Abstract
Exposure to natural environments can have calming and stress-reducing effects on humans. Moreover, previous studies suggest that these benefits may be greater in areas with higher species richness. Our study took advantage of a “natural experiment” to examine people’s behavioral, physiological, and psychological reactions to increases in levels of marine biota in a large aquarium exhibit during three stages of restocking: Unstocked, Partially stocked, and Fully stocked. We found that increased biota levels were associated with longer spontaneous viewing of the exhibit, greater reductions in heart rate, greater increases in self-reported mood, and higher interest. We suggest that higher biota levels, even in managed settings, may be associated with important well-being and health benefits, particularly for individuals not able to access the natural analogues of managed environments.
Keywords: restoration, aquatic environment, aquarium, health and well-being, biodiversity
Introduction
Overview
Psychological research suggests that individuals tend to prefer natural, rather than built, settings (Hartig, Evans, Jamner, David, & Gärling, 2003; Ulrich et al, 1991). These environmental preferences appear to be mediated by perceptions that nature provides elements of psychological well-being such as positive emotions, reduced stress, and cognitive fascination (Ulrich, 1984; van den Berg, Koole, & van der Wulp, 2003; also see reviews, Bowler, Buyung-Ali, Knight, & Pullin, 2010; Velarde, Fry, & Tveit, 2007). The current research aims to assess the well-being benefits (psychological and physiological) that people derive from different levels of biota (defined here as the plant and animal life of a particular region; “biota,” n.d.), and thus add to the body of work attempting to understand potential dose–response relationships for natural ecosystems and human well-being (Millennium Ecosystem Assessment, 2005; UK National Ecosystem Assessment, 2011).
The opportunity for the current research arose due to a complete refurbishment and restocking of a large exhibit in the United Kingdom’s National Marine Aquarium. As restocking was conducted in three stages, it provided a rare opportunity for a “natural experiment” (Medical Research Council, 2011) to compare people’s responses with different levels of biota in precisely the same setting. Questions we sought to address included, how long people would stay in front of the exhibit as stocking level increased (reflecting intrinsic fascination), how would physiological markers of stress alter in relation to different levels of biota, and what emotional states people reported during different stages of restocking. Parts of the research required incidental visitor observation (e.g., length of stay), whereas other aspects required close monitoring of experimental participants (e.g., heart rate, mood) during exposure to the exhibit.
Previous studies suggest that greater actual or perceived species richness is associated with greater psychological well-being (Dallimer et al, 2012; Fuller, Irvine, Devine-Wright, Warren, & Gaston, 2007). The current study extends these findings in three key ways. First, we believe it is the first study to consider reactions to different levels of subaquatic, rather than terrestrial, biota by examining reactions to increases in marine life during a restocking event. There is currently a lack of research relating to the health and well-being benefits of aquatic environments (Sandifer, Sutton-Grier, & Ward, 2015) and, although people may be less likely to visit subaquatic environments than terrestrial ones, the tens of thousands of people who learn to dive (PADI, 2015), visit public aquariums (Gusset & Dick, 2011), own their own aquaria, or watch underwater documentaries, is testament to their appeal (note aquariums and aquaria are both acceptable plurals of aquarium—we will refer to public “aquariums” and home “aquaria”). Moreover, precisely because most people will not regularly encounter these species and ecosystems, there is a need to understand what benefits they provide to ensure we understand the value they confer. Second, as the water in the exhibit needed to “settle” before any animals were introduced, it provided a “control” condition (i.e., water, but no living specimens) that enabled us to begin teasing apart the relative importance of the physic-chemical environment from the animals present within it. This is particularly important in the context of aquatic environments as recent work suggests that they may be especially good at providing psychological benefits such as feelings of calm and mental well-being, irrespective of issues of wildlife considerations (White, Alcock, Wheeler, & Depledge, 2013; White, Cracknell, Corcoran, Jenkinson, & Depledge, 2014; White, Pahl, Ashbullby, Herbert, & Depledge, 2013; White et al, 2010). Even research not specifically exploring preferences for aquatic environments but, for instance, exploring scenic preferences for different environment types, have found that water features strongly as a preferred environmental variable (e.g., Han, 2007; Kweon, Ulrich, Walker, & Tassinary, 2008; Purcell, Peron, & Berto, 2001).
Third, by examining people’s reactions to different levels of restocking after both 5 and 10 min of exposure, we were also able to explore potential duration of dose–response effects. Recent field studies indicate that people may gain additional psychological benefits from longer self-selected visits to natural environments (e.g., White et al, 2013). The current, quasi-experimental study, therefore, is the first to examine psychophysiological responses to different “doses” of underwater marine life over different time periods.
Stress Recovery and Psychological Restoration From Nature Experiences
Many studies have investigated the links between environment type and/or composition, and people’s psychological responses. This research found high correlations between preferences for natural environments and perceived restoration (e.g., Purcell et al, 2001; van den Berg et al, 2003). It is clear that human beings are drawn to, and may gain benefits from, natural environments. There are three main approaches that attempt to explain why this might be: the Biophilia Hypothesis, the Psychophysiological Stress Recovery Theory (PSRT), and Attention Restoration Theory (ART). Although all three approaches maintain that people find natural environments more restorative than urban or artificial settings, their mechanisms and motivations appear to differ. Biophilia is the “innately emotional affiliation of human beings to other living organisms” (Wilson, 1993, p. 31). It suggests that more than one million years of evolution has resulted in humans being genetically programmed to positively respond to natural environments that support success and survival. PSRT also proposes an evolutionary basis for the immediate and involuntary emotional and physiological responses to aspects of natural environments after stressful experiences. It suggests that humans have an unlearned predisposition to recognize and respond in a positive way to natural components (e.g., water, vegetation) and arrangements of these components, that aid survival and promote well-being (Hartig et al, 2003; Ulrich et al, 1991). It is proposed that, following a stressor, unthreatening natural settings provide a valuable “breather” from stress. This facilitates a more positive emotional state, decreases levels of physiological arousal, and recharges energy expended used to cope with the stressor (Ulrich, 1993; Ulrich et al, 1991). Finally, ART suggests that prolonged or intense periods of directed attention, the type of forced concentration that leads to mental fatigue, distraction, and irritability, can be alleviated by experiencing a restorative setting (Herzog, Maguire, & Nebel, 2003). According to Kaplan (1995), restorative settings have four key components: fascination (the environment holds one’s attention effortlessly—particularly “soft” fascination—see Kaplan, 1995), being away (the environment is psychologically or physically removed from a person’s daily routine), extent (the environment is rich and coherently connected), and compatibility (the environment is compatible with a person’s expectations and inclinations). Although ART tends to be driven by the need to restore mental fatigue and PSRT is prompted by the mitigation of psychological stress (Berto, 2014), these two antecedents are not mutually exclusive. Stress-induced physiological arousal or negative affect can occur in the presence or absence of attentional fatigue, and vice versa (Hartig et al, 2003). There is also some evidence that the underlying processes for these two restorative theories are associated with different parts of the brain. Voluntary, directed attention has been linked to the right frontal cortex of the brain (Kastner, De Weerd, Desimone, & Ungerleider, 1998), whereas the involuntary reaction to psychophysiological stress has been located in the limbic system (Ulrich, 1983). Of these approaches, the PSRT aligns most closely with our research interests (i.e., exploring psychophysiological responses following a stressor, rather than cognitive recovery), and is therefore the main conceptual framework for the current study.
Although most research carried out on restorative environments focuses on the restorative benefits of real or simulated (e.g., photographs) natural ecosystems, compared with more urban settings, a smaller number of studies have explored alternative potentially restorative environments, such as botanical gardens, zoos, museums, and houses of worship (e.g., Ballantyne, Packer, & Hughes, 2008; Bennett & Swasey, 1996; Herzog, Ouellette, Rolens, & Koenigs, 2010; Kaplan, Bardwell, & Slakter, 1993; Packer & Bond, 2010; Pals, Steg, Siero, & van der Zee, 2009). Some of these weather-independent places may provide important access points to nature that would not be accessible ordinarily.
