Abstract
Gemcitabine is an antimetabolite chemotherapy agent with schedule-dependent metabolism and efficacy. The purpose of this study was to identify the fixed-dose-rate (FDR) of gemcitabine administration in cancer-bearing cats that achieved a target plasma concentration (TPC) of 10 to 20 μM. Fifteen client-owned cats received gemcitabine infusions administered at various FDR for 1 to 6 hours. Plasma gemcitabine and dFdU (2′,2′-difluorodeoxyuridine), the major gemcitabine metabolite, were quantitated by high performance liquid chromatography. Cats treated with an FDR less than 2.5 mg/m2 per minute failed to achieve TPC, whereas cats treated with an FDR of 10 mg/m2 per minute quickly exceeded the target range. An FDR of 5 mg/m2 per minute provided the longest duration of exposure without exceeding the upper limit of the TPC. Plasma dFdU concentration mirrored plasma gemcitabine concentrations. These data suggest that in order to maintain TPC of gemcitabine in cats the FDR lies between 2.5 and 5 mg/m2 per minute. A Phase II study to evaluate efficacy and toxicity of this approach is underway.
Résumé
Administration de gemcitabine à vitesse et à dose fixes chez des chats atteints du cancer : une étude pilote. La gemcitabine est un agent de chimiothérapie antimétabolite ayant un métabolisme et une efficacité qui dépendent du plan thérapeutique. Cette étude visait à identifier la vitesse et la dose fixes (VDF) d’administration de la gemcitabine chez des chats atteints du cancer qui avaient atteints une concentration plasmatique cible (CPC) de 10 à 20 μM. Quinze chats appartenant à des clients ont reçu des infusions de gemcitabine administrées à diverses VDF pendant 1 à 6 heures. La gemcitabine et la dFdU (2′,2′-difluorodeoxyuridine) dans le plasma, le métabolite majeur de la gemcitabine, ont été quantifiés par chromatographie liquide à haute performance. Les chats traités à l’aide de VDF de moins de 2,5 mg/m2 par minute n’ont pas réussi à atteindre la CPC, tandis que les chats traités à l’aide de VDF de 10 mg/m2 par minute ont rapidement dépassé la zone cible. Des VDF de 5 mg/m2 par minute ont fourni la durée d’exposition la plus longue sans dépasser la limite supérieure de la CPC. La concentration de dFdU dans le plasma a reflété les concentrations de gemcitabine dans le plasma. Ces données suggèrent qu’fin de maintenir la CPC de la gemcitabine chez les chats, les VDF doivent se situer entre 2,5 et 5 mg/m2 par minute. Une étude de phase II pour évaluer l’efficacité et la toxicité de cette approche est actuellement en cours.
(Traduit par Isabelle Vallières)
Introduction
Gemcitabine (2′,2′-difluorodeoxycytidine or dFdC) is an antimetabolite chemotherapy agent with clinical activity against a number of solid tumors in humans, in particular carcinomas, and hematopoietic cancers (1–6). It is a synthetic pyrimidine nucleoside analog, which resembles cytosine arabino-side. This cell cycle phase specific chemotherapeutic drug blocks G1-S transition (1,3,4,7). The inactive form of gemcitabine, dFdC, is sequentially phosphorylated to dFdCTP after it gains entry into the cell. This active form competes with native deoxycytidine triphosphate (dCTP) for incorporation into DNA and inhibits DNA replication and repair, which leads to programmed cell death (1,4).
The formation of dFdCTP is a multi-step process. The first step is rate-limiting and saturable (1,7), and is regulated by deoxycytidine kinase (dCK). In humans, the optimum plasma gemcitabine concentration that results in peak dCK activity and maximal intracellular accumulation of the cytotoxic metabolite, dFdCTP, is 10 to 20 μM (3,6,7). Importantly, excess gemcitabine plasma concentrations have been shown to inhibit the activity of dCK (1,4). This balance has been tested in vitro in cell lines, ex vivo in freshly isolated human leukemia cells, and in vivo in cancer-bearing humans, and is best achieved by continuous (10 mg/m2 per minute), long-term (> 30 min) exposure (3,6–8).
Historically, gemcitabine has been administered to humans at high doses over short time intervals, once weekly for 3 consecutive weeks with 1 wk off between cycles (9). In the last decade, the dosing schedule for gemcitabine has transitioned to a FDR with lower concentrations (10 mg/m2 per minute) and prolonged infusion times (100 to 150 min). This method of administration is postulated and proven to increase the active metabolite, dFdCTP, within circulating human leukemia cells (3,6,7); however, data for cells within solid tumors are lacking, most likely due to the inability to easily sample such tissues repeatedly.
Catabolism of gemcitabine occurs through deamination by cytidine deaminase (CDA) to difluorodeoxyuridine (dFdU), which is then excreted in the urine (10). A study has evaluated gemcitabine metabolism ex vivo in fresh whole blood from various species (human, dog, cat, horse). This study showed that catabolism of gemcitabine in fresh whole blood is slower in the cat compared to fresh whole blood from dogs, humans, and horses, suggesting the potential need for shorter treatment infusions, lower doses, or longer infusions at lower doses in the cat (11). There is no current standard dosing scheme for the use of gemcitabine in feline patients with cancer. Although its use as a 20- to 30-minute infusion has been reported in several previous studies, gemcitabine has failed to clearly demonstrate clinical efficacy (12–15).
The purpose of this study was to administer gemcitabine as a FDR to cancer-bearing cats and to report, for the first time in this species, plasma concentrations of dFdC and dFdU during infusions.
