston (R.T.P., M.J.B.); University of Oklahoma, Oklahoma City (J.L.W.); Women and Infants Hospital, Providence, RI (P.A.D.S.); University of Pennsylvania (S.C.R.) and Fox Chase Cancer Center (L.P.M.) — both in Philadelphia; University of Colorado Cancer Center, Aurora (S.A.D.); University of Alabama at Birmingham, Birmingham (W.K.H.); James Cancer Center, Ohio State University, Columbus (D.M.O.); Minnesota Oncology/Hematology–Oncology Service, Edina (M.P.B.); Indiana University School of Medicine, Carmel (H.M.); and University of Arizona Cancer Center, Creighton University School of Medicine, and St. Joseph’s Hospital and Medical Center (B.J.M.) — all in Phoenix.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 3.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 4.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 6.Milross CG, Mason KA, Hunter NR, Chung WK, Peters LJ, Milas L. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J Natl Cancer Inst. 1996;88:1308–1314. doi: 10.1093/jnci/88.18.1308. [DOI] [PubMed] [Google Scholar]
- 7.Lau DH, Xue L, Young LJ, Burke PA, Cheung AT. Paclitaxel (Taxol): an inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother Radiopharm. 1999;14:31–36. doi: 10.1089/cbr.1999.14.31. [DOI] [PubMed] [Google Scholar]
- 8.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59:3776–3782. [PubMed] [Google Scholar]
- 9.Lopes NM, Adams EG, Pitts TW, Bhuyan BK. Cell kill kinetics and cell cycle effects of taxol on human and hamster ovarian cell lines. Cancer Chemother Pharmacol. 1993;32:235–242. doi: 10.1007/BF00685842. [DOI] [PubMed] [Google Scholar]
- 10.Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–5992. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 11.Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 12.Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 13.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–1026. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 14.Pignata S, Scambia G, Katsaros D, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15:396–405. doi: 10.1016/S1470-2045(14)70049-X. [DOI] [PubMed] [Google Scholar]
- 15.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the Functional Assessment of Cancer Therapy–Ovarian. J Clin Oncol. 2001;19:1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 17.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28:172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 18.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. 2001;19:4054–4057. doi: 10.1200/JCO.2001.19.20.4054. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 22.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 23.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–376. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Chan JK, Cheung MK, Husain A, et al. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108:521–528. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 26.Fuh KC, Shin JY, Kapp DS, et al. Survival differences of Asian and Caucasian epithelial ovarian cancer patients in the United States. Gynecol Oncol. 2015;136:491–497. doi: 10.1016/j.ygyno.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Hertz DL. Germline pharmacogenetics of paclitaxel for cancer treatment. Pharmacogenomics. 2013;14:1065–1084. doi: 10.2217/pgs.13.90. [DOI] [PubMed] [Google Scholar]
- 28.Bosó V, Herrero MJ, Santaballa A, et al. SNPs and taxane toxicity in breast cancer patients. Pharmacogenomics. 2014;15:1845–1858. doi: 10.2217/pgs.14.127. [DOI] [PubMed] [Google Scholar]
- 29.Seidman AD, Conlin AK, Bach A, et al. Randomized phase II trial of weekly vs. every 2 weeks vs. every 3 weeks nanoparticle albumin-bound paclitaxel with bevacizumab as first-line chemotherapy for metastatic breast cancer. Clin Breast Cancer. 2013;13(4):239.e1–246.e1. doi: 10.1016/j.clbc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Jain RK. An indirect way to tame cancer. Sci Am. 2014;310:46–53. doi: 10.1038/scientificamerican0214-46. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 34.Chan JK, Herzog TJ, Hu L, et al. Bevacizumab in treatment of high-risk ovarian cancer — a cost-effectiveness analysis. Oncologist. 2014;19:523–527. doi: 10.1634/theoncologist.2013-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohn DE, Kim KH, Resnick KE, O’Malley DM, Straughn JM., Jr At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. J Clin Oncol. 2011;29:1247–1251. doi: 10.1200/JCO.2010.32.1075. [DOI] [PubMed] [Google Scholar]
- 36.Dalton HJ, Yu X, Hu L, et al. An economic analysis of dose dense weekly paclitaxel plus carboplatin versus every-3-week paclitaxel plus carboplatin in the treatment of advanced ovarian cancer. Gynecol Oncol. 2012;124:199–204. doi: 10.1016/j.ygyno.2011.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.