Abstract
Lymphatic vessels play vital roles in immune surveillance and immune regulation by conveying antigen loaded dendritic cells, memory T cells, macrophages and neutrophils from the peripheral tissues to draining lymph nodes where they initiate as well as modify immune responses. Until relatively recently however, there was little understanding of how entry and migration through lymphatic vessels is organized or the specific molecular mechanisms that might be involved. Within the last decade, the situation has been transformed by an explosion of knowledge generated largely through the application of microscopic imaging, transgenic animals, specific markers and function blocking mAbs that is beginning to provide a rational conceptual framework. This article provides a critical review of the recent literature, highlighting seminal discoveries that have revealed the fascinating ultrastructure of leucocyte entry sites in lymphatic vessels, as well as generating controversies over the involvement of integrin adhesion, chemotactic and haptotactic mechanisms in DC entry under normal and inflamed conditions. It also discusses the major changes in lymphatic architecture that occur during inflammation and the different modes of leucocyte entry and trafficking within inflamed lymphatic vessels, as well as presenting a timely update on the likely role of hyaluronan and the major lymphatic endothelial hyaluronan receptor LYVE-1 in leucocyte transit.
Keywords: Lymphatic endothelium, Lymph node, Adhesion receptor, Transmigration, Dendritic cell, Chemokine, Chemotaxis, Haptotaxis, Inflammation, Hyaluronan, Confocal imaging
Introduction
The lymphatic system is comprised of an extensive network of vessels that permeates virtually every tissue in the body, frequently intertwining with the blood vessels and nerve fibres. Taking up plasma that leaks from blood vessels, the lymph capillaries convey this through the lymph nodes to the thoracic duct for return to the venous circulation as lymph, a clear fluid containing leucocytes and various tissue derived macromolecules [1]. The physiological importance of the lymphatics for fluid drainage and their clinical relevance for dissemination of tumours in cancer metastasis are both well recognized [2,3]. More importantly, the lymphatics are a major conduit for conveying free antigen and antigen loaded dendritic cells from the tissues to local draining lymph nodes for immune surveillance and immune activation [4,5]. Indeed, several other leucocyte populations including T and B lymphocytes and neutrophils also migrate via the lymphatics to modulate the quality and polarity of immune responses in lymph nodes [6–11] while macrophages are cleared from the tissues via lymph during the resolution phase of inflammation [12–14]. Surprisingly little is known however about the basic mechanisms by which leucocytes enter lymphatic vessels and migrate to lymph nodes, and whether or how the entry of each leucocyte subpopulation is regulated. Partly because the process of fluid transport in lymph has been regarded as an indolent process, it has been assumed that the entry of leucocytes from the tissues is also passive. However, a wave of research in this area over the last decade has provided exciting new insights into the ultrastructural features of lymphatic capillaries and the specialized interendothelial junctions that permit leucocyte entry. Amongst these is the discovery that lymphatic endothelial cells can tune their expression of adhesion molecules and chemokines to meet the differing demands for leucocyte entry in resting and inflamed tissues [4,5,15–17]. These findings have led to the realisation that leucocyte trafficking in lymphatics is an active process akin to that in the blood vasculature and one that is subject to complex regulation to allow fine control over cell transit. This review will cover what is currently known about the unusual endothelial junctions within lymphatic vessels and the various processes that regulate leucocyte chemotaxis and transendothelial migration at these sites. Such processes allow both the entry of cells to peripheral lymphatic vessels and their exit to the paracortex and cortex in lymph nodes. However, I shall mostly confine my discussion to peripheral vessels and the transit of dendritic cells, the leucocyte subpopulation about which we know the most.
Lymphatic transmigration–specialised endothelial junctions in lymphatic capillaries for constitutive transit of DCs
Earlier studies using cannulated domestic animals [18] showed that the migrating cell populations in afferent lymph are mostly lymphocytes (85-90% CD4 positive effector memory T cells) and dendritic cells (10-15%). Given the constant interstitial flow created by distal lymphatic pumping, the apparently discontinuous nature of lymphatic endothelium and its high permeability to fluids and dissolved macromolecules it was tacitly assumed that leucocyte entry into the lymphatic capillaries was driven solely by hydrodynamic forces. However, electron microscopic images of skin explants that have captured particularly the larger DC in the process of transmigrating afferent lymphatic vessels have shown that the dimensions of the cell body are many times greater than those of the endothelial “gaps” through which they enter, underlining the fact that DC make intimate contacts with lymphatic endothelial cells and must constrict their nuclei and cell bodies during entry [19]. Further insight into the architecture of these entry points came from the seminal work of Baluk et al. who imaged the lymphatics of the mouse trachea using confocal microscopy [20]. These studies revealed that the endothelial junctions of initial blind-ended capillaries had a distinct architecture, quite different to that of junctions in both blood vessels and larger downstream lymphatic capillaries and collectors. Whereas the endothelia of blood vessels are connected by continuous zipper-like arrangements of conventional tight and adherens junctions, the oakleaf shaped endothelia of initial lymphatics are joined at their scalloped edges by discontinuous button-like junctions interspersed with loose flap like openings. Unlike the buttons, which contain molecules associated with tight junctions and adherens junctions such as VE-Cadherin, ZO-1, claudin-5, occludins, JAM-A and ESAM pinning their sides, the loose openings at their tips are decorated with CD31, the lymphatic hyaluronan receptor LYVE-1 and likely other proteins that have yet to be determined. Interestingly, this discrete arrangement of CD31 was lost upon genetic ablation of VE-cadherin, suggesting that CD31 is specifically corralled at these sites [20]. Moreover, migrating DC most frequently targeted these specialized junctions in initial lymphatic capillaries and appeared to enter the vessel lumen at the loose flaps, squeezing themselves through the 2-3 μm apertures they formed. Due to the extent of the overlap, these entry points resemble deep endothelial-lined crevices, in which CD31, LYVE-1 and likely other receptors have the potential to mediate transient contacts with transmigrating cells (see below).
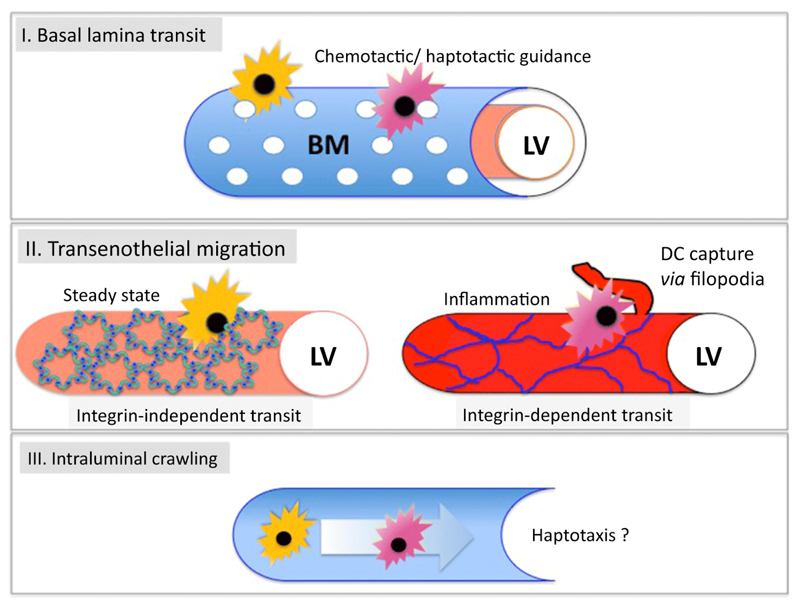
Even before reaching the vessel endothelium to initiate adhesion and transmigration, leucocytes migrating in the tissues have to negotiate the perilymphatic basement membrane, the matrix structure comprised of collagens, laminins, nidogen and perlecan that surrounds lymphatic capillaries and collectors. Unlike blood vessels however, the basement membrane of lymphatic capillaries is loosely organized [21,22]. Notably, this basement membrane is enriched for α4 laminins, which lack the short c-terminal arm required for network formation, and this may explain their characteristically low density and high permeability [23]. In an intriguing study on the subject, Pflicke et al. imaged the collagen IV and laminin enriched perilymphatic matrix surrounding lymphatic vessels in skin explants using spinning disc confocal microscopy and observed a discontinuous structure permeated by small gaps or portals (Figure 1).
Figure 1.
Stages in the transit of dendritic cells from tissue to lymph. The three main steps during transit are represented as shown as I. migration through the loose perforated basal lamina membrane (BM) surrounding initial lymphatic vessels (LV), a process that is thought to involve migration along haptotactic gradients of CCL21 and other chemokines II. Basal to luminal transmigration, a process that is reported to involve integrin-independent migration for steady state vessels and integr-independent adhesion/migration via ICAM-1 and VCAM-1 in the case of inflamed vessels. III. Intraluminal crawling, a process that directs DC migration in low flow regions of the initial capillaries before they reach the downstream valved collectors, where flow increases by two orders of magnitude.
Furthermore, using ex vivo “crawl-in assays” with exogenous fluorescently labelled bmDC they showed that leucocytes transiting from the dermis targeted these gaps to access the basolateral surface of the lymphatic vessel prior to transmigration. The gaps appear to form spontaneously rather than as a result of remodeling by DC, as they were observed also in the basement membrane of initial capillaries in CCR7-/- mice where DC traffic is ablated. Curiously this process is the inverse of the mechanisms used by neutrophils to extravasate from inflamed blood capillaries where exit occurs by transient MMP-mediated remodeling of the vascular basement membrane in discrete regions specifically depleted of collagen IV and laminin [24]. In the lymphatics however, the portals are devoid of all matrix components and DC entry did not appear to require MMP activity. Moreover, having squeezed through these basement membrane portals, the adoptively transferred bmDC appeared to transmigrate the vessel endothelium by squeezing through the flap valves while maintaining the integrity of the lateral buttons, confirming the findings of Baluk et al. [20].
Given the intimate contacts that must occur between DC and endothelium during transmigration at flap valves, one would predict involvement of typical leucocyte adhesion molecules such as β1 and β2 integrins and their counter-receptors ICAM-1 and VCAM-1. Surprisingly and somewhat counter-intuitively this does not appear to be the case. DC genetically deleted for all major leucocyte integrin chains displayed normal transmigration in vivo and trafficked to skin draining lymph nodes via afferent lymphatics without any obvious defects [25]. Hence it was concluded that DC migrate solely by amoeboid movement, guided by a combination of lymph flow and chemotaxis (see below, and Figure 1). An important feature of these experiments is that DC migration was assessed under steady-state conditions, in the absence of any overt inflammatory stimulus [25]. Nevertheless the data suggest that during normal homeostasis, the lymph-borne trafficking of DC proceeds in an unhindered manner, at least within proximal initial capillaries and that the novel discontinuous junctional architecture in these structures presents little if any barrier to transit.
This free mode of interstitial migration and vessel entry may well be necessary to allow efficient low level trafficking of immature and semi-mature tissue resident DC during immune surveillance and the homeostatic maintenance of lymph node HEVs [26]. However, the mode of DC transmigration during inflammation, involving e.g. epidermal Langerhans cells and monocyte-derived DC recruited from the blood circulations appears to be rather different (Figure 1).
A distinct mechanism for transmigration in inflamed lymphatics
Whereas resting DC migrate at a low rate from tissues to lymph nodes under conditions of normal homeostasis, the rate of migration increases an order of magnitude during conditions of inflammation. The release of various inflammatory mediators, leukotrienes and prostaglandins, leads to an increase in the permeability of blood vessels that provokes an influx of plasma to the interstitium, raising interstitial fluid pressure and lymph flow. Furthermore, classical inflammatory cytokines such as IL-18, IL-1 and TNFα following tissue injury, and pathogen associated molecular patterns including LPS and other toll-like receptor ligands produced in microbial infection trigger activation of the lymphatic endothelium, conditioning lymphatic vessels for enhanced leucocyte transit in much the same way as documented for blood endothelium. Earlier in vivo studies had already indicated ICAM-1 induction in inflamed lymphatics and its involvement in the migration of Langerhans cells to skin draining lymph nodes following contact hypersensitisation or hapten administration [27,28]. Such findings have been both confirmed and greatly extended by studies documenting considerable changes in the transcriptional profile of human lymphatic endothelial cells treated with TNFα, and of murine LEC isolated from the skin subjected to either oxazolone contact hypersensitivity or Freund’s complete adjuvant. These revealed dramatic upregulation of classical adhesion molecules including ICAM-1, VCAM-1, E and P-selectin and chemokines such as CCL21 and CXCL12 that promote DC and lymphocyte trafficking, together with transient loss of lymphatic differentiation markers such as PROX-1 and LYVE-1 consistent with a reversible change to an intermediate vascular phenotype. Curiously, similar upregulation of ICAM-1, VCAM-1 and E-selectin, together with an increase in CCL21 and de-localisation of junctional VE-cadherin may also be achieved by increasing the rate of transmural flow in vitro, suggesting that in inflamed tissues, such flow may be a primary stimulus for LEC activation in vivo [29]. In particular, the role of ICAM-1 in supporting β2 integrin mediated DC adhesion to inflamed endothelium has been confirmed both in vitro and in vivo, and ICAM-1 blocking mAbs were shown to impair DC trafficking from skin to lymph nodes during contact hypersensitivity reactions by arresting their entry to lymphatic capillaries at the basolateral surface [15]. Similar blockade has also been shown to impair lymphatic uptake of antigen loaded DC after dermal vaccination with flu nucleoprotein and subsequent T cell priming in downstream lymph nodes [5,15,30]. Curiously the ICAM-1 integrin adhesive interaction may also influence DC maturation [31].
Further details of how ICAM-1 and VCAM-1 support integrin-mediated adhesion of DC have emerged from confocal imaging of the inflamed dermal lymphatic vessels of oxazolone-hypersensitised mice in vivo. Here, DCs were seen to adhere and transmigrate preferentially at endothelia that had upregulated ICAM-1 [32]. Furthermore, ICAM-1 and VCAM-1 were shown to be concentrated on filopodia-like protrusions that were extended from the endothelial cells to envelope the transmigrating DCs and engage them via interaction with β1 and β2 integrins (see Figure 1). Interestingly, these interactions appeared to precede mostly transcellular rather than paracellular transit through the endothelium. Clearly the process has close parallels with the transcellular migration of mononuclear cells across blood vascular endothelium, where leucocytes were observed to engage transmigratory cups lined with ICAM-1 which translocated to the basolateral surface via caveolin-1 rich vesicular structures in a mechanism co-ordinated by actin and vimentin fibres [33–35]. Whether ICAM-mediated leucocyte transmigration across lymphatic endothelium can also proceed via a transcellular route in vivo is not known, but the possibility exists that individual populations such as macrophages or neutrophils might use the mechanism.