Materials and methods
Animals
Client-owned cats with various malignancies confirmed by cytology or histopathology were enrolled in the study. Cats had to be at least 1 y old and > 1.5 kg body weight (BW) (Table 1). Baseline complete blood (cell) counts (CBC) and serum biochemistry analyses were obtained to evaluate for any co-morbidities within 2 wk of enrollment into the trial. This was deemed to be sufficient pre-treatment patient assessment in the context of the aims of the study; however, full staging was not carried out. Concurrent supportive therapy, which was prescribed at the discretion of the attending clinician, such as pain medication or antibiotics was permitted. Complete blood (cell) counts were obtained only prior to each subsequent infusion of gemcitabine. Cats had to have at least 5000 peripheral blood mononuclear cells (PBMCs)/μL and > 2000 neutrophils/μL of blood to be considered for entry into the trial and before each infusion. Cats were permitted to receive infusions at different fixed dose rates (FDR) no more than once every 2 wk. Cats were not allowed to receive the same FDR more than once. Clients provided signed informed consent with understanding of the investigational goals of the study and could withdraw their cat from the study for any reason and at any time. This study was approved by both the Institutional Animal Care and Use Committee (protocol 16192) and the Clinical Trial Review Board.
Table 1.
Subject characteristics
| Subject | Disease | Age (years) | Number of infusions |
|---|---|---|---|
| 1 | OSCC | 12 | 6 |
| 2 | CA, nasal | 13 | 14 |
| 3 | ISS | 8 | 1 |
| 4 | SCC, planum | 15 | 8 |
| 5 | SCC, tonsil | 6 | 1 |
| 6 | CA, mammary | 12 | 7 |
| 7 | Melanoma, oral | 14 | 5 |
| 8 | CA, lung | 15 | 1 |
| 9 | OSCC | 15 | 2 |
| 10 | OSCC | 7 | 3 |
| 11 | OSCC | 13 | 2 |
| 12 | SCC, ocular | 7 | 3 |
| 13 | OSCC | 14 | 1 |
| 14 | ISS | 14 | 1 |
| 15 | CA, pancreas | 15 | 6 |
OSCC — oral squamous cell carcinoma; CA — carcinoma; ISS — injection site sarcoma; SCC — squamous cell carcinoma.
Drug preparation
Gemcitabine (dFdC) is a commercially available chemotherapeutic agent and supply is consistent. It was reconstituted using sterile saline per the manufacturer’s (Sandoz, Princeton, New Jersey, USA) recommendations (final concentration 38 mg/mL).
Plasma preparation
Cats treated in this study had a sterile indwelling catheter placed in a peripheral vein. Blood (1 mL) was drawn before infusion, hourly during infusion, and at the end of infusion (EOI) into a lithium heparin tube and the plasma was separated immediately by centrifugation at 400 × g at room temperature for 5 min. The plasma proteins were precipitated by mixing with an equal volume of 6% sulfasalicylic acid (SSA). Precipitated proteins were separated by centrifugation as described and the protein-free plasma was frozen (−80°C) until the dFdU and gemcitabine were quantitated by high performance liquid chromatography (HPLC).
Fixed-dose-rate administration
The concentration of the commercially prepared gemcitabine was high (38 mg/mL); therefore, the resulting volume to be infused was small. Thus, the total calculated drug dose was further diluted with normal saline to a final volume permitting an infusion rate of 10 mL/h by syringe pump (Medfusion 3500; Smith Medical ADS, St. Paul, Minnesota, USA). For instance, a cat that was scheduled to receive 380 mg of gemcitabine over 6 h would have 10 mL of prepared gemcitabine brought to a volume of 60 mL.
Analytical procedures
To determine the concentration of plasma gemcitabine and dFdU, an HPLC chromatograph (Model ALC-204; Waters Associates, Milford, Massachusetts, USA) equipped with 2 pumps (Model 6000A; Waters Associates), and a gradient programmer (Model 660; Waters Associates) was used to fractionate plasma extracts. Samples (200 μL) were maintained at 10°C and were then applied to a column (Atlantis 46 × 100 mm μC18 3 μm; Waters Associates) and eluted at a rate of 1.7 mL/min using a concave gradient (curve #6) as follows: 100% Buffer A (10 mM NaH2PO4, pH 3) for 7 min, 97% Buffer A and 3% Buffer B (100% acetonitrile) for 7 to 14 min, 100% Buffer A for 14 to 18 min. The column eluate was monitored by photodiode array (PDA Detector Model 2998; Waters Associates) (200 λ and 320 λ and a sampling rate of 1 and a resolution of 1.2) and the dFdC and dFdU analytes were identified by comparisons of their retention profiles and absorption spectra with those of the standards and quantitated by electronic integration with reference to external standards. The lower limit of detection of this assay is 0.05 nmol in 100 μL of acid deproteinated plasma. Commercial software (Empower 2 version 6.0; Waters Associates) was used to integrate peaks.
Determination of plasma levels of gemcitabine and dFdU
The study was carried out in 2 parts. In the first part, a 1-hour infusion was administered at various dose rates to determine if the proposed TPC could be achieved. In the second part of the study, the duration of infusion was varied to assess the threshold for and duration of the TPC.
1-hour infusion
While unknown in the cat, in vitro and in vivo work have identified the optimal plasma concentration of gemcitabine to range between 10 μM and 20 μM and this concentration is reached within 1 h of a 10 mg/m2 per minute infusion in humans (3,6,7). Thus, the threshold for achieving the target plasma concentration (TPC) for the cats in this study was also set to 10 to 20 μM. Cats in this arm were started at the first visit with 1 mg/m2 per minute and were allowed to receive 2.5, 5, 7.5 and then 10 mg/m2 per minute for 1 h at each subsequent infusion. Seventeen gemcitabine infusions were delivered to 5 cats and the plasma gemcitabine and dFdU concentrations were quantitated as described in analytical procedures.
Multiple-hour infusions
In vitro, ex vivo, and in vivo human studