Abstract
Practice guidelines that recommend active patient involvement in decisions about preventive health interventions are becoming increasingly common. These decisions frequently involve difficult trade‐offs between competing risks and benefits that require easily accessible information about the expected outcomes, superb doctor–patient communication, and effective integration of objective outcome data with individual values and preferences. Successful implementation of recommendations for shared decision‐making in preventive health care will require the development of efficient methods for making these complex decisions in busy practice settings. This article describes how the analytic hierarchy process, a multiple criteria decision‐making method, could facilitate successful implementation of shared decision‐making regarding preventive health care in clinical practice. The method is illustrated using recent guidelines for colorectal cancer screening for average risk patients issued by the American Gastroenterological Association.
Keywords: analytic hierarchy process, colorectal cancer screening, decision‐making, decision‐aiding methods, prevention, shared decision‐making
Introduction
In 1997 new guidelines for colorectal cancer screening were issued by the American Gastroenterological Association on behalf of a consortium of organizations that included the American Society for Gastrointestinal Endoscopy, the American Society of Colon and Rectal Surgeons, the American College of Gastroenterology, and the Society of American Gastrointestinal Endoscopic Surgeons. The guidelines recommend that all average risk patients [Link] consider following one of five screening programmes beginning at age 50: annual faecal occult blood tests, flexible sigmoidoscopy every 5 years, annual faecal occult blood tests and flexible sigmoidoscopy every 5 years, double‐contrast barium enema every 5–10 years, and colonoscopy every 10 years. The guideline notes that these options differ in a number of ways, including strength of supporting scientific evidence, safety, patient acceptability, effectiveness, simplicity, and cost. As no single screening programme was deemed clearly superior, and all were judged likely to reduce mortality associated with colorectal cancer, the guideline endorses all five programmes and recommends that decisions regarding colorectal cancer screening be made by patients and clinicians using a shared decision‐making process. 1
Practice guidelines, such as this one, recommending active patient involvement in decisions about preventive health interventions are becoming increasingly common. Recent examples from the United States include the American College of Physicians guidelines regarding hormone replacement therapy 2 and prostate cancer, 3 and the United States Preventive Services Task Force recommendations regarding aspirin for primary prophylaxis of myocardial infarction. 4 Recommendations such as these are appropriate whenever a preventive health intervention has the potential to produce both good and bad outcomes and there is no single course of action that is superior to all others in every important respect. In these cases decisions whether or not to adopt the intervention depend on trade‐offs between competing risks and benefits that are most appropriately made by the individuals who will be affected by the outcomes of the decision. 5
In addition to improving the quality of the clinical decision‐making process, involving patients in decisions regarding preventive interventions may have other benefits. Studies in a variety of settings have shown that patients with chronic diseases who participate in decisions about their care have better outcomes than less involved patients. 6 Since there is a large overlap between the patient‐related tasks associated with managing chronic diseases and many preventive interventions, it is possible that patients who actively participate in preventive decision‐making will experience similar beneficial effects.
Despite these advantages, there are major barriers to be overcome before shared decision‐making can become the norm for decisions regarding preventive health care. One set of difficulties stems from the nature of the decision itself. As noted above, decisions about prevention are frequently complex and require difficult trade‐offs between competing risks and benefits. Moreover, the information needed to make preventive decisions – data regarding the risks, benefits, and costs of the alternative management strategies – is seldom readily available in a format that can easily support clinical decision‐making. The situation is further complicated by the fact that benefits provided by third party payers such as managed care organizations or other health insurance plans frequently affect decisions regarding preventive interventions. This adds another interested party or ‘stakeholder’ to the clinical, decision‐making process.
A second source of difficulties relates to the feasibility of introducing a shared decision‐making approach into clinical practice. Currently, it is not clear whether patients want to accept a more active role in decision‐making. 7 Even if patients want to participate, most decisions about prevention are currently made in busy primary care settings where there is little time available to have the in‐depth discussion required for successful shared decision‐making. Finally, it has not been established whether the benefits associated with shared decision‐making outweigh the costs involved. 8
The purpose of this paper is to describe a promising approach to resolving some of these difficulties. The approach is based on multiple criteria decision‐making, the branch of the decision sciences devoted to the development of methods for helping people make better decisions when faced with complex choices involving conflicting objectives, several options, and multiple stakeholders. Multiple criteria decision‐making is an active research area and several decision‐aiding techniques are available. For this paper, I will illustrate how a multiple criteria decision‐making based approach can be used to promote shared decision‐making for preventive interventions using the analytic hierarchy process (AHP).
The AHP is a well‐known multiple criteria decision‐making method that has been successfully applied to a wide range of problems in many different contexts, including medical decision‐making. 9 , 10, 11, –12 Factors contributing to its success include its ability to simplify a complex problem in a concise and easily understood fashion, procedural simplicity, and several excellent computer software programmes. Although I will use the AHP to illustrate the use of multiple criteria decision‐making methods in implementing shared decision‐making, it should be noted that the AHP and other multiple criteria decision‐making methods share many similarities and the best method(s) to use to promote shared decision‐making in clinical practice has yet to be determined.
AHP overview
An AHP analysis is a four‐step process. In the first step a decision model is created. A decision model is a schematic diagram that describes the decision being made. In the AHP, the model contains the goal of the decision, the options being considered, and the considerations or criteria that will be used to determine how well the options meet the goal. These elements are arranged in a diagram that has the goal at the top, the options at the bottom and the criteria (broken down into subcriteria if necessary) in between. Figure 1 illustrates the basic format of an AHP model.
Figure 1.
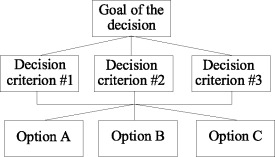
The basic AHP model format.
The second step of an AHP analysis consists of the collection and summarization of information regarding how well the options fulfil the criteria. The model is used to focus the research questions and appropriately summarize the resulting information.
The third step consists of a series of comparisons amongst the elements of the decision. The options are compared according to how well they satisfy each criterion and the criteria are compared according to their importance in achieving the goal. The comparisons can proceed in either a ‘bottom up’ fashion, starting with the comparisons amongst the options relative to the criteria and then proceeding up the hierarchy, or a ‘top down’ fashion beginning with the comparisons amongst the criteria and then continuing down the hierarchy. In practical applications, the bottom up approach is usually used. When multiple decision‐makers are involved, it is possible to divide the comparisons according to individual areas of expertise and use the AHP model framework as a way to combine their judgements.
The comparisons are made between two elements at a time and continue until every pair of elements has been evaluated. These pairwise comparisons are made relative to the importance, likelihood, or desirability of the decision elements being evaluated, depending on the context. The comparisons are made using a nine‐point scale that can be implemented using verbal, graphic, or numeric formats. On the commonly used verbal scale, the two elements being compared can be equal (corresponding to a 1 on the numeric scale), or one element can be moderately more important (or preferable or likely), strongly more important, very strongly more important, or extremely more important. These comparisons correspond to 3, 5, 7 and 9 on the nine‐point scale. Comparisons intermediate to these points are allowed and assigned the corresponding even numbers. When the graphic formats are used, relative areas are used to assign numeric values.
After the comparisons are elicited, they are converted to the numeric scale, if necessary, and entered into a matrix. The relative importance, likelihood or desirability of the elements is then obtained by calculating the right eigenvector of the matrix, a procedure that is analogous to taking the average of all the comparisons. The results are then normalized so that they sum to one. For options, the results indicate how well they fulfil the criteria; for criteria, the results indicate their relative importance in achieving the goal of the decision.
In addition to deriving a quantitative scale that reflects the relationships amongst the decision elements being compared, the comparison matrix is used to calculate a measure of the consistency within each set of comparisons. This measure, called the inconsistency ratio, represents the ratio of the amount of inconsistency in the comparisons compared with a set of completely random comparisons. By convention, inconsistency ratios of 0.1 or less are considered acceptable.
When quantitative data are available about the relationship between options and criteria, the analysis can proceed in one of two ways. The first is to make judgmental comparisons about the significance of the differences between options, using the standard pairwise comparison format described above. The other is to enter exact ratio comparisons of the available data directly into the analysis. The latter strategy has two practical advantages: (a) the reduction in the number of comparisons can significantly shorten the time required to perform an analysis and (b) the effects of new information on the results of the analysis can be determined quickly and easily. Its disadvantage is that it fails to capture individual differences in perceptions about the significance of the differences amongst the options.
After all of the comparisons are completed, the results are combined into a composite score that indicates how well each of the options fulfils the goal. The AHP has two options for performing this operation called the distributive and the ideal modes. The distributive mode is used when the objective of an analysis is to prioritize a set of options to determine how to distribute something amongst them. Examples of this type of analysis include determining an optimal investment portfolio or allocating research and development funds. The ideal mode is used when the object of the analysis is to identify the best of a set of options and is the more appropriate mode to use in most medical decision‐making situations.
The fourth and final step of an AHP analysis consists of making a decision. An AHP analysis is not intended to dictate what should be done, but to help a decision‐maker gain insight into his/her values and the relative merits of the available decision options. When multiple decision‐makers are involved, the AHP can enhance communication by providing a way to compare different points of view and elicit individual values and preferences. To further promote insight and communication, several sensitivity analysis techniques are available that allow decision‐makers to determine how changes in the pairwise comparisons or criteria weights affect the results.
AHP analysis of the colorectal cancer screening decision
To illustrate how the AHP and similar multiple‐criteria decision‐making techniques could form the basis of an approach for shared decision‐making regarding preventive interventions, this section will show how the AHP could be used to help 50‐year‐old, average risk patients and their clinicians make decisions amongst the five recommended colorectal screening options discussed above. This approach to clinical decision‐making would proceed in two stages, an initial preparatory stage and a subsequent decision‐making stage.
Preparatory stage of the implementation process
The goal of this stage is to create a decision model that can be used in practice. Activities included would be the first two steps in the AHP analysis sequence: creation of a preliminary decision model and the summarization of pertinent information.
Figure 2 shows one way the decision for choosing amongst the five recommended screening programmes could be framed as an AHP model. At the top is the goal of the decision: ‘make the best decision about colorectal cancer screening’. At the bottom are the five recommended screening options plus an additional option –‘No Screening’– which represents a decision to not screen actively for colorectal cancer at the current time. In the middle are four criteria for evaluating how well the options meet the goal.
Figure 2.
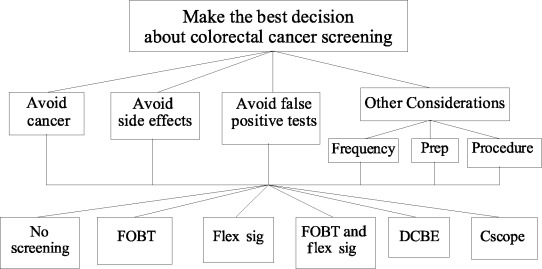
Example AHP model regarding the selection of a colorectal cancer screening programme. Prep = test preparation; FOBT = annual faecal occult blood tests; Flex Sig = flexible sigmoidoscopy every 5 years; DCBE = double contrast barium enema every 5 years; and Cscope = colonoscopy every 10 years.
• Avoid colorectal cancer – this criterion indicates that a good screening strategy is one that results in a high likelihood of never developing colorectal cancer.
• Avoid side‐effects – this criterion indicates that a good screening strategy is one that has little, if any, chance of causing harm. For the purpose of this illustration, only major side‐effects related to screening – death, major bleeding, and colonic perforation – will be considered.
• Avoid false positives – this criterion means that a good screening test is one that rarely results in a false positive result that leads to unnecessary additional tests and anxiety.
• Other considerations – this last criterion is broken down into three subcriteria: (1) frequency of screening tests; (2) the preparation required for screening tests; and (3) the nature of the screening procedure(s).
The three considerations included in the other considerations category may influence people’s decisions regarding their preferred screening method, but are likely to be less important than the three preceding criteria. Therefore, they are grouped together so that more meaningful comparisons between their collective importance and the other criteria can be made.
The other component of the preparatory stage is gathering and summarizing relevant data. In this case, information is required concerning the abilities of the screening options to fulfil the criteria and subcriteria located on the adjacent levels of the model. Fortunately, this information is provided in the published guideline; 1 these data, which are based on cumulative events over a 35‐years screening period beginning at age 50 and stopping at age 85, are summarized in Table 1.
Table 1.
Information describing relationship between options and decision criteria*
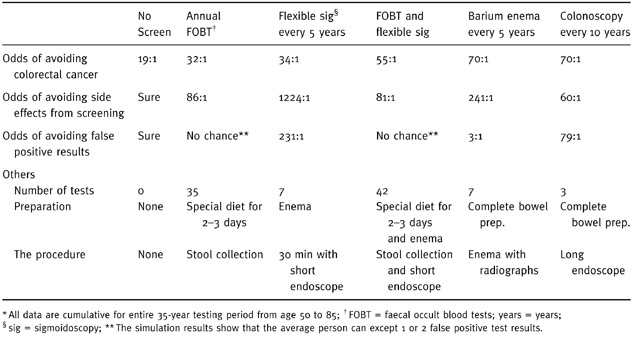
To shorten the time needed for the subsequent pairwise comparisons and to help focus the analysis on the elicitation of preferences amongst the criteria, this information could be directly entered into the preliminary model during this stage as direct ratio comparisons. For example, the comparison between no screening and annual faecal occult blood tests with regard to avoiding colorectal cancer would be the ratio of the odds of avoiding colorectal cancer with annual faecal occult blood tests (32:1), divided by the odds with no screening (19:1), or 1.7. Direct ratio comparisons amongst the options relative to avoid colorectal cancer, avoid screening side‐effects, and avoid false positives are summarized in Table 2. [Link]
Table 2.
Results of direct data comparisons amongst the options
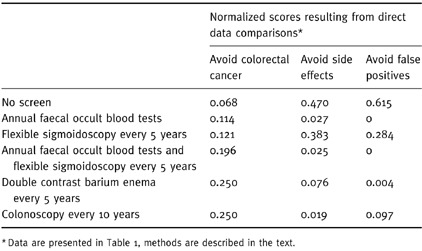
Table 1 also includes summary information concerning the three considerations included under other considerations: the frequency of screening, test preparation, and the nature of the procedure for each option. No direct comparisons can be entered for these subcriteria because the relationship between this information and the relative desirability of the screening options is not readily apparent. For example, some people might prefer infrequent screening intervals to minimize inconveniences, whereas others might feel better if they had more frequent screenings to make sure nothing was missed or had developed since the last screening test. As a result of this, subjective comparisons amongst the options regarding these criteria are necessary.
Shared decision‐making stage of the implementation process
Once the preparatory stage is completed, the resulting decision model and supporting data would be distributed to clinical settings for use by patients and clinicians as part of a shared decision‐making process.
The first step in the shared decision‐making stage would be to review the structure of the model. Depending on the perspectives of the individuals involved, it might be appropriate to add new criteria, remove existing ones, or alter their relationships. To improve the clarity of the illustration, I will assume that the decision‐maker(s) decided to modify the preliminary model by eliminating the other considerations criterion because the collective importance of the three subcriteria involved was judged to be trivial compared to the other three criteria under consideration.
After the structure of the model is reviewed (and modified if necessary) the pairwise comparison process would begin. Using a ‘bottom‐up’ approach, the analysis would start by comparing the options relative to their ability to fulfil the decision criteria. In the example, this would consist of a quick review of the results of the direct data comparisons that were entered into the preliminary model regarding avoiding colorectal cancer, side‐effects, and false positive screening test results. This information is shown in Table 2. If these results were deemed reasonable, the analysis would then proceed up the hierarchy to the comparisons amongst the three remaining criteria.
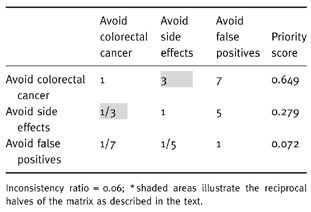
With three criteria to compare, three pairwise comparisons are needed. The first comparison in the example would be between avoid colorectal cancer and avoid side‐effects. To make this comparison, the decision‐maker would first decide if these two criteria are equally important in terms of making the best decision regarding colorectal cancer screening. Let’s assume they are not equal and that avoid colorectal cancer is judged to be moderately more important. This verbal scale judgement is then converted to its numeric equivalent, three, and entered into a comparison matrix, as shown in the highlighted cell in Table 3. The reciprocal of this comparison is used to fill in the highlighted corresponding cell on the lower half of the matrix. The same procedure would then be used to make the other two comparisons. To continue the example, suppose the decision‐maker thought that avoid colorectal cancer was very strongly more important than avoid false positives and that avoid side‐effects was strongly more important than avoid false positives. The resulting comparison matrix, criteria weights, and inconsistency index are illustrated in Table 3.
Table 3.
Comparison matrix for the three example criteria and the resulting priority scores*
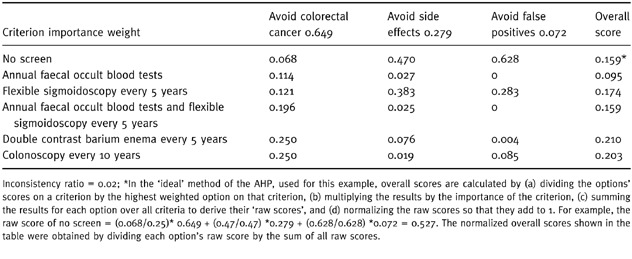
After all comparisons are completed, overall scores for the options would be calculated, as illustrated in Table 4, and the results discussed. The doctor and patient would then make a decision. This could happen in one of several ways. Depending on the circumstances, doctor and patient could choose to implement the best choice revealed by the patient’s analysis (in the example, double contrast barium enema every 5 years), combine the patient’s analysis with one performed separately by the clinician, perform various sensitivity analyses to see how different comparisons amongst criteria or options affect the results, or choose to defer an immediate decision to ponder questions and issues raised by the analysis. At this point, the perspectives of other interested parties, such as third party payers or managed care plans, could be introduced and combined with those of the patient and the clinician. Regardless of the approach taken, the results of the patient’s AHP analysis, including their judgements regarding what considerations are pertinent and their relative importance, will be known and can be openly discussed and examined as part of the decision‐making process.
Table 4.
Example analysis results
Based on previous studies (discussed below), the process described in the example would take an average patient about 35 min.
Discussion
As illustrated in the example, shared decision‐making approaches based on multiple criteria decision‐making have the potential for overcoming many of the cognitive and practical problems with implementing shared decision‐making regarding preventive and other health care interventions. The decision model can help ensure that all important considerations, including those that are unique to individual patients, are addressed. It can also serve as a guide to the collection and summarization of pertinent information. By reducing a complex problem into small, easily managed parts, the analysis helps avoid errors that result from attempts to make highly complex decisions intuitively. It also provides a mechanism for integrating multiple viewpoints into the decision‐making process in an explicit and unbiased manner.
Along with these potential advantages, several important questions exist regarding the feasibility and usefulness of shared decision‐making programmes based on the AHP and other multiple‐criteria, decision‐making methods. One set of questions relates to the best design for implementing this type of approach in practice settings. Design issues range from the best format, location, and timing for the analysis to determining the best method to use. Other largely unexplored issues include how this type of approach affects the process and outcomes of clinical decision‐making and whether its benefits outweigh the costs.
Although the clinical effectiveness of shared decision‐making programmes based on multi‐criteria, decision‐making methods has not yet been fully determined, preliminary data suggest that they can be implemented with beneficial results. In a study examining the diagnostic management of patients hospitalized with acute upper gastrointestinal bleeding, we found that both patients and primary care physicians were able to successfully complete a moderately complex AHP analysis. 13 Study results showed that doctors and patients had substantially different perspectives regarding two of the four decision criteria – the importance of identifying the source of bleeding and avoiding diagnostic test side‐effects – and that there were several distinct preference patterns amongst the decision criteria represented in the patients’ responses. This study showed the feasibility of using this approach to examine medical decisions and uncovered a number of important differences in values and preferences that would not have been apparent under normal circumstances.
Two subsequent studies have had similar results. The first examined patients’ preferences for colorectal cancer screening in an out‐patient general medicine clinic. 14 The other involved asking groups of obstetricians, paediatricians and mothers attending high risk pregnancy clinics to compare alternative practice guidelines regarding the management of neonatal group B streptococcal infections. 15 In addition to confirming the earlier findings, these two studies also found that the patients involved were overwhelmingly capable and in favor of implementing this approach to assist with medical decisions.
In summary, there is a close correspondence between the needs of successfully implementing a shared decision‐making programme in clinical practice and the functions provided by multiple‐criteria, decision‐making methods. This relationship, along with the encouraging early results noted above, suggest that multiple criteria decision‐making methods such as the AHP can provide an effective basis for developing successful shared decision‐making programmes regarding preventive health and similar medical interventions. Such programmes have the potential to improve the quality of clinical decision‐making, improve the outcomes of preventive health services, and strengthen doctor‐patient relationships.
Footnotes
The guidelines define a patient as average risk if they do not have one or more conditions that place them in a high risk category. High risk conditions include: a first degree relative with colorectal cancer or an adenomatous polyp, a family history of familial adenomatous polyposis or hereditary non‐polyposis colorectal cancer, or a personal history of adenomatous polyps, colorectal cancer, or inflammatory bowel disease.
Entering direct ratio comparison in the example problem is complicated by finding a suitable numeric representation for the complete lack of side‐effects and false positive results associated with no screening. This problem can be resolved either by using a suitable estimate when calculating direct comparisons or by obtaining subjective pairwise comparison judgements amongst the options. To illustrate the direct comparison method in the example, I used the former strategy: 1500:1 was used for the odds of avoiding side‐effects with no screening and 500:1 for the odds of avoiding false positive test results.
References
- 1. Winawer SJ, Fletcher RH, Miller L et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology, 1997; 112 : 594 642. [DOI] [PubMed] [Google Scholar]
- 2. Anonymous . Guidelines for counselling post‐menopausal women about preventative hormone therapy. Annals of Internal Medicine, 1992; 117 : 1038 1041. [DOI] [PubMed] [Google Scholar]
- 3. Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II. Estimating the risks, benefits and costs. Annals of Internal Medicine, 1997; 126 : 468 479. [DOI] [PubMed] [Google Scholar]
- 4. Pendergrass PW, DiGuiseppi C. Aspirin prophylaxis for primary prevention of myocardial infarction. In: DiGuiseppi C, Atkins D, Woolf S (eds) US Preventative Services Task Force Guide to Clinical Preventive Services (2nd edn.). Alexandria (VA): International Medical Publishing, 1996, 845–851.
- 5. Forrow L, Wartman S, Brock D. Science, ethics and the making of clinical decisions. Implications for risk factor intervention. Journal of the American Medical Association, 1988; 259 : 3161 3167. [PubMed] [Google Scholar]
- 6. Kaplan S, Greenfield S, Ware J. Assessing the effects of physician–patient interactions on the outcomes of chronic disease. Medical Care, 1989; 3 (Suppl.): S110 S127. [DOI] [PubMed] [Google Scholar]
- 7. Deber RB. Physicians in health care management: 8. The patient‐physician partnership: decision making, problem solving and the desire to participate. Canadian Medical Association Journal, 1994; 151 : 423 427. [PMC free article] [PubMed] [Google Scholar]
- 8. Hersey J, Matheson J, Lohr K. Consumer health informatics and patient decision‐making. Rockville (MD): AHCPR, 1997.
- 9. Zahedi F. The analytic hierarchy process – a survey of the method and its applications. Interfaces, 1986; 16 : 96 108. [Google Scholar]
- 10. Golden B, Wasil E, Harker P. The Analytic Hierarchy Process. Applications and studies. Berlin: Springer‐Verlag, 1989.
- 11. Dolan JG, Isselhardt BJ Jr, Cappuccio JD. The analytic hierarchy process in medical decision making: a tutorial. Medical Decision Making, 1989; 9 : 40 50. [DOI] [PubMed] [Google Scholar]
- 12. Saaty T. How to make a decision: the analytic hierarchy process. Interfaces, 1994; 24 : 19 43. [Google Scholar]
- 13. Dolan JG, Bordley DR, Miller H. Diagnostic strategies in the management of acute upper gastrointestinal bleeding: patient and physician preferences. Journal of General Internal Medicine, 1993; 8 : 525 529. [DOI] [PubMed] [Google Scholar]
- 14. Dolan JG. Are patients capable of using the analytic hierarchy process and willing to use it to help make clinical decisions? Medical Decision Making, 1995; 15 : 76 80. [DOI] [PubMed] [Google Scholar]
- 15. Peralta‐Carcelen M, Fargason CA, Coston D, Dolan JG. Preferences of pregnant women and physicians for two strategies for prevention of early‐onset group B streptococcal sepsis in neonates. Archives of Paediatric and Adolescent Medicine, 1997; 151 : 712 718. [DOI] [PubMed] [Google Scholar]