Aquariums and Health and Well-Being
Public aquariums worldwide welcome around 700 million visitors annually (Gusset & Dick, 2011), and smaller aquaria are present in homes, health care settings, and businesses. Although many people visit aquariums for entertainment or educational purposes (Packer & Ballantyne, 2002; Wyles et al, 2013), aquariums’ potential to provide psychological benefits is considerable. Given the literature reviewed above, all three approaches (Biophilia, PSRT, and ART) may be, to some extent, relevant for aquarium contexts. Although not “natural” environments, public aquariums certainly contain “nature” and, as such, present opportunities to affiliate with living things (Biophilia). Aquariums potentially fulfill the four criteria of ART, and are thus capable of providing broader restorative experiences. They hold an array of animals of all shapes, sizes, and colors that may hold one’s attention effortlessly (fascination), the setting is physically removed from a person’s everyday life (being away); there are many different live exhibits, interactive displays, and educational panels to explore (extent); and, they are a place a person has chosen to visit (compatibility). Finally, and of relevance to PSRT, aquariums may trigger physiological, as well as psychological responses, that are indicative of calming and stress-reducing effects.
Anecdotal evidence and some data support the view that people gain relaxation and psychological well-being from large public aquariums (Falk, Heimlich, & Bronnenkant, 2008; Packer & Bond, 2010). In the home context, Kidd and Kidd (1999) found that 70% of aquaria owners described their fish as calming and stress reducing. Indeed, the assumption that fish tanks are often present in health care settings because of their potentially relaxing and calming properties has already prompted research in this area. Although findings from some studies only approached significance (e.g., Barker, Rasmussen, & Best, 2003; DeSchriver & Riddick, 1990) or were simply a testing ground for animal-assisted therapy in a clinical setting (Cole & Gawlinski, 1995), other studies produced significant results. For instance, Katcher, Segal, and Beck (1984) found that participants who were instructed to contemplate an aquarium before dental surgery, experienced greater relaxation and reduced anxiety than control conditions. Riddick (1985) added an aquarium to the home of seven non-institutionalized elderly people and found that the aquarium group experienced a significant decrease in diastolic blood pressure (DBP) compared with two other groups (a “visitor” and a “control” group—eight and seven participants each, respectively) with no fish tank to care for. Following a stressor task, significant decreases in blood pressure and/or heart rate were observed in participants who watched a tropical fish tank (Katcher, Friedman, Beck, & Lynch, 1983) or videotapes of fish, birds, or primates (Wells, 2005). Finally, Edwards and Beck (2002) observed beneficial changes in patients with Alzheimer’s disease following the introduction of a fish tank in the activity/dining room. Individuals who tended to pace or wander spent more time sitting at the table watching the fish whereas those who were usually lethargic were more alert. Both responses resulted in a significant and important increase in food intake, a decrease in nutritional supplementation and weight gain for most residents. Thus, there is some indicative evidence for the benefits aquaria can provide but these mostly relate to small aquaria and health care settings: The effects of changes in a large public aquarium context have not been investigated.
The Role of Biodiversity, Species Richness, or Abundance
The contents of an aquarium may be of crucial importance if we are aiming to understand beneficial (or otherwise) effects of aquariums. There is a growing literature on psychological benefits of blue/aquatic environments, mostly derived from testing landscape scenery (via photographs or in situ). Thus, one question is whether the presence of water, before any marine life is added, provides benefits. Obviously, the presence of marine life is vital in public aquariums, but what does this add to the tank full of water? And, is it important how much marine wildlife there is, and how varied this is? To our knowledge, these questions have not yet been examined. There is, however, research in terrestrial contexts that has looked at the role of “biodiversity.” This research has shown that greater well-being outcomes (e.g., Dallimer et al, 2012; Fuller et al, 2007; Luck, Davidson, Boxall, & Smallbone, 2011) and aesthetic preferences (e.g., Lindemann-Matthies & Bose, 2007; Lindemann-Matthies, Junge, & Matthies, 2010) can be positively associated with higher species richness (e.g., plants, birds). However, a recent systematic review of studies researching the health and well-being benefits of “biodiverse” environments (Lovell, Wheeler, Higgins, Irvine, & Depledge, 2014) concluded that much of the evidence was weak and equivocal and that further research was required.
At this point, it is worth highlighting the confusion surrounding the term “diversity.” According to Tuomisto (2010), “the term ‘diversity’ has been used in at least four conceptually different ways in the ecological literature, primarily because indices of diversity have been equated with diversity itself” (p. 853). These issues over terminology have made communicating about diversity appear excessively complicated (Tuomisto, 2010). In view of this confusion, and because our field study could not control for, or separately test the influence of, species richness or the abundance of individuals (causing what psychologists term a confound), we simply refer to increases in stocking level as increases in “biota” (defined earlier).
In sum, differences in aquarium content and composition may be very important in understanding the psychological effects of aquariums, but no research to date has investigated this question.
Current Research and Hypotheses
The current research extends earlier aquarium studies by using a substantially larger, public exhibit, and by exploring the additive or synergistic effects of adding marine life, over and above an aquatic environment per se. We examined psychophysiological reactions to the exhibit across three stages of restocking: “Unstocked”—seawater only and artificial decoration; “Partially stocked”—moderate levels of biota; and “Fully stocked”—approximately double the number of species and individuals (as the Partially stocked condition). Although a small number of studies have explored psychological responses to species richness (e.g., Dallimer et al, 2012), species abundance has less frequently been taken into consideration. Although our study also expanded on previous work by obtaining a precise measure of species abundance (as well as species richness), we had little control over restocking and, therefore, species richness and abundance were still confounded.
The study had two parts. Part A consisted of observing the amount of time a subsample of aquarium visitors spent looking at the exhibit at each restocking stage. As previous studies have demonstrated positive relationships between psychological well-being and (a) level of species richness and (b) length of self-selected visits to natural environments, we explored whether there was a relationship between stocking level and voluntary exposure time to the exhibit. Part B consisted of monitoring the physiological and psychological reactions of experimental participants seated in front of the exhibit after 5 and 10 min across the three conditions. Again, based on earlier research, we explored the relationship between indices of psychophysiological well-being (e.g., positive mood, heart rate) and the stocking level in the exhibit. Furthermore, by monitoring these indices at 5 and 10 min, we were able to see whether longer exposures conferred more benefits. Exposure times of 5 and 10 min were selected partly due to experimental time constraints and partly based on previous studies. Wells (2005) noted significant changes in physiological measure when using a 10 min video intervention. Katcher et al. (1983; Katcher et al, 1984) used longer interventions (≥20 min) but felt that maximal relaxation could be obtained in less time after noting that participants watching just water and plants in a previous study became bored and restless within 20 min. Other studies exploring reactions to restorative environments have found that physiological responses can occur within a few minutes (e.g., <4 min; Ulrich et al, 1991) and psychological changes can be immediate (e.g., Pretty, Peacock, Sellens & Griffin, 2005). Furthermore, from an adaptive evolutionary viewpoint, it would be expected that restoration should occur quite quickly (in minutes, rather than hours), depending on the stress response (Ulrich et al, 1991). These sudden changes also highlight the importance of employing a repeated-measures strategy (Hartig et al, 2003).
Based on previous research, we hypothesized the following:
Hypothesis 1 (Part A): Voluntary exposure time would reflect intrinsic fascination and would be positively correlated with the level of biota present within the exhibit.
As earlier studies suggest that psychological and physiological benefits may be gained from viewing aquaria, we hypothesized the following:
Hypothesis 2 (Part B): There would be a positive relationship between psychophysiological responses and viewing the exhibit when it contained marine life.