These findings have led to general acceptance that the trafficking of DC in inflamed as opposed to resting lymphatics is dependent on integrin-adhesion although the contributions of individual components may vary according to the nature of the inflammatory stimulus [5]. Why should it be necessary to induce a separate adhesion-dependent pathway when initial lymphatic capillaries already possess overlapping junctions specifically adapted for transit? One likely answer is that additional entry sites may be required to accommodate the large increase in leucocyte trafficking that occurs in response to inflammation. Whether these are located in proximal lymphatic capillaries or within distal capillaries and pre-collectors where conventional zipper junctions predominate is still uncertain. In this regard, Baluk and co-workers have shown that during inflammation-induced lymphangiogenesis provoked by infection with M. pulmonis, the newly sprouting lymphatic vessels have predominantly zipper type junctions rather than buttons and flaps, recapitulating the pattern seen during embryonic development [20,36]. More surprisingly, they also found that pre-existing lymphatic capillaries with button junctions underwent a transformation to zipper junctions over a period of 14 days post-infection [36]. The switch from buttons to zippers could also be reversed by application of dexamethasone, indicating regulation by glucocorticoid receptors. Hence, integrin-mediated leucocyte transmigration may actually be the default mode within inflamed tissue.
Additional receptors contributing to leucocyte transmigration
The button and zipper junctions of lymphatic capillaries contain a number of receptors that are characteristic of the tight junctions and adherens junctions of vascular endothelia including CD31 (PECAM-1), CD99, JAMs, ESAM, claudins and others that are specific to lymphatic endothelium as listed in Table 1.
Table 1.
Major adhesion molecules on lymphatic endothelial cells associated with leucocyte transmigration.
| ICAM-1 | Endothelial cell Receptor for β2 integrins |
| VCAM-1 | Endothelial cell Receptor for β1 integrins |
| E-selectin | Endothelial cell lectin-like cell adhesion molecule |
| L1CAM | Integral membrane protein. Homotypic adhesion receptor |
| CD47 | Integrin-associated protein, binds thrombospondin-1 and signal-regulatory protein alpha (SIRPα) |
| MMR | Macrophage mannose receptor. Binds and internalizes glycoproteins with sugar chains containing mannose residues |
| CLEVER-1/FEL-1 | Rapidly recycling scavenger receptor for LDL, bacteria, advanced glycation end-products etc. |
| ALCAM | Activated leucocyte cell adhesion molecule. Ig superfamily receptor, mediates homotypic interactions and binds scavenger receptor CD6 on lymphocytes, L1CAM and galectin-8 |
| JAM-1 | Endothelial tight junction adhesion receptor. Engages in homotypic and heterotypic interactions |
| Neuropilin-1 | Endothelial cell receptor for Semaphorin 3A |
| CD31 (PECAM-1) | Endothelial cell homotypic adhesion receptor. |
| CD99 | Endothelial cell homotypic adhesion receptor. |
| Podoplanin | Lymphatic endothelial cell sialomucin. Binds CLEC-2 on leucocytes and mediates blood lymphatic separation during development. Binds chemokines via O-glycans |
| LYVE-1 | Lymphatic endothelial cell hyaluronan receptor, also binds certain growth factors via N-glycans |
Hence it is likely that some of these mediate events during both integrin-independent leucocyte transmigration at buttons and integrin-dependent transmigration at zipper junctions-subsequent to engagement with ICAM-1 or VCAM-1. During leucocyte transmigration of the blood vasculature, CD31 mediates adhesion to the luminal surface of the endothelium before handover to CD99 in the convoluted lateral border recycling compartment (LBRC) where they each mediate subsequent diapedesis [37–40]. In lymphatic endothelium CD31 is at the basolateral surface and lines the flap valves between button junctions that constitute DC entry sites, while CD99 is mostly on the luminal surface [41].
Interestingly, DC transmigration over TNFα activated HDLEC monolayers or whole skin explants is significantly impaired after treatment with either CD31 or CD99 blocking mAbs [41]. The dynamics of CD31 and CD99 in lymphatic endothelium have yet to be investigated, however it is conceivable that an interplay between these molecules in button junctions forms a conveyor belt for leucocyte diapedesis. One might even speculate that the relatively large area of endothelium within the crevice-like flap valves of button junctions (where most contacts with transmigrating DC occur) is a lymphatic equivalent of the vascular LBRC.
The tight junctional adhesion molecule, JAM-A, a component of button and zipper junctions and a constituent of the LBRC has received relatively little attention in terms of its role in lymphatic transmigration. Expressed also in DC and other leucocyte populations, JAM-A can engage in homotypic interactions in cis that stabilize tight junctions and in trans that allow modulation of tight junctions for leucocyte transmigration in the blood vasculature [20]. In the only study to date, Cera et al. used constitutive (LoxP/CAG.Cre) and endothelial specific (LoxP/Tie-2 Cre) knockout approaches [42]. Surprisingly, they found that endothelial specific deletion of JAM-A resulted in no significant impairment in DC trafficking in vivo and no disruption of lymphatic vessel structure or junctional stability. However, isolated JAM-A-/- DC displayed increased transmigration through JAM-A+/+ LEC monolayers in vitro and enhanced trafficking to lymph nodes in response to skin contact hypersensitivity in vivo when adoptively transferred to wild-type mice [42]. These findings suggest that JAM-A on DC mediates heterotypic interactions with LEC that regulate important steps in the transmigratory pathway, although the identity of the endothelial ligand(s) and their overall significance remain obscure.
A further set of receptors that are induced specifically in cytokine activated LEC has also been found to play roles in leucocyte transmigration. These include the Ig superfamily receptor L1CAM, the integrin associated protein CD47 and the lymphocyte co-receptor molecule 4-1BB each of which is outlined briefly below.
L1CAM, (CD171) a homotypic adhesion molecule associated with neuronal axon guidance was shown to mediate transmigration across TNFα activated lymphatic endothelium in vitro as well as trafficking to draining lymph nodes in contact hypersensitised mouse skin in vivo [43]. The participation of L1CAM in transmigration was apparently specific to inflamed lymphatic endothelium as the receptor was absent from resting cells and required incubation with inflammatory cytokine for its induction. Which specific step L1CAM controls during adhesion and transmigration and its location in relation to buttons and zippers, remains to be determined.
CD47, an integral membrane Ig superfamily protein, has been reported to regulate retention of Langerhans cells in the epidermis via its ligand SIRPα and to mediate T cell transendothelial migration under flow [44,45]. Although expressed on both leucocytes and endothelia, genetic deletion of the receptor in DC as opposed to lymphatics impaired DC transmigration and subsequent trafficking to lymph nodes in FITC sensitized skin inflammation. The identities of the CD47 ligands in lymphatic endothelium however remain unknown.
Lastly 4-1BB, a TNF superfamily receptor normally associated with haematopoietic cells and a co-receptor for T lymphocyte activation was reported to play an indirect role in promoting leucocyte transmigration. Inducibly expressed in LEC treated with TNFα, IL-1 or LPS in vitro and inflamed skin lymphatics in vivo, 4-1BB was shown to potentiate DC transmigration by eliciting expression of VCAM-1 and E-selectin, and secretion of CCL21 when ligated with the agonist mAb 6B4 [46]. It may be that repeated interactions between 4-1BB and its natural ligands in migrating DC condition lymphatic vessels for increased cell transit in inflammation.
Finally, a subset of constitutively expressed scavenger receptors that includes the mannose receptor (CD206) [47,48] and the Combined Lymphatic Endothelial and Vascular Endothelial receptor CLEVER-1 [49] has been reported to play roles in lymphatic trafficking. The mannose receptor (MR) which is largely expressed in macrophages and absent from blood vascular endothelium contains an N-terminal carbohydrate recognition domain that binds sulphated glycans including sialyl Lewis X and mediates uptake and clearance of immunoglobulins, hormones and certain pathogens. In the lymphatics, MR is expressed both in peripheral vessels and lymph node sinuses, and is reported to mediate lymphocyte and tumour cell adhesion to lymphatic endothelium through interaction with L-selectin as shown by in vitro binding and in vivo lymph node trafficking studies [50]. Likewise CLEVER-1, a multidomain scavenger receptor expressed in peripheral lymphatics in addition to lymph node HEV and type 2 macrophages was shown to support adherence of lymphocytes, monocytes and granulocytes and its blockade by antibody impaired lymphocyte migration to draining lymph nodes during antigen-driven lymph node hypertrophy [51]. As yet the ligands for CLEVER-1 on leucocytes that mediate trafficking in lymphatics are not known. It is tempting to speculate that CLEVER-1 and perhaps MR in lymphatic endothelium, through their association with scavenging activity mediate the clearance of phagocytes or cell debris from peripheral tissues via afferent lymph.
In summary, a relatively large number of adhesion or adhesion related molecules appear to play roles in leucocyte transit that are accessory to the key integrin counter receptors lICAM-1 and VCAM-1 which mediate DC firm adhesion durjng inflammation. Indeed individual blockade of these molecules by antibody administration or genetic deletion can impair transmigration to a significant extent, often as much as 70%. Hence there is either some redundancy or each receptor may control sequential steps in the transit process. One needs to exercise caution in interpreting whether these receptors contribute to the transmigration process itself or to subsequent trafficking events as the data are still in many cases incomplete and much further characterisation will be needed to determine A) the precise location of each component in relation to button and zipper junctions, B) their involvement in homeostatic and/or inflammatory trafficking and ultimately C) their mechanisms of action.
Orchestration of DC entry and trafficking by chemotaxis and haptotaxis
In addition to the adhesion machinery described in the preceding sections, leucocytes require orchestration from chemokines and other chemoattractant molecules not only to migrate through the interstitial spaces towards lymphatic vessels, but to guide them towards gaps in the basal lamina to trigger their arrest beneath the endothelium and to direct their polarity for diapedesis. Lymphatic endothelial cells themselves provide many of these cues through their synthesis of a wide range of different chemokines (reviewed in [52]) including the immature/mature DC, monocyte and T cell attractants CCL2 (MCP-1), CCL5 (RANTES), CCL20 (MIP 1α) and CCL21 (secondary lymphoid chemokine), and the neutrophil attractants CXCL1 (Groα), CXCL2 (Groβ), CXCL5 (ENA-78) and CXCL8 (IL-8), all of which are upregulated upon stimulation with inflammatory cytokines [5,15]. Hence in terms of chemotaxis, lymphatic vessels display a pleiotropic response to inflammation and can in principle attract the recruitment of almost any tissue migrating leucocyte subpopulation.
Of these many chemokines, the most widely studied is CCL21, which is expressed primarily in lymphatic endothelium, and its G protein coupled receptor CCR7 expressed by DC and certain other migratory leucocytes. Indeed CCR7 and its ligands has also been reported to direct the trafficking of effector memory T cells from skin and lung via afferent lymphatics [10,11] and just recently, the lymphatic trafficking of subpopulations of neutrophils in skin [7]. Mice lacking CCR7 had delayed T cell immune responses and abnormal skin contact hypersensitivity responses in which Langerhans cells fail to exit the skin through afferent lymphatic vessels and migrate to draining lymph nodes [53,54]. Similarly, exerting blockade of CCL21 by administering neutralizing mAbs impaired DC trafficking in skin lymphatics [55]. Mice unlike humans express two different isoforms of the chemokine, CCL21ser and CCL21leu that differ by a single residue, are encoded by separate genes and localize exclusively to lymph node sinuses or peripheral lymphatics respectively [56–58]. Notably, plt mice, which carry a deletion of CCL21 leu, have a paucity of lymph node T cells and impaired immune responsiveness, consistent with a requirement for lymphatic endothelial derived CCL21 for entry and trafficking of antigen loaded DC.
Normally stored within intracellular vesicles and secreted at low levels by resting LEC, CCL21 is transcriptionally upregulated by inflammatory cytokines such as TNFα. After secretion, CCL21 induces both chemotaxis of DC towards lymphatic capillaries and ICAM-1 dependent adhesion to the endothelium via conformational activation of β2 integrins on the DC [59]. In common with other chemokines, CCL21 has a heparin-binding tract and is sequestered by heparan sulphate proteoglycans and collagen in the extracellular matrix (see below). Indeed this property appears to be critical in facilitating translymphatic migration as well as permitting microscopic imaging of secreted CCL21 in and around initial lymphatic capillaries in tissues.
Intravital imaging of resting and inflamed skin in the mouse footpad revealed the presence of discrete puncta of secreted CCL21 on the basolateral surface of initial lymphatics, close to the flap-like openings at button junctions, where it is tethered by collagen IV within the loose subendothelial matrix [60]. Curiously, migrating DC were observed to extend filopodia towards these puncta after which they docked to the underlying lymphatic endothelium and transmigrated. In contrast, CCR7 deficient DC migrated past the CCL21 puncta and failed to dock [60]. These findings suggest that CCL21 prompts the arrest and adhesion of DC at lymphatic vessels rather than exerting fluid phase chemotaxis, transiently docking the migrating cells next to perforations in the loose basal lamina from which they can initiate transendothelial migration.
A separate study using intravital microscopy to image DC trafficking in the more superficial layers of unstimulated mouse ear skin explants, revealed CCL21 to be distributed in the perilymphatic interstitium along a steep gradient that decayed with distance from the lymphatic capillary wall [61]. Furthermore, migratory DC in the skin explants were observed to change from random to directional movement at a point some 90 µm from the vessel wall, the maximal effective distance that the gradient was estimated to extend from the vessel perimeter. Importantly, the CCL21 gradient was not disrupted by washing the explants or by short-term exposure to brefeldin A, an inhibitor of membrane re-cycling, but was flattened by adding exogenous CCL21 or by enzymatic digestion with heparitinase. The discrepancies in these two studies seem to indicate subtle differences in the distribution of CCL21 in the upper and lower dermal layers due to immobilization on different matrix components [60,61]. However both studies imply a haptotactic rather than chemotactic function in which CCL21 signals adhesion close to the basolateral surface of lymphatic capillaries and subsequent transendothelial migration, presumably by activating β2 integrins in DC through CCR7 engagement and inside out signaling. How these features can be reconciled with a model of DC migration that relies purely on amoeboid movement is unclear [25]. Indeed, conformational activation of β2 integrins by CCL21 has been shown to promote adhesion and transmigration across human LEC monolayers in vitro [59,62]. Furthermore, depots of CCL21 have been observed next to the ICAM-1 microvilli that envelop transmigrating DCs in inflamed lymphatics, (as described above) and development of these protrusions is blocked by CCL21 neutralising mAbs [32].
Besides CCL21, the chemokine and survival-inducing factor CXCL12 (SDF-1) has also been shown to direct trafficking of DC in lymphatic vessels. Expressed at low levels in resting LEC and normal skin and upregulated in inflamed skin lymphatics, CXCL12 is a potent chemoattractant for monocyte-derived DCs and stimulates basolateral-to-luminal transmigration across hDLEC monolayers in vitro [63,64]. Furthermore, its receptor CXCR4 is highly expressed in cutaneous MHC class II+ DC and a synthetic CXCR4 antagonist (4-F-benzoyl-TN14003) was reported to impair migration of dermal DC and Langerhans cells to skin draining nodes during the sensitization phase of contact hypersensitivity in mice [63]. In contrast, CXCL12 does not appear to control the exit of T cells to afferent lymphatic vessels in inflamed skin, despite expressing CXCR4 and retaining chemokine responsiveness [65]. Hence CXCL12 may be rather specific for DC. Given some of the similarities with CCL21, and the fact that CXCL12 also binds heparan sulphate via a tract of basic residues at the c-terminus, it is tempting to speculate that the two molecules regulate similar steps in DC transmigration through haptotactic guidance.
Recently a third chemokine CX3CL1 (fractalkine) and its sole receptor CX3CR1 have also been reported to direct leucocyte trafficking in lymphatics. Unusually, CX3CL1 shares the dual properties of an adhesion molecule and a conventional chemoattractant, in both cases through interaction with its receptor expressed in cells of the CD14+ monocyte/macrophage/DC lineage and subsets of tissue resident DCs and epidermal Langerhans cells [66–69]. Synthesized as a type I integral membrane protein with an extracellular domain containing a novel C-X-X-X-C chemokine motif and a mucin-like stalk, the full-length molecule induces tight integrin-independent adhesion of leucocytes to blood vascular endothelium [66,70–72]. The soluble chemokine form is generated by cleavage of membrane-anchored CX3CL1 with the disintegrin and metalloproteinases ADAM10 and ADAM17 and promotes conventional integrin-mediated chemotaxis [73,74]. Significantly, lymphatic endothelial cells were shown to express CX3CL1 only after activation with inflammatory cytokines and it is the soluble chemokine rather than the membrane-bound form that predominates [75]. Notably, CX3CL1 was also found to mediate transmigration of DC across LEC monolayers, and disruption of CX3CR1 in these cells results in impaired entry to lymphatics and delayed trafficking to draining lymph nodes. However CX3CL1 appears to have a more subtle effect on migration and unlike CCR7-/- DC, CX3CR1-/- DC do not accumulate at the basolateral surface of lymphatic capillaries during skin contact hypersensitivity responses in mice. The polarity of CX3CL1 secretion by HDLEC is similar to that of CCL21 and the majority is released from the basolateral surface of the endothelium in vitro [75]. However, CX3CL1 does not bind heparin sulphate and so cannot establish similar haptotactic gradients near lymphatic capillaries. It may therefore assist in directing DC migration close to the basolateral surface of the vessel, perhaps guiding cells towards ICAM-1 rich microvillar projections in inflamed tissues.
Interestingly, the activity of CCL21, CXCL12 and several other C-C chemokines in directing leucocyte entry is also regulated by a group of recently discovered atypical chemokine receptors that includes ACKR1 (Duffy antigen), ACKR2, formerly known as D6, ACKR3 (CXCR7) and ACKR4 (CCRL1). These non-signalling receptors endocytose their chemokine ligands and thus regulate their levels in the extracellular milieu. In the case of ACKR2, which binds as many as eighteen different CC chemokines, the receptor prevents their accumulation in lymphatic endothelium during inflammation that would otherwise cause inappropriate logjamming of recruited leucocytes at the vessel surface [76–78]. In contrast, CXCR7, which binds CXCL12 and CCRL1, which binds CCL21 are thought to control cell migration by shaping chemotactic gradients of these chemokines near lymphatic vessels and between the floor and ceiling of lymph node subcapsular sinuses [79,80]. Undoubtedly, further investigation into these novel receptors will reveal additional subtleties in the way that leucocyte chemotaxis is regulated by lymphatic endothelia.
Besides conventional chemokines, a number of other chemotactic agents can also direct leucocyte trafficking via lymph. For example the semaphorins, a group of membrane bound and secreted chemorepellants that direct axonal guidance via their receptors, the plexins and neuropilins were reported to play a critical role during transmigration by regulating the contraction and squeezing of DC that allows their migration through the lymphatic endothelium to the vessel lumen. In particular, the soluble semaphorin Sema 3A was shown to be secreted by LEC and to bind neuropilin 1 at the posterior surface of transmigrating DC, delivering a signal via RhoA and its downstream effector kinase ROCK for actomyosin contraction and amoeboid movement as opposed to adhesion [81]. Hence semaphorins appear to mediate diapedesis and are likely to participate in this important event under both resting (integrin-independent) and inflammatory (integrin-dependent) conditions.
Finally, the lipid mediator sphingosine 1 phosphate and its receptor S1P1 that regulate lymphocyte egress in the lymph node medulla have been shown also to control leucocyte transit across lymphatic vessels in the tissues. Dermal lymphatic vessel endothelial cells were reported to release sphingosine 1 phosphate in response to inflammatory stimuli, and its interaction with T cells led to their arrest via β2 integrin mediated adhesion in the underlying parenchyma [82].
Migration within lymphatic vessels-Intraluminal crawling
Having reached the vessel lumen, transmigrated DCs maintain contact with the endothelium and have been observed to crawl along its inner surface. Curiously, this crawling appeared to lack directionality, with some DCs migrating in the opposite direction to lymph flow rather than advancing downstream towards collectors [60]. However, a large component of this random behaviour may be due to the reduction in lymph flow induced by the ketamine/xylazine anaesthesia used during the intravital imaging experiments, Indeed, more directional migration appeared to predominate when mice were anaesthetized with isoflurane, which had a lesser effect on lymphatic contraction [60].
Further details of DC crawling along the luminal surface of lymphatic endothelium have emerged from studies in which events were closely monitored in the skin of mice by intravital microscopy. Specifically, Nitschke et al [83] found that inhibition of the Rho-associated protein kinase ROCK that controls cell motility and triggers release from β2 integrin mediated adhesion had little effect on DC crawling under conditions of normal homeostasis, but massively disrupted the process under conditions of inflammation. Consistent with earlier published findings that the integrin ligand ICAM-1 is upregulated in inflamed lymphatic endothelium, where it allows initial adhesion of DC to the basolateral floor and subsequent transmigration [15], these findings suggest that integrin:ICAM interactions may also be essential for adhesion and crawling within the vessel lumen (see Figure 1).
Given that chemokines orchestrate DC migration towards lymphatic vessels and their initial entry during diapedesis, they may well also influence migration within the vessel lumen. Besides CCL21 and CX3CL1, most of the chemokines synthesized by lymphatic endothelial cells including CCL2, CCL5, CCL20, CXCL8 are secreted luminally rather than basolaterally [15,17,59]. Sequestration on the luminal surface of initial lymphatics could potentially establish haptotactic gradients to orchestrate migration of DC or other leucocyte populations towards downstream collectors where the higher rate of flow is sufficient to convey them to draining nodes by passive drift [60]. One likely candidate for mediating sequestration is the 43 kDa sialomucin podoplanin, a classical lymphatic marker that is expressed primarily on the luminal surface of the endothelium [84]. Indeed podoplanin has been reported to bind CCL21 (Kd 70 nM) and other CC chemokines, presumably via its O-linked glycan chains and hence could function as a low-affinity binding partner. Alternatively chenokines could be sequestered via heparin sulphate proteoglycans [85–87]. Consistent with this notion, a recent report demonstrated that CCL21-dependent DC adhesion to the luminal surface of primary LEC under flow was reduced after disruption of heparan sulphate biosynthesis by knockdown of the enzymes N-deacetylase sulphotransferase (NDST) or xylose transferase 2 (XylT2), or following treatment with heparitinase [85]. As yet the identities of such HSPGs are unknown.
Hyaluronan and lymphatic trafficking–the role of LYVE-1
One of the most abundant surface proteins in lymphatic endothelium LYVE-1, the lymphatic vessel hyaluronan receptor has long been implicated in regulating leucocyte trafficking. A member of the Link superfamily, the LYVE-1 polypeptide incorporates an extended consensus binding module for the large polymeric matrix glycosaminoglycan hyaluronan (GlcNAc-GlcUA)n at the N-terminus of an approximately 130 residue mucin-like stalk [88,89]. The selective expression of LYVE-1 in discontinuous “loose” endothelia including liver and spleen sinusoids coupled with its segregation within those regions of the vessel network - the initial capillaries and lymph node sinuses where leucocytes enter and exit, attest to a function in regulating leucocyte transit. Moreover, the particular location of LYVE-1 at known leucocyte entry points between button junctions implies intimate contact with transiting cells [20]. Added to this, LYVE-1 is a close homologue of the leucocyte hyaluronan receptor CD44 that mediates recruitment of lymphocyte, monocyte and neutrophils to inflamed tissues through adhesion to HA synthesized by activated vascular endothelial cells [90,91]. Our initial hypothesis was that sequestration of HA by LYVE-1 would promote adherence of migrating leucocytes bearing the major haemopoietic HA receptor CD44 and subsequent transmigration. Although formation of this tripartite complex was validated using recombinant soluble receptor preparations in plate binding assays [88], there was little evidence of any HA binding when primary lymphatic endothelial cells expressing native LYVE-1 were investigated [92]. One explanation for this apparent paradox is that lymphatic endothelial cells decorate the stalk region of LYVE-1 with sialylated O-glycans that mask HA binding in the endogenous receptor [92,93]. Although we originally predicted that LYVE-1 might become re-activated by an endogenous neuraminidase activity in vivo, it is more likely that sialylation is a constitutive modification that serves to tune the receptor for selective HA binding rather than its silencing. Accordingly we found that the sialylated receptor can indeed bind HA after appropriate ordering on the surface of native LEC or engagement with HA organised in appropriate supramolecular complexes (Lawrance, Banerji and Jackson unpublished). The basis for these properties is that the binding affinity of the large HA polymer for an individual LYVE-1 molecule is relatively weak (100 μM), so simultaneous interactions with many neighbouring receptors are required to achieve stable binding through receptor avidity. We predict the true physiological function of LYVE-1 is to bind HA synthesised and presented on the plasma membrane of migrating DC [94–96]. Indeed the fact that LYVE-1 can be internalized or shed from lymphatic endothelium after activation would allow for subsequent detachment during transit [97]. It is also worth noting that the formation of a tripartite LYVE-1:HA:CD44 adhesion complex could extend the distance between the plasma membranes of DCs and LECs by 100-200 nm at the site of contact, based on the likely dimensions of HA and its receptors (see e.g. ref. [98]). In inflamed tissues this would have consequences for the generation of firm adhesion between more compact molecules such as β2 integrins and ICAM-1. Such considerations will have to be factored into future models of LYVE-1 mediated leucocyte adhesion and transendothelial migration.
Surprisingly, LYVE-1 gene deletion yielded no obvious defects in lymphatic vessel development or drainage function and no overt defects in trafficking of DC when this was tested under conditions close to normal homeostasis [99,100]. However, measurement of DC trafficking under inflammatory conditions in our laboratory is beginning to reveal differences between LYVE-1-/- and wild-type mice, and mice treated with LYVE-1 function blocking mAbs consistent with a role for the receptor in regulating lymphatic trafficking (Johnson and Jackson unpublished).
Intriguingly the carbohydrate chains of LYVE-1 have also been reported to bind certain growth factors. In their original studies, Huang and co-workers described interactions with PDGF-BB, VEGF-A and IGFBP3 separate from the HA binding site in LYVE-1, via conserved basic heparin-binding tracts present within the mitogens [101,102]. In addition, they presented evidence for an interaction between LYVE-1 and the tyrosine kinase-linked growth factor receptor PDFGR, inferring a role for LYVE-1 as a low affinity co-receptor analogous to heparin sulphate proteoglycans and FGFRs/VEGFRs. Some of these findings were confirmed recently by Platonova et al. who showed the binding interaction involved LYVE-1 N-linked sugars, based on sensitivity to N-glycanase cleavage, and furthermore that ligation with these growth factors promoted LEC proliferation in vitro and lymphangiogenesis in vivo (corneal vascularization assay) [103]. The significance for leucocyte transmigration is that the growth factors also induced VE-cadherin phosphorylation and redistribution at endothelial junctions, culminating in elevated junctional permeability [104]. Importantly, experiments in our laboratory indicate that direct ligation of LYVE-1 with its main ligand hyaluronan or with cognate LYVE-1 mAbs also leads to intracellular signaling and modulation of VE-cadherin. Given that VE-cadherin underpins the button junctions either side of the LYVE-1 lined flap valves in initial capillaries, it is easy to imagine how such effects could facilitate the transit of DC by loosening restraints at the entry site. Of course, these hypotheses will need to be tested and further experimentation will be required before such a mechanism can be confirmed. Whether these events also mediate leucocyte transit under conditions of inflammation at zipper junctions will clearly need to be investigated.
Conclusions
It has become increasingly clear that the entry of leucocytes to initial lymphatic capillaries is an intricate multistep process that involves integrin-dependent and integrin-independent mechanisms, contingent on steady-state or inflammatory conditions. Nevertheless our understanding of the process is still rudimentary, and a number of questions remain to be answered. For example in inflammation, it is not clear whether integrin-mediated mechanisms permit leucocyte transmigration in more distal vessels including valved pre-collectors in deeper tissues, or whether the process is confined to proximal blind-ended capillaries in the superficial layers; do ICAM-1 lined filopodia form in the vicinity of button junctions or only in the continuous zippered junctions ? Even though transit at button junctions may be independent of ICAM-1 and VCAM-1 adhesion, it is likely that adhesion to other molecules such as CD31, CD99 and LYVE-1 plays a role in the process. In fact we know relatively little about how the many “accessory” molecules outlined in this review contribute to the discrete transmigratory machineries that mediate steady state and inflammatory trafficking. There is also uncertainty as to whether DC or other leucocytes can exit as well as enter lymphatic vessels and whether they receive signals for differentiation or immune function from the endothelium during transit.
With regard to intraluminal crawling, do the many chemokines secreted from the luminal surface of lymphatic endothelium serve to guide the process through haptotaxis or do they act downstream to condition draining lymph nodes for leucocyte recruitment?
Resolution of these issues and elucidation of the detailed choreography of cell-cell interactions during lymphatic trafficking will be greatly aided by the ready availability of lymphatic reporter mice (eg Prox tom) and transgenic lines (eg CD11c YFP) that allow visualization of events in live mice, or in tissue explants by 2 photon microscopy. No doubt we can look forward to many new insights within the next ten years.
Acknowledgements
This work was supported by Unit funding from the Medical Research Council (MRC) and by MRC Research Grants G1100134 and MR/L008610/1 at the University of Oxford.
Footnotes
This article was originally published in a special issue, entitled: “Role of Lymphatics in Immunity”, Edited by Dr. David G Hancock, Flinders University, Australia
References
- 1.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 2.Swartz MA, Hubbell JA, Reddy ST. Lymphatic drainage function and its immunological implications: from dendritic cell homing to vaccine design. Semin Immunol. 2008;20:147–156. doi: 10.1016/j.smim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Swartz MA, Skobe M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microsc Res Tech. 2001;55:92–99. doi: 10.1002/jemt.1160. [DOI] [PubMed] [Google Scholar]
- 4.Russo E, Nitschké M, Halin C. Dendritic cell interactions with lymphatic endothelium. Lymphat Res Biol. 2013;11:172–182. doi: 10.1089/lrb.2013.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigl B, Aebischer D, Nitschké M, Iolyeva M, Röthlin T, et al. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood. 2011;118:205–215. doi: 10.1182/blood-2010-12-326447. [DOI] [PubMed] [Google Scholar]
- 6.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- 7.Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood. 2011;117:1196–1204. doi: 10.1182/blood-2009-11-254490. [DOI] [PubMed] [Google Scholar]
- 8.Maletto BA, Ropolo AS, Alignani DO, Liscovsky MV, Ranocchia RP, et al. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood. 2006;108:3094–3102. doi: 10.1182/blood-2006-04-016659. [DOI] [PubMed] [Google Scholar]
- 9.Megiovanni AM, Sanchez F, Robledo-Sarmiento M, Morel C, Gluckman JC, et al. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J Leukoc Biol. 2006;79:977–88. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- 10.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 11.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 13.Bellingan GJ, Laurent GJ. Fate of macrophages once having ingested apoptotic cells: Lymphatic clearance or in situ apoptosis? 2008 [Google Scholar]
- 14.Gautier EL, Ivanov S, Lesnik P, Randolph GJ. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood. 2013;122:2714–2722. doi: 10.1182/blood-2013-01-478206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, et al. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson LA, Jackson DG. Cell traffic and the lymphatic endothelium. Ann N Y Acad Sci. 2008;1131:119–133. doi: 10.1196/annals.1413.011. [DOI] [PubMed] [Google Scholar]
- 17.Johnson LA, Jackson DG. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis. 2014;17:335–345. doi: 10.1007/s10456-013-9407-0. [DOI] [PubMed] [Google Scholar]
- 18.Young AJ. The physiology of lymphocyte migration through the single lymph node in vivo. Semin Immunol. 1999;11:73–83. doi: 10.1006/smim.1999.0163. [DOI] [PubMed] [Google Scholar]
- 19.Stoitzner P, Pfaller K, Stössel H, Romani N. A close-up view of migrating Langerhans cells in the skin. J Invest Dermatol. 2002;118:117–125. doi: 10.1046/j.0022-202x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- 20.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leak LV, Burke JF. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am J Anat. 1966;118:785–809. doi: 10.1002/aja.1001180308. [DOI] [PubMed] [Google Scholar]
- 22.Schmid-Schönbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 23.Vainionpää N, Bützow R, Hukkanen M, Jackson DG, Pihlajaniemi T, et al. Basement membrane protein distribution in LYVE-1-immunoreactive lymphatic vessels of normal tissues and ovarian carcinomas. Cell Tissue Res. 2007;328:317–328. doi: 10.1007/s00441-006-0366-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–32. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lämmermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Söldner R, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 26.Wendland M, Willenzon S, Kocks J, Davalos-Misslitz AC, Hammerschmidt SI, et al. Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity. 2011;35:945–957. doi: 10.1016/j.immuni.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Wang JH, Guo YJ, Sy MS, Bigby M. In vivo treatment with anti-ICAM-1 and anti-LFA-1 antibodies inhibits contact sensitization-induced migration of epidermal Langerhans cells to regional lymph nodes. Cell Immunol. 1994;158:389–399. doi: 10.1006/cimm.1994.1285. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Guan H, Zu G, Bullard D, Hanson J, et al. The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node. Eur J Immunol. 2001;31:3085–3093. doi: 10.1002/1521-4141(2001010)31:10<3085::AID-IMMU3085>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teoh D, Johnson LA, Hanke T, McMichael AJ, Jackson DG. Blocking development of a CD8+ T cell response by targeting lymphatic recruitment of APC. J Immunol. 2009;182:2425–2431. doi: 10.4049/jimmunol.0803661. [DOI] [PubMed] [Google Scholar]
- 31.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. 2009;183:1767–1779. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teijeira A, Garasa S, Peláez R, Azpilikueta A, Ochoa C, et al. Lymphatic endothelium forms integrin-engaging 3D structures during DC transit across inflamed lymphatic vessels. J Invest Dermatol. 2013;133:2276–2285. doi: 10.1038/jid.2013.152. [DOI] [PubMed] [Google Scholar]
- 33.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 35.Millán J, Hewlett L, Glyn M, Toomre D, Clark P, et al. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 36.Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol. 2012;180:2561–2575. doi: 10.1016/j.ajpath.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenkel AR, Dufour EM, Chew TW, Sorg E, Muller WA. The murine CD99-related molecule CD99-like 2 (CD99L2) is an adhesion molecule involved in the inflammatory response. Cell Commun Adhes. 2007;14:227–237. doi: 10.1080/15419060701755966. [DOI] [PubMed] [Google Scholar]
- 38.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 39.Dufour EM, Deroche A, Bae Y, Muller WA. CD99 is essential for leukocyte diapedesis in vivo. Cell Commun Adhes. 2008;15:351–363. doi: 10.1080/15419060802442191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan DP, Muller WA. Neutrophil and monocyte recruitment by PECAM, CD99, and other molecules via the LBRC. Semin Immunopathol. 2014;36:193–209. doi: 10.1007/s00281-013-0412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torzicky M, Viznerova P, Richter S, Strobl H, Scheinecker C, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) and CD99 are critical in lymphatic transmigration of human dendritic cells. J Invest Dermatol. 2012;132:1149–1157. doi: 10.1038/jid.2011.420. [DOI] [PubMed] [Google Scholar]
- 42.Cera MR, Del Prete A, Vecchi A, Corada M, Martin-Padura I, et al. Increased DC trafficking to lymph nodes and contact hypersensitivity in junctional adhesion molecule-A-deficient mice. J Clin Invest. 2004;114:729–738. doi: 10.1172/JCI21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maddaluno L, Verbrugge SE, Martinoli C, Matteoli G, Chiavelli A, et al. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J Exp Med. 2009;206:623–635. doi: 10.1084/jem.20081211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van VQ, Lesage S, Bouguermouh S, Gautier P, Rubio M, et al. Expression of the self-marker CD47 on dendritic cells governs their trafficking to secondary lymphoid organs. EMBO J. 2006;25:5560–5568. doi: 10.1038/sj.emboj.7601415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW. Endothelial CD47 interaction with SIRPgamma is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood. 2008;112:1280–1289. doi: 10.1182/blood-2008-01-134429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teijeira Á, Palazón A, Garasa S, Marré D, Aubá C, et al. CD137 on inflamed lymphatic endothelial cells enhances CCL21-guided migration of dendritic cells. FASEB J. 2012;26:3380–3392. doi: 10.1096/fj.11-201061. [DOI] [PubMed] [Google Scholar]
- 47.Irjala H, Alanen K, Grenman R, Heikkila P, Joensuu H, et al. Mannose receptor (MR) and common lymphatic endothelial and vascular endothelial receptor (CLEVER)-1 direct the binding of cancer cells to the lymph vessel endothelium. Cancer Res. 2003;63:4671–4676. [PubMed] [Google Scholar]
- 48.Irjala H, Johansson EL, Grenman R, Alanen K, Salmi M, et al. Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium. J Exp Med. 2001;194:1033–1042. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salmi M, Koskinen K, Henttinen T, Elima K, Jalkanen S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood. 2004;104:3849–3857. doi: 10.1182/blood-2004-01-0222. [DOI] [PubMed] [Google Scholar]
- 50.Marttila-Ichihara F, Turja R, Miiluniemi M, Karikoski M, Maksimow M, et al. Macrophage mannose receptor on lymphatics controls cell trafficking. Blood. 2008;112:64–72. doi: 10.1182/blood-2007-10-118984. [DOI] [PubMed] [Google Scholar]
- 51.Irjala H, Elima K, Johansson EL, Merinen M, Kontula K, et al. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur J Immunol. 2003;33:815–824. doi: 10.1002/eji.200323859. [DOI] [PubMed] [Google Scholar]
- 52.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 53.Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 54.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- 56.Vassileva G, Soto H, Zlotnik A, Nakano H, Kakiuchi T, et al. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J Exp Med. 1999;190:1183–1188. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen SC, Vassileva G, Kinsley D, Holzmann S, Manfra D, et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002;168:1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 58.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson LA, Jackson DG. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int Immunol. 2010;22:839–849. doi: 10.1093/intimm/dxq435. [DOI] [PubMed] [Google Scholar]
- 60.Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208:2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 62.Eich C, de Vries IJ, Linssen PC, de Boer A, Boezeman JB, et al. The lymphoid chemokine CCL21 triggers LFA-1 adhesive properties on human dendritic cells. Immunol Cell Biol. 2011;89:458–465. doi: 10.1038/icb.2010.103. [DOI] [PubMed] [Google Scholar]
- 63.Kabashima K, Shiraishi N, Sugita K, Mori T, Onoue A, et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol. 2007;171:1249–1257. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kabashima K, Sugita K, Shiraishi N, Tamamura H, Fujii N, et al. CXCR4 engagement promotes dendritic cell survival and maturation. Biochem Biophys Res Commun. 2007;361:1012–1016. doi: 10.1016/j.bbrc.2007.07.128. [DOI] [PubMed] [Google Scholar]
- 65.Geherin SA, Wilson RP, Jennrich S, Debes GF. CXCR4 is dispensable for T cell egress from chronically inflamed skin via the afferent lymph. PLoS One. 2014;9:e95626. doi: 10.1371/journal.pone.0095626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, et al. Identification and molecular characterization of fractalkine receptor CX3CR,which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 67.Chinnery HR, Ruitenberg MJ, Plant GW, Pearlman E, Jung S, et al. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2007;48:1568–1574. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niess JH, Brand S, Gu X, Landsman L, Jung S, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 69.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 71.Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haskell CA, Cleary MD, Charo IF. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem. 1999;274:10053–10058. doi: 10.1074/jbc.274.15.10053. [DOI] [PubMed] [Google Scholar]
- 73.Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 74.Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 75.Johnson LA, Jackson DG. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J Cell Sci. 2013;126:5259–5270. doi: 10.1242/jcs.135343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, et al. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 77.Nibbs RJ, Gilchrist DS, King V, Ferra A, Forrow S, et al. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J Clin Invest. 2007;117:1884–1892. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitehead GS, Wang T, DeGraff LM, Card JW, Lira SA, et al. The chemokine receptor D6 has opposing effects on allergic inflammation and airway reactivity. Am J Respir Crit Care Med. 2007;175:243–249. doi: 10.1164/rccm.200606-839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neusser MA, Kraus AK, Regele H, Cohen CD, Fehr T, et al. The chemokine receptor CXCR7 is expressed on lymphatic endothelial cells during renal allograft rejection. Kidney Int. 2010;77:801–808. doi: 10.1038/ki.2010.6. [DOI] [PubMed] [Google Scholar]
- 80.Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat Immunol. 2014;15:623–630. doi: 10.1038/ni.2889. [DOI] [PubMed] [Google Scholar]
- 81.Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, et al. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol. 2010;11:594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 83.Nitschké M, Aebischer D, Abadier M, Haener S, Lucic M, et al. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood. 2012;120:2249–2258. doi: 10.1182/blood-2012-03-417923. [DOI] [PubMed] [Google Scholar]
- 84.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 85.Yin X, Johns SC, Kim D, Mikulski Z, Salanga CL, et al. Lymphatic specific disruption in the fine structure of heparan sulfate inhibits dendritic cell traffic and functional T cell responses in the lymph node. J Immunol. 2014;192:2133–2142. doi: 10.4049/jimmunol.1301286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yin X, Johns SC, Lawrence R, Xu D, Reddi K, et al. Lymphatic endothelial heparan sulfate deficiency results in altered growth responses to vascular endothelial growth factor-C (VEGF-C) J Biol Chem. 2011;286:14952–62. doi: 10.1074/jbc.M110.206664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin X, Truty J, Lawrence R, Johns SC, Srinivasan RS, et al. A critical role for lymphatic endothelial heparan sulfate in lymph node metastasis. Mol Cancer. 2010;9:316. doi: 10.1186/1476-4598-9-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banerji S, Ni J, Wang SX, Clasper S, Su J, et al. LYVE-,a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-,the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- 90.Jackson DG. The lymphatic endothelial hyaluronan receptor LYVE-1. Glycoforum. 2004 doi: 10.1074/jbc.M702889200. [DOI] [PubMed] [Google Scholar]
- 91.Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009;230:216–231. doi: 10.1111/j.1600-065X.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- 92.Nightingale TD, Frayne ME, Clasper S, Banerji S, Jackson DG. A mechanism of sialylation functionally silences the hyaluronan receptor LYVE-1 in lymphatic endothelium. J Biol Chem. 2009;284:3935–3945. doi: 10.1074/jbc.M805105200. [DOI] [PubMed] [Google Scholar]
- 93.Functional regulation of the lymphatic endothelial hyaluronan receptor LYVE-1: The role of N-glycosylation. In: Nightingale TD, Banerji S, Jackson DG, editors; Balazs EAaH, C V., editors. Hyaluronan , Structure, Metabolism, Biological Activities, Therapeutic Applications. I ed. II. Matrix Biology Institute; New Jersey: 2005. pp. 615–618. [Google Scholar]
- 94.Mummert DI, Takashima A, Ellinger L, Mummert ME. Involvement of hyaluronan in epidermal Langerhans cell maturation and migration in vivo. J Dermatol Sci. 2003;33:91–97. doi: 10.1016/s0923-1811(03)00160-9. [DOI] [PubMed] [Google Scholar]
- 95.Mummert ME. Immunologic roles of hyaluronan. Immunol Res. 2005;31:189–206. doi: 10.1385/IR:31:3:189. [DOI] [PubMed] [Google Scholar]
- 96.Mummert ME, Mummert D, Edelbaum D, Hui F, Matsue H, et al. Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J Immunol. 2002;169:4322–4331. doi: 10.4049/jimmunol.169.8.4322. [DOI] [PubMed] [Google Scholar]
- 97.Johnson LA, Prevo R, Clasper S, Jackson DG. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. J Biol Chem. 2007;282:33671–33680. doi: 10.1074/jbc.M702889200. [DOI] [PubMed] [Google Scholar]
- 98.Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–325. doi: 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 101.Huang SS, Tang FM, Huang YH, Liu IH, Hsu SC, et al. Cloning, expression, characterization, and role in autocrine cell growth of cell surface retention sequence binding protein-1. J Biol Chem. 2003;278:43855–43869. doi: 10.1074/jbc.M306411200. [DOI] [PubMed] [Google Scholar]
- 102.Boensch C, Huang SS, Connolly DT, Huang JS. Cell surface retention sequence binding protein-1 interacts with the v-sis gene product and platelet-derived growth factor beta-type receptor in simian sarcoma virus-transformed cells. J Biol Chem. 1999;274:10582–10589. doi: 10.1074/jbc.274.15.10582. [DOI] [PubMed] [Google Scholar]
- 103.Platonova N, Miquel G, Regenfuss B, Taouji S, Cursiefen C, et al. Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker LYVE-1. Blood. 2013;121:1229–1237. doi: 10.1182/blood-2012-08-450502. [DOI] [PubMed] [Google Scholar]
- 104.Hou WH, Liu IH, Tsai CC, Johnson FE, Huang SS, et al. CRSBP-1/LYVE-1 ligands disrupt lymphatic intercellular adhesion by inducing tyrosine phosphorylation and internalization of VE-cadherin. J Cell Sci. 2011;124:1231–1244. doi: 10.1242/jcs.078154. [DOI] [PMC free article] [PubMed] [Google Scholar]