More specifically, we anticipated a decrease in heart rate and blood pressure (e.g., as per Katcher et al, 1983; Wells, 2005), an improvement in mood, and a decrease in arousal (e.g., as per Katcher et al, 1984), suggestive of greater relaxation and reduced stress. We also anticipated more positive responses to the evaluation statements. Moreover, we hypothesized that these benefits would be greater in the Fully stocked condition as previous work (e.g., Dallimer et al, 2012) has associated greater psychological well-being with higher species richness. As field studies have found a relationship between psychological benefits and length of self-selected visits to natural environments, we hypothesized the following:
Hypothesis 3 (Part B): Longer exposure time to the exhibit would improve psychophysiological responses.
Overall, although facets of Biophilia (affiliation with living things) and ART (elements of fascination, being away, extent, and compatibility) are found in an aquarium environment and are relevant to this research, our hypotheses (i.e., psychophysiological responses to a stressor) are most applicable to PSRT.
Materials and Methods
Study Site and Experimental Conditions
The exhibit undergoing refurbishment was a 550,000-L aquarium exhibit (14.3 m length × 6.2 m width × 6 m height), viewed predominately through a single, huge acrylic window (14 m × 4.25 m). While drained of water, the exhibit was decorated with artificial seaweed (e.g., kelp—Laminaria spp.) and temperate corals (e.g., pink sea fans—Eunicella verrucosa) to recreate a local U.K. underwater habitat. The exhibit was then filled with seawater that was left to mature for 3 weeks before any marine life was added. Skylights above the tank allowed some natural light to penetrate the exhibit. The sheer size of the exhibit, together with the subtle changes in natural light and the gentle movement of the artificial seaweed (generated by hidden pumps), created a natural looking underwater scene that was pleasant, soothing, and immersive.
Each stage of the study was conducted on week days, during normal aquarium opening hours. In Condition 1 (Unstocked), normal visitors and participants in the experiment viewed the exhibit when it contained only seawater and artificial decoration. After Condition 1 was completed, the first fish and invertebrates were introduced to the exhibit. Although every effort was made to ensure that all visitors/participants in Condition 2 (Partially stocked) were presented with the same biota this was not possible. The practicalities of coordinating a field study in a working aquarium resulted in some fish being introduced to the exhibit throughout the time taken to collect all visitors/participants in Condition 2, although not while visitors/participants were actually present. Simpson’s Index of Diversity (1-D) for the exhibit, therefore, increased from 0.615 to 0.808 as the number of fish species increased to 10 over a 3-day period (80 individual fish, for example, Orders: Gadiformes, Pleuronectiformes, and Rajiformes—see online appendix for full species list). Nevertheless, the majority of visitors/participants (>88%) viewed the exhibit when it contained approximately six species of fish (~60 individuals) and two species of crustaceans (14 individuals—Order: Decapoda). For these visitors/participants Simpson’s Index of Diversity averaged 0.730.
Before the start of Condition 3 (Fully stocked, Figure 1), a further period of restocking was undertaken resulting in a total of 22 species: 19 fish species (138 individual fish) and 3 invertebrate species (13 individual crustaceans). All visitors/participants in Condition 3 were exposed to the same level of biota (1-D = 0.881). Recalculation with Shannon Weiner’s Diversity Index also indicated that the Fully stocked condition (H’ = 2.51) was more diverse than the Partially stocked condition (H’ = 1.93). Ethical approval for the study was provided by the University of Plymouth’s Faculty of Science Ethics Committee.
Figure 1.

Condition 3—Fully stocked exhibit.
Part A
Participants
Participants (visitors) were 112 randomly selected members of the public who were observed during a visit to the National Marine Aquarium. Observations were carried out during the three stages of refurbishment: Unstocked (n = 41), Partially stocked (n = 31), and Fully stocked (n = 40). Visitors were informed at the aquarium entrance that the exhibit was being refurbished.
Procedure and measures
A rectangular area of 84 m2 (14 m × 6 m) in front of the exhibit was defined as the “target area.” Observations were made by two trained researchers who were positioned unobtrusively at the back of the exhibit area. On commencement of a data collection session, the first adult visitor to enter the target area (either a lone visitor or one individual from a couple or group) was timed (in seconds) from the moment he or she entered the target area to the time he or she left it. The researchers then waited 1 min before selecting the next adult who entered the target area. Observations were carried out on a time schedule to reduce potential bias. Measurements were taken twice weekly, at varying times of the day. Numbers of observations differed across conditions due to visitor volume on sampling days.
Part B
Participants
Eighty-four students from a U.K. university elected to take part in the study for course credit (M age = 24 years, 64 females). Due to some “no shows” participant numbers differed slightly across conditions: Condition 1, n = 29; Condition 2, n = 26; Condition 3, n = 29. As this study was a “natural experiment,” we were unable to randomly allocate participants to condition. Restocking took place over a 10-month period and, because participants were recruited for each condition at different time points, it is only quasi-experimental.
Procedure and measures
Participants made their own way to the aquarium and were collected from the entrance, in pairs, at an agreed time. As equipment and paperwork needed to be monitored and administered throughout the study, one researcher was allocated per participant. Participants were taken directly to the study area (without stopping to view any other exhibits on the way) and were seated in a curtained booth located in front of the exhibit but which obstructed the view of the exhibit. Participants were seated and briefed at the start of the study that we were interested in how their psychological and physiological measures changed in response to watching an aquarium exhibit; they were not informed about the different stocking levels at this point. We informed participants that mood, heart rate, and blood pressure would be taken before, after, and while watching the aquarium exhibit. Participants were informed of the confidentiality of their responses and their right to withdraw. If happy to proceed, participants signed a consent form. A heart rate monitor (Cateye PL-6000 with ear clip) and blood pressure monitor (Omron HEM705C) were attached to each participant.
Participants then sat quietly behind the curtain for 5 min, and blood pressure and heart rate were measured twice, after 2 min and after 5 min. In the 3-min interval, participants completed a series of anagrams ranging from very simple to impossible. We anticipated that this task would increase participants’ stress arousal. However, as no significant effects on either blood pressure or heart rate were found between the two time periods, we collapsed both measures together to form a more robust estimate of baseline physiological states before the curtain was drawn and the exhibit revealed (i.e., Time 1: “Baseline”).
Just after the second set of physiological measurements was taken, and before the curtain was withdrawn, two measures of psychological mood were taken: the Feeling Scale (Hardy & Rejeski, 1989) and the Felt Arousal Scale (adapted from Svebak & Murgatroyd, 1985). The Feeling Scale is a single-item 11-point bipolar scale (very bad to very good, −5 to +5), designed to measure affective valence. The Felt Arousal Scale uses a single-item 7-point scale (low to high arousal, 0 to +6). These measures recognize the fact that mood is related to two orthogonal dimensions (valence and arousal), so for instance, one can be in a positive mood with high arousal (excited) or low arousal (calm) or a negative mood with high arousal (angry) or low arousal (depressed). Combined, these scales have been validated in many studies examining the impact of exercise on mood at multiple time points and show reliable patterns of change over time (e.g., Ekkekakis, Hall, Van Landuyt, & Petruzzello, 2000). Once completed, the curtain was drawn back to reveal the exhibit in one of the three experimental conditions (Unstocked, Partially stocked, or Fully stocked), and participants were instructed to watch the exhibit until the curtain was drawn again.
After a period of 5 min, researchers discretely noted the participants’ heart rate before asking them to complete the mood scales again (Time 2: “+5 min”). Blood pressure readings were not taken at this point so as not to disturb participants. Participants then watched the exhibit for a further 5 min after which the final heart rate and blood pressure readings were recorded. Mood scales were also completed for the last time (Time 3: “+10 min”). Finally, participants were asked to complete five evaluation statements: “I enjoyed watching the exhibit,” “I feel better after watching this exhibit,” “I would be happy to watch this exhibit again” (on a 7-point scale: not at all to very much, 0 to +6), “I found watching this exhibit” (very boring to very interesting, 0 to +6), and “I would be happy to watch this exhibit for another (5, 10, 15 or 20) minutes.” At the end of the study the participants were debriefed and thanked for their time.
Analysis strategy
Part A
Preliminary analysis of visit duration found a large positive skew (1.89), reflecting the fact that although the majority (66%) of visitors spent less than 4 min at the exhibit, a few spent much longer, up to 20 min. Nevertheless, time was approximately lognormal, so was transformed to log base 10 to enable greater confidence in the outputs of the subsequent ANOVA. Planned repeated contrasts were also used to compare each subsequent stage of restocking.
Part B
For the psychophysiological part of the study, we began by examining participants’ evaluations of their experiences after watching the exhibit for 10 min (with one-sample t tests used to compare responses with zero). We then examined the effects of watching the exhibit on blood pressure, heart rate, and mood. As blood pressure was only taken twice, we used paired-samples t tests for each condition separately to establish changes associated with watching an Unstocked, Partially, or Fully stocked exhibit for 10 min. To investigate the relative effects of the different stocking levels, we computed the change in blood pressure scores over time (from Baseline to +10 min) for each participant and analyzed these change scores using a one-way ANOVA, with level of stocking as the between-participant factor and planned contrasts if the main effect was significant. As heart rate and mood (valence and arousal) were measured at three time points we used one-way repeated-measures ANOVAs, with planned repeated contrasts, to explore the effects of each condition separately over time. Then, to explore the relative effects of the different conditions, we again derived difference scores by subtracting baseline scores from those at 5 and 10 min. These change scores were then analyzed using a series of 2 (Time: +5 min/+10 min) by 3 (Condition: Unstocked/Partially/Fully stocked) mixed factorial ANOVAs with repeated measures on Time and planned repeated contrasts (reflecting the predicted order of Stocking and Time). Change scores were used for several analyses because there was a pattern of non-equivalence across conditions at Baseline. In particular, participants who arrived at the aquarium in the Fully stocked condition had, by chance, higher average levels of (diastolic) blood pressure, significantly higher heart rate, and less positive mood (Table 1). Potential reasons for these differences are presented in the discussion and possibly reflect the studies’ status as a “natural experiment.”
Table 1.
M (SD) for Post-Viewing Evaluation Statements, Blood Pressure and Heart Rate Readings, and Mood.
| Unstocked (n = 26) |
Partially stocked (n = 29) |
Fully stocked (n = 26) |
|
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| Evaluation statement | |||
| 1. I enjoyed watching this exhibit | 3.14 (1.48) | 4.58 (1.17) | 5.00 (0.80) |
| 2. I found this exhibit very boring–very interesting | 4.14 (1.43) | 5.38 (0.98) | 6.00 (0.76) |
| 3. I feel better after watching this exhibit | 3.34 (1.68) | 4.19 (1.23) | 4.38 (1.05) |
| 4. I would be happy to watch this exhibit again | 3.10 (1.76) | 4.46 (1.42) | 5.24 (0.91) |
| 5. I would be happy to watch this exhibit for another . . . 5, 10, 15, 20 min | 7.41 (3.69) | 11.35 (5.93) | 11.38 (5.33) |
| Blood pressure (mm HG)a | |||
| Systolic | |||
| T1: Pre-exposure (Baseline) | 115.71 (10.51) | 114.21 (12.39) | 114.50 (9.47) |
| T3: @10 min exposure | 113.54 (9.73) | 109.67 (15.20) | 111.67 (8.06) |
| Diastolic | |||
| T1: Pre-exposure (Baseline) | 68.98 (6.28) | 68.31 (9.05) | 71.85 (8.30) |
| T3: @10 min exposure | 67.50 (7.18) | 65.79 (10.71) | 70.89 (7.22) |
| Heart rate (bpm)b | |||
| T1: Pre-exposure (Baseline) | 74.61 (11.89) | 76.62 (11.86) | 81.59 (13.69) |
| T2: @5 min exposure | 72.04 (10.93) | 71.38 (10.56) | 76.57 (11.79) |
| T3: @10 min exposure | 72.43 (12.02) | 71.08 (10.76) | 75.61 (11.29) |
| Mood | |||
| Valence | |||
| T1: Pre-exposure (Baseline) | 2.11 (1.77) | 2.27 (1.64) | 1.41 (1.88) |
| T2: @5 min exposure | 2.29 (1.56) | 2.96 (1.40) | 2.24 (1.57) |
| T3: @10 min exposure | 2.54 (1.53) | 3.12 (1.21) | 2.66 (1.72) |
| Arousal | |||
| T1: Pre-exposure (Baseline) | 2.86 (1.38) | 2.77 (1.28) | 2.66 (1.40) |
| T2: @5 min exposure | 2.04 (1.37) | 2.23 (1.31) | 2.31 (1.39) |
| T3: @10 min exposure | 1.50 (1.14) | 1.85 (1.41) | 1.97 (1.32) |
Note. Evaluation statements values are the mean of reported scores on a 7-point scale (0 = not at all, 6 = very much—Statements 1, 3, and 4, 0 = very boring, 6 = very interesting—Statement 2). Valence values are the mean of reported scores on a single-item 11-point scale (−5 = very bad, +5 = very good). Arousal values are the mean of reported scores on a single-item 7-point scale (0 = low arousal, 6 = high arousal). SD = standard deviation.
Excludes two outliers with changes >3 SDs from the mean.
Excludes one outlier with changes >3 SDs from the mean.
Results
Part A
Time spent in front of the exhibit increased as the stocking level increased (Unstocked M = 3.01 min, SD = 3.57 min; Partially stocked M = 4.08 min, SD = 3.66 min; Fully stocked M = 5.94 min, SD = 4.16 min). Using the log base 10 transformation, we found, as predicted, that duration was greatest in the Fully stocked condition (log10 M = 0.66, SD = 0.35), followed by the Partially stocked condition (log10 M = 0.48, SD = 0.35), and then the Unstocked condition (log10 M = 0.26; SD = 0.47). ANOVA analysis (using log transformed scores) found a significant main effect of condition, F(2, 109) = 10.15, p < .001, = .157. Planned contrasts revealed that the time spent in front of the Fully and Partially stocked conditions was significantly greater than the Unstocked condition (p < .001 and p = .022, respectively). Furthermore, there was a marginally significant difference between the Partially and Fully stocked conditions (p = .065). In summary, and in support of Hypothesis 1, visitors stayed significantly longer in front of the exhibit with the highest stocking level suggesting that they found it more interesting.
Part B
Evaluation statements
Participants in all three conditions found 10 min in front of the exhibit was an enjoyable and interesting experience that made them feel better. Furthermore, participants stated that they were happy to continue watching the exhibit for another 7½ (Unstocked) or 11½ min (Partially and Fully stocked)—see Table 1 for test statistics. We compared participants’ evaluations across the three conditions using one-way ANOVAs on each of the five statements (see Figure 2 for Evaluation Statements 1-4; the time estimates for Statement 5 are reported separately below). As predicted (Hypothesis 2), there was a significant increase in all five ratings. The planned repeated contrasts revealed that responses to all five evaluation statements were significantly more positive in the Partially versus Unstocked condition (enjoyed watching, p < .001; found interesting, p < .001; feel better, p = .023; happy to watch again, p = .001; and watch for an additional 3.94 min, p = .005). Means in the Fully stocked condition were also all higher than in the Partially stocked condition, but were only significantly higher for level of interest (p = .041) and willingness to watch the exhibit again (p = .043). In other words, people rated watching an Unstocked exhibit positively, Partial restocking significantly improved these ratings, and Fully stocking the exhibit improved some of the ratings further.
Figure 2.
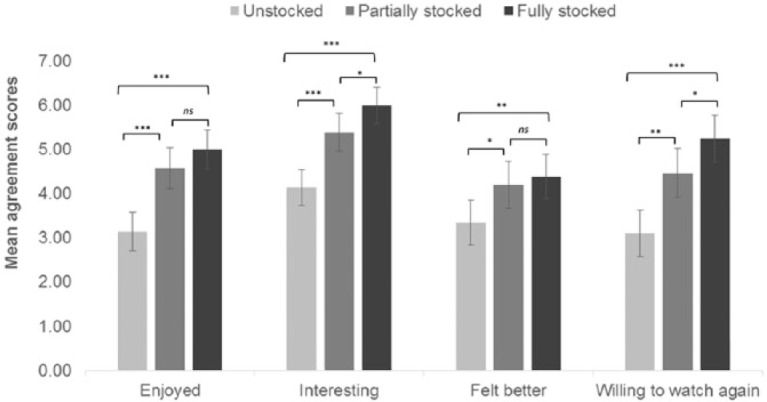
Mean agreement scores for evaluation statements.
Note. ns = not significant. Error bars: 95% confidence intervals.
*p ≤ .05. **p ≤ .01. ***p ≤ .001.
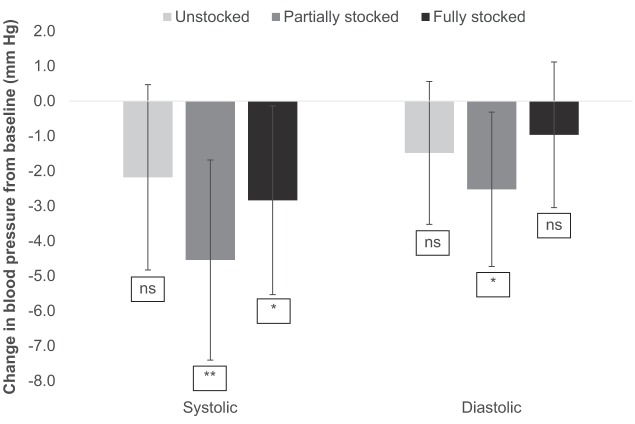
Blood pressure
Two participants, one in the Unstocked and one in the Partially stocked condition, were excluded from blood pressure analysis due to erratic readings suggesting measurement error (changes >3 SDs from the mean). Analyses of the remaining participants found drops in both systolic blood pressure (SBP) and DBP in all three conditions (Table 1 and Figure 3) but only some of these were statistically significant. Specifically, SBP dropped significantly for the Partially, t(23) = 2.96, p = .007, and Fully stocked, t(26) = 2.59, p = .016, conditions, and DBP dropped significantly only in the Partially stocked condition, t(23) = 2.27, p = .033. The ANOVAs found no significant main effect of condition for either SBP, F(2, 76) = 0.77, p = .467, or DBP, F(2, 76) = 0.54, p = .584, and no significant repeated contrasts. In short, there was only partial support for Hypothesis 2 on this measure: Watching the exhibit, irrespective of condition, generally decreased blood pressure but the role of stocking level was unclear.
Figure 3.
Change in systolic and diastolic blood pressure (mm Hg) from baseline at 10 min as a function of exhibit stocking level.
Note. ns = not significant. Error bars: 95% confidence intervals.
*p ≤ .05. **p ≤ .01. ***p ≤ .001.
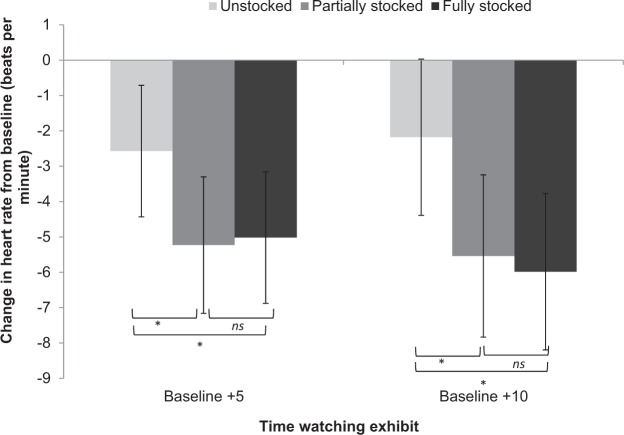
Heart rate
Data from one participant, in the Fully stocked condition, were excluded due to erratic readings (changes >3 SDs from the mean). The one-way ANOVAs for each condition separately revealed a similar basic pattern over time, with heart rate dropping substantially during the first 5 min followed by more modest changes over the next 5 min (Table 1). Specifically, there was a main effect of time in all three conditions. In each case, the planned repeated contrasts showed significant drops in heart rate over the first 5 min but no further significant change from 5 to 10 min, although the drop in the Fully stocked condition was somewhat greater than the other two conditions (Figure 4). The mixed factorial ANOVA found a significant effect of condition, F(2, 79) = 3.38, p = .039, = .053. The planned contrasts revealed that both the Partially and Fully stocked conditions resulted in significantly greater drops in heart rate than the Unstocked (control) condition (Partially, p = .032; Fully, p = .024). There was no significant effect of Time, F(1, 79) = 0.50, p = .482, = .006, or Time × Condition interaction, F(2, 79) = 0.91, p = .405, = .023. Nevertheless, the significant Intercept for Time, F(1, 79) = 62.23, p < .001, = .441, reflects the overall drop in heart rate compared with baseline. In short, as with blood pressure there is a tendency for heart rate to drop in all three conditions, drops in heart rate were significantly greater in the two conditions containing biota, supporting Hypothesis 2. Despite some indication of a duration dose–response effect, the majority of the effects took place in the first 5 min with only marginal gains subsequently, thus only partially supporting Hypothesis 3.
Figure 4.
Change in heart rate (beats per minute) from baseline at 5 and 10 min as a function of exhibit stocking level.
Note. ns = not significant.
*p ≤ .05. **p ≤ .01. ***p ≤ .001.
We noted that mean baseline heart rate was, by chance, higher in the Fully stocked condition than the other two. In part, this may have been due to generally poorer weather during the testing period, which (as well as potentially worsening the mood of participants) tends to make the aquarium more crowded and noisy. Given this possibility, we reran all our analyses controlling for visitor numbers on test days (MN = 421, minN = 288, maxN = 656) and found no significant main effect of attendance numbers or any interactions with attendance on any of the physiological or psychological variables. Nevertheless, attendance figures are per day, and thus we cannot be sure how participants were affected by ambient visitor numbers at the precise time of testing.
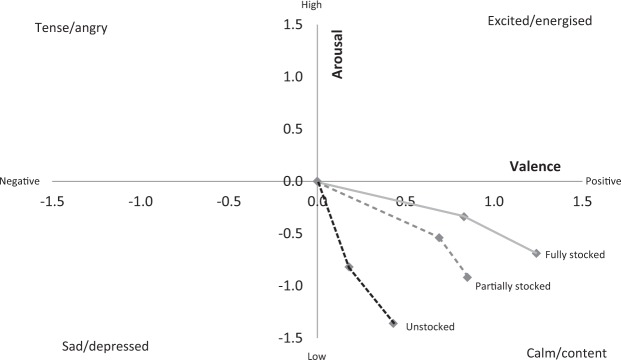
Mood
In general, valence increased over time (people felt more positive) and arousal fell (people became more relaxed)—see Table 1 and Figure 5. The one-way repeated-measures ANOVAs for each condition revealed that whereas valence improved considerably in both the Fully, F(2, 56) = 24.80, p < .001, = .470, and Partially stocked, F(2, 50) = 8.97, p < .001, = .264, conditions, the improvement in the Unstocked condition was not significant, F(2, 54) = 1.52, p = .228, = .053. Importantly, for the duration-related dose–response discussion, the planned repeated contrasts revealed that although valence became significantly more positive after 5 min in the Partially stocked condition, F(1, 25) = 9.88, p = .004, = .283, it did not further improve after 10 min, F(1, 25) = 1.35, p = .256, = .051. However, in the Fully stocked condition valence increased both from baseline to 5 min, F(1, 28) = 17.31, p < .001, = .382, and from 5 to 10 min, F(1, 28) = 10.67, p = .003, = .276. Given the non-significant main effect of time in the Unstocked condition, neither of the planned contrasts was significant. In other words, and in support of Hypotheses 2 and 3, there is a suggestion here of a double dose–response effect: Longer exposure to a greater stocking level produced the greatest improvement in valence.
Figure 5.
Psychological reaction to the three different levels of stocking presented in two-dimensional affective space.
Arousal decreased significantly in all three conditions: Fully stocked, F(2, 56) = 5.40, p = .007, = .162; Partially stocked, F(2, 50) = 9.27, p < .001, = .270; and Unstocked, F(2, 54) = 17.44, p < .001, = .393. However, the planned contrasts found that whereas the drops from baseline to 5 min and from 5 to 10 min were associated with significant drops in arousal in the Unstocked, Baseline to 5 min: F(1, 27) = 12.72, p = .001, = .320; 5 to 10 min: F(1, 27) = 9.45, p = .005, = .259, and Partially stocked, Baseline to 5 min: F(1, 25) = 7.70, p = .010, = .236; 5 to 10 min: F(1, 25) = 5.95, p = .022, = .192 conditions, the drop in arousal in the Fully stocked condition was only significant from 5 to 10 min exposure, F(1, 28) = 4.70, p = .039, = .144, and not in the first 5 min, F(1, 28) = 2.38, p = .134, = .078. Referring to Figure 5 (differences from baseline), it seems that while watching the Fully stocked exhibit resulted in a greater increase in positive emotions than the Partially stocked exhibit, arousal was less affected. Contrary to our original Hypotheses (2 and 3), these findings suggest that watching the Fully stocked condition created a less calming but more energizing effect.
Regarding comparisons over time across condition, two mixed factorial ANOVAs (one each for valence and arousal) again found significant main effects of time, valence: F(1, 80) = 10.33, p = .002, = .114; arousal: F(1, 80) = 19.75, p < .001, = .198, only a marginally significant effect of condition for valence, F(2, 80) = 2.66, p = .076, = .062, and no effect for arousal, F(2, 80) = 1.65, p = .198, = .040. Neither of the interactions approached statistical significance, valence: F(2, 80) = 0.803, p = .451, = .020; arousal: F(2, 80) = 0.383, p = .683, = .009. Planned contrasts found that the only reliable difference for valence was between Unstocked and Fully stocked conditions (p = .025). There was no significant difference between conditions for arousal although there was a marginal effect between the Unstocked and Fully stocked conditions (p = .075). Again, then, it seems that there is some effect of biota with higher levels of restocking “tipping the balance” compared with the Unstocked exhibit.
Discussion
Summary of Findings
We had a unique opportunity to test a number of psychological and physiological responses to different levels of marine biota in a very large aquarium exhibit and to also examine the relative importance of the physical setting separate from the marine life contained within it. We were also able to examine people’s reactions after both 5 and 10 min of exposure, enabling us to explore potential duration dose–response effects.
Visitor observations in Part A confirmed that, on average, visitors stayed longer in front of the exhibit when it contained the greatest level of marine life, supporting Hypothesis 1. If we assume that behavior reflects preferences this suggests that people gained more benefit from the exhibit when it was fully stocked. From a psychological perspective it might be that the greater levels of biota provided greater levels of interest and fascination and the opportunity to disengage from the mundane, all elements which have previously been shown to aid psychological restoration.
Evaluations of experimental participants who were closely monitored during their exposure to the exhibit in Part B supported these perspectives. Specifically, as stocking levels in the exhibit increased, participants’ interest in the exhibit and willingness to watch it again significantly increased, indicating a dose–response relationship (supporting Hypothesis 2). Furthermore, it was also evident in both the observation and evaluation data that the exhibit alone, although unstocked and containing only seawater and artificial decoration, appeared to be sufficiently interesting to confer some benefits in line with previous studies showing the psychologically restorative effects of aquatic environments in general (e.g., White et al, 2013).
The physiological evidence in support of the dose–response effects of stocking level (Hypothesis 2) and exposure time (Hypothesis 3) was weaker. All three stages of exhibit restocking were associated with significant drops in blood pressure and heart rate, indicating that exposure in all conditions was calming and physiologically restorative. Note that these decreases were observed after a rest period, and so cannot be attributed simply to sitting quietly. Most of these gains occurred within the first 5 min, with only diminishing returns following a further 5 min of exposure. Nonetheless, the greatest drop in heart rate occurred in the Fully stocked condition and this drop was significantly different from the Unstocked condition. The mixed blood pressure results may result from the relative responses of SBP and DBP to a given situation: An increase in SBP characterizes a beta-adrenergic response linked to stressors involving active coping or defense whereas an increase in DBP represents an alpha-adrenergic response linked to stressors involving vigilance or passive coping (Hartig et al, 2003). Hence, although there was no effect of condition, potentially the decreases in SBP and DBP (some of which were significant) may reflect the overall benign and non-threatening setting.
Potential dose–response relationships were more evident in the mood data where people reported how they were currently feeling in terms of valence (positivity) and arousal. In general, as duration of exposure increased people became both more positive and calmer but as biota levels increased, people became more positive but relatively less calm. This latter finding is concordant with the notion that the greater levels of biota are associated with more interest and fascination. In fact, when viewed from this perspective, there might not be any reason to expect greater drops in blood pressure and heart rate from watching more versus less marine life as the former is more stimulating (e.g., see Katcher et al, 1983). Indeed, Pilotti, Klein, Golem, Piepenbrink, and Kaplan (2015) found a significant increase in participants’ SBP following exposure to a nature video (but not an urban video) and suggest that changes in blood pressure may be related to pre-intervention (baseline) levels and participants’ expectations.
The current data add to the still small body of literature, which suggests that individuals may gain psychological well-being from exposure to environments that contain relatively high levels of biota. Furthermore, these findings provide some support for all three previously mentioned hypotheses: The aquarium provided contact with nature (Biophilia) and fulfilled the criteria of ART, either fully for visitors who had chosen to visit the aquarium, or partially for participants for whom the aquarium still provided fascination and an escape from their daily routine. Overall, although participants tended to leave feeling happier and more relaxed (psychologically and physiologically) after viewing the exhibit, the unsuccessful stressor task (and our resultant inability to demonstrate a measurable effect on “elevated stress”) would appear to lessen the relevance of this work to PSRT, compared with the two other theoretical models, Biophilia and ART. However, self-rating data from a study using unstressed university students found that everyday natural scenes (particularly those containing water) held the participants’ attention and produced more positive emotional states than urban scenes lacking nature (Ulrich, 1981). Furthermore, the self-ratings in Ulrich (1981) were largely convergent with results of brain electrical activity (electroencephalography [EEG]) recordings in the alpha frequency range, suggesting that participants were more relaxed and wakeful when viewing natural scenes. Consistency between psychological and physiological measures in our study likewise justifies greater confidence in the findings and also suggests that findings are not a result of social desirability effects.
Although the current data pertain to a very large, and thus presumably immersive exhibit, our findings may inspire further research, which examines whether similar effects are found for smaller aquaria with generally smaller fish and lower stocking levels. This could be important for locations where reducing psychophysiological stress is a key objective; not only in traditional health care settings (e.g., dental waiting rooms) but in everyday places of stress such as office buildings. Our results suggest that an individual does not need to spend long in front of an exhibit (just 5 min) to derive significant benefits.
Limitations and Future Research
Key advantages of our research included a control condition (no biota present) and precise knowledge about the actual level of marine life at both stages (unlike most field studies). This enabled us to say what added advantage the presence of fauna had over and above the background environment.
The research also benefited from taking place in a working aquarium during normal opening hours. Although we could have conducted the research “out-of-hours” when peace and quiet could be assured, we felt it was important to examine the potential of the exhibit to promote well-being under normal conditions to enable generalization. However, this also presented challenges. We noted, for instance, that participants in the Fully stocked condition had, at baseline, generally higher heart rates and more negative moods than those in the other two conditions. Given these baseline differences, potentially due to poor weather and/or higher ambient visitor numbers, we also cannot rule out regression to the mean effects as a possible explanation for the particularly strong gains (e.g., in heart rate and valence) in the Fully stocked group. We also note that our stressor task (anagrams) did not sufficiently stress our participants: There was no significant increase in heart rate or blood pressure following the stressor task, and averaged readings (see Table 1 for means) fell within typically accepted “normal” rates for adults (i.e., resting heart rate: 60-100 bpm; blood pressure: <120/80 mm Hg—www.heart.org). The lack of stressor means that this study is therefore unlike other studies investigating stress reduction. Had the task been more effective at increasing stress arousal, it is possible that some of our findings may have been stronger. Replications of our findings are thus desirable.
A further limitation of our study was that, due to its opportunistic nature, species richness was naturally confounded with species abundance. As mentioned, this limits our ability to understand the relative impact of species richness versus abundance. As highlighted by Dallimer et al. (2012) who considered reactions to species richness only, “it is equally possible that the abundance of a given taxonomic group is more important or noticeable than the number of different species” (p. 51). However, such studies would be almost impossible in situ and would probably require more controlled laboratory studies. At best, we could at least be sure that participants were responding to current, known measures of species richness and abundance rather than estimates obtained during past surveys.
Finally, although we avoided collecting data during feeding times (when many species are particularly active) we also wonder how important certain types of behavior are in terms of the potential restorative effects for humans. Some species are relatively shy or have a limited behavior repertoire whereas others are more gregarious or exhibit intriguing behaviors, which may enhance the experience. Future work is therefore needed to explore whether there might be different levels of well-being associated with different types and/or combinations of underwater species.
Conclusion
In summary, the current work provides tentative support for previous, limited research that has suggested there are psychological and physiological benefits of watching aquaria. It extends earlier studies by exploring the potential influence of increasing biota levels on well-being measures recorded at several time points. The evidence that greater levels of stocking had positive effects on experience evaluations and mood extends findings from terrestrial studies that suggest dose–response relationships between biota levels and immediate psychological well-being. Further work is now needed to investigate different potential benefits across different aquarium types, sizes, and contexts. Just as research on plant array preferences may improve the design of green spaces for well-being, a greater understanding of aquarium biota and exhibit composition may maximize the restorative potential of aquaria in health care environments and other stressful settings such as the workplace.
Finally, the findings further highlight that restorative effects can be derived from artificial, as well as “real” nature experiences. Opportunities for engaging with nature, even in “managed” settings, may be key in helping urban populations connect with natural environments. Furthermore, as marine ecosystems can be adversely affected by visitors, the ability to connect people to marine environments by proxy, for example, through aquariums, could be extremely important. Aquariums may therefore be important for delivering psychological well-being, enhancing perceptions of the value of natural ecosystems, and ultimately encouraging support for conservation efforts in the wild.
Acknowledgments
The authors thank Paul Cox, formerly at the National Marine Aquarium, for identifying the opportunity for this research, and Abigail Corcoran, Julie Goodhew, and James Manthorp for help with data collection. They also thank Paul Somerfield at Plymouth Marine Laboratory for his help with diversity indices data and interpretation.
Author Biographies
Deborah Cracknell is a marine biologist and lead researcher at the National Marine Aquarium, Plymouth, United Kingdom. She is also undertaking a psychology-based PhD student at Plymouth University researching the relationship between aquarium environments (specifically, types and levels of subaquatic biodiversity), and human health and well-being outcomes.
Mathew P. White is an environmental/health psychologist interested in the impacts different types and quality of environment have on people’s health and well-being. He is particularly interested in the role of aquatic environments.
Sabine Pahl is an associate professor (reader) in the School of Psychology at Plymouth University. Her research interests are social cognition, restorative effects of natural environments, and the psychology of sustainable attitudes and behavior especially applied to energy and protecting the marine environment.
Wallace J. Nichols is a marine biologist interested in ocean conservation, especially relating to sea turtles. He is also interested in the psychological benefits and cognitive effects of engaging with aquatic environments.
Michael H. Depledge holds the chair of environment and human health at the University of Exeter Medical School and is visiting professor at University College, London. He is a former commissioner of the Royal Commission on Environmental Pollution and former chief scientist of the Environment Agency of England and Wales. His research interests include the impact of environment on health and well-being, the effects of chemical body burdens on human health and the environment, and finding ways of communicating scientific information to policymakers and politicians.
Footnotes
Authors’ Note: The authors confirm that National Aquarium Limited (NAL) played no part in the study design, data collection, or analysis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was conducted as part of the first author’s PhD, funded by National Aquarium Limited (NAL).
References
- Ballantyne R., Packer J., Hughes K. (2008). Environmental awareness, interests and motives of botanical gardens visitors: Implications for interpretive practice. Tourism Management, 29, 439-444. [Google Scholar]
- Barker S. B., Rasmussen K. G., Best A. M. (2003). Effect of aquariums on electroconvulsive therapy patients. Anthrozoös, 16, 229-240. [Google Scholar]
- Bennett E. S., Swasey J. E. (1996). Perceived stress reduction in urban public gardens. HortTechnology, 6, 125-128. [Google Scholar]
- Berto R. (2014). The role of nature in coping with psycho-physiological stress: A literature review on restorativeness. Behavioral Sciences, 4, 394-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- biota. (n.d.) In Collins English Dictionary. Retrieved from http://www.thefreedictionary.com/biota
- Bowler D. E., Buyung-Ali L. E., Knight T. M., Pullin A. S. (2010). A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health, 10, Article 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K., Gawlinski A. (1995). Animal-assisted therapy in the intensive care unit: A staff nurse’s dream comes true. Nursing Clinics of North America, 30, 529-537. [PubMed] [Google Scholar]
- Dallimer M., Irving K. N., Skinner A. M. J., Davies Z. G., Rouquette J. R., Maltby L. L., Gaston K. J. (2012). Biodiversity and the feel-good factor: Understanding associations between self-reported human well-being and species richness. BioScience, 62, 47-55. [Google Scholar]
- DeSchriver M. M., Riddick C. C. (1990). Effects of watching aquariums on elders’ stress. Anthrozoös, 4, 44-48. [Google Scholar]
- Edwards N. E., Beck A. M. (2002). Animal-assisted therapy and nutrition in Alzheimer’s disease. Western Journal of Nursing Research, 24, 697-712. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P., Hall E. E., Van Landuyt L. M., Petruzzello S. J. (2000). Walking in (affective) circles: Can short walks enhance affect? Journal of Behavioural Medicine, 23, 245-275. [DOI] [PubMed] [Google Scholar]
- Falk J. H., Heimlich J., Bronnenkant K. (2008). Using identity-related visit motivations as a tool for understanding adult zoo and aquarium visitor’s meaning making. Curator: The Museum Journal, 51, 55-79. [Google Scholar]
- Fuller R. A., Irvine K. N., Devine-Wright P., Warren P. H., Gaston K. J. (2007). Psychological benefits of green space increase with biodiversity. Biology Letters, 3, 390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusset M., Dick G. (2011). The global reach of zoos and aquariums in visitor number and conservation expenditures. Zoo Biology, 30, 566-569. [DOI] [PubMed] [Google Scholar]
- Han K.-T. (2007). Responses to six major terrestrial biomes in terms of scenic beauty, preference and restoration. Environment and Behavior, 39, 529-556. [Google Scholar]
- Hardy C. J., Rejeski W. J. (1989). Not what, but how one feels: The measurement of affect during exercise. Journal of Sport & Exercise Psychology, 11, 304-317. [Google Scholar]
- Hartig T., Evans G. W., Jamner L. D., David D. S., Gärling T. (2003). Tracking restoration in natural and urban field settings. Journal of Environmental Psychology, 23, 109-123. [Google Scholar]
- Herzog T. R., Maguire C. P., Nebel M. B. (2003). Assessing the restorative components of environments. Journal of Environmental Psychology, 23, 159-170. [Google Scholar]
- Herzog T. R., Ouellette P., Rolens J. R., Koenigs A. M. (2010). Houses of worship as restorative environments. Environment and Behavior, 42, 395-419. [Google Scholar]
- Kaplan S. (1995). The restorative benefits of nature: Toward an integrative framework. Journal of Environmental Psychology, 15, 169-182. [Google Scholar]
- Kaplan S., Bardwell L. V., Slakter D. B. (1993). The museum as a restorative environment. Environment and Behavior, 25, 725-742. [Google Scholar]
- Kastner S., De Weerd P., Desimone R., Ungerleider L. G. (1998). Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science, 282, 108-111. [DOI] [PubMed] [Google Scholar]
- Katcher A. H., Friedman E., Beck A. M., Lynch J. J. (1983). Looking, talking and blood pressure: The physiological consequences of interaction with the living environments. In Katcher A. H., Beck A. M. (Eds.), New perspectives on our lives with companion animals (pp. 351-359). Philadelphia: University of Pennsylvania Press. [Google Scholar]
- Katcher A., Segal H., Beck A. (1984). Comparison of contemplation and hypnosis for the reduction of anxiety and discomfort during dental surgery. American Journal of Clinical Hypnosis, 27, 14-21. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Kidd R. M. (1999). Benefits, problems, and characteristics of home aquarium owners. Psychological Reports, 84, 998-1004. [Google Scholar]
- Kweon B.-S., Ulrich R. S., Walker V. D., Tassinary L. G. (2008). Anger and stress: The role of landscape posters in an office setting. Environment and Behavior, 40, 355-381. [Google Scholar]
- Lindemann-Matthies P., Bose E. (2007). Species richness, structural diversity and species composition in meadows created by visitors of a botanical garden in Switzerland. Landscape and Urban Planning, 79, 298-307. [Google Scholar]
- Lindemann-Matthies P., Junge X., Matthies D. (2010). The influence of plant diversity on people’s perception and aesthetic appreciation of grassland vegetation. Biological Conservation, 143, 195-202. [Google Scholar]
- Lovell R., Wheeler B. W., Higgins S. L., Irvine K. N., Depledge M. H. (2014). A systematic review of the health and well-being benefits of biodiverse environments. Journal of Toxicology and Environmental Health, Part B: Critical Reviews, 17, 1-20. [DOI] [PubMed] [Google Scholar]
- Luck G. W., Davidson P., Boxall D., Smallbone L. (2011). Relations between urban bird and plant communities and human well-being and connection to nature. Conservation Biology, 25, 816-826. [DOI] [PubMed] [Google Scholar]
- Medical Research Council. (2011). Using natural experiments to evaluate population health interventions: Guidance for producers and users of evidence. Retrieved from http://www.mrc.ac.uk/documents/pdf/natural-experiments-guidance
- Millennium Ecosystem Assessment. (2005). Ecosystems and human well-being: Synthesis. Washington, DC: Island Press. [Google Scholar]
- Packer J., Ballantyne R. (2002). Motivational factors and the visitor experience: A comparison of three sites. Curator: The Museum Journal, 45, 183-198. [Google Scholar]
- Packer J., Bond N. (2010). Museums as restorative environments. Curator: The Museum Journal, 53, 421-436. [Google Scholar]
- Pals R., Steg L., Siero F. W., van der Zee K. I. (2009). Development of the PRCQ: A measure of perceived restorative characteristics of zoo attractions. Journal of Environmental Psychology, 29, 441-449. [Google Scholar]
- Pilotti M., Klein E., Golem D., Piepenbrink E., Kaplan K. (2015). Is viewing a nature video after work restorative? Effects on blood pressure, task performance and long-term memory. Environment and Behavior, 47, 947-969. [Google Scholar]
- Pretty J., Peacock J., Sellens M., Griffin M. (2005). The mental and physical outcomes of green exercise. International Journal of Environmental Health Research, 15, 319-337. [DOI] [PubMed] [Google Scholar]
- PADI Worldwide Corporate Statistics. 2015. PADI Statistics Retrieved from http://www.padi.com/scuba-diving/about-padi/statistics/
- Purcell T., Peron E., Berto R. (2001). Why do preferences differ between scene types? Environment and Behavior, 33, 93-106. [Google Scholar]
- Riddick C. C. (1985). Health, aquariums, and the non-institutionalised elderly [Special issue: Pets and the family]. Marriage & Family Review, 8, 163-173. [Google Scholar]
- Sandifer P. A., Sutton-Grier A. E., Ward B. P. (2015). Exploring connections among nature, biodiversity, ecosystem services, and human health and well-being: Opportunities to enhance health and biodiversity conservation. Ecosystem Services, 12, 1-15. [Google Scholar]
- Svebak S., Murgatroyd S. (1985). Metamotivational dominance: A multimethod validation of reversal theory constructs. Journal of Personality and Social Psychology, 48, 107-116. [Google Scholar]
- Tuomisto H. (2010). A consistent terminology for quantifying species diversity? Yes, it does exist. Oecologia, 164, 853-860. [DOI] [PubMed] [Google Scholar]
- Ulrich R. S. (1981). Natural versus urban scenes: Some psychophysiological effects. Environment and Behavior, 13, 523-556. [Google Scholar]
- Ulrich R. S. (1983). Aesthetic and affective response to natural environment. In Altman I., Wohwill J. F. (Eds.), Behaviour and the natural environment (pp. 85-125). New York, NY: Plenum. [Google Scholar]
- Ulrich R. S. (1984). View through a window May influence recovery from surgery. Science, 224, 420-421. [DOI] [PubMed] [Google Scholar]
- Ulrich R. S. (1993). Biophilia, biophobia and natural landscapes. In Kellert S., Wilson E. O. (Eds.), The biophilia hypothesis (pp. 73-137). Washington, DC: Island Press. [Google Scholar]
- Ulrich R. S., Simons R. F., Losito B. D., Fiorito E., Miles M. A., Zelson M. (1991). Stress recovery during exposure to natural and urban environments. Journal of Environmental Psychology, 11, 201-230. [Google Scholar]
- UK National Ecosystem Assessment. (2011). Understanding nature’s value to society. Oxford, UK: Information Press. [Google Scholar]
- van den Berg A. E., Koole S. L., van der Wulp N. Y. (2003). Environmental preference and restoration: (How) are they related? Journal of Environmental Psychology, 23, 135-146. [Google Scholar]
- Velarde M. D., Fry G., Tveit M. (2007). Health effects of viewing landscapes—Landscape types in environmental psychology. Urban Forestry & Urban Greening, 6, 199-212. [Google Scholar]
- Wells D. L. (2005). The effect of videotapes of animals on cardiovascular responses to stress. Stress & Health, 21, 209-213. [Google Scholar]
- White M. P., Alcock I., Wheeler B. W., Depledge M. H. (2013). Coastal proximity and health: A fixed effects analysis of longitudinal panel data. Health & Place, 23, 97-103. [DOI] [PubMed] [Google Scholar]
- White M. P., Cracknell D., Corcoran A., Jenkinson G., Depledge M. H. (2014). Do preferences for waterscapes persist in inclement weather and extend to sub-aquatic scenes? Landscape Research, 39, 339-358. [Google Scholar]
- White M. P., Pahl S., Ashbullby K., Herbert S., Depledge M. H. (2013). Feelings of restoration from recent nature visits. Journal of Environmental Psychology, 35, 40-51. [Google Scholar]
- White M., Smith A., Humphryes K., Pahl S., Snelling D., Depledge M. (2010). Blue space: The importance of water for preference, affect, and restorativeness ratings of natural and built scenes. Journal of Environmental Psychology, 30, 482-493. [Google Scholar]
- Wilson E. O. (1993). Biophilia and the conservation ethic. In Kellert S., Wilson E. O. (Eds.), The biophilia hypothesis (pp. 31-41). Washington, DC: Island Press. [Google Scholar]
- Wyles K. J., Pahl S., White M., Morris S., Cracknell D., Thompson R. C. (2013). Towards a marine mindset: Visiting an aquarium can improve attitudes and intentions regarding marine sustainability. Visitor Studies, 16, 95-110. [Google Scholar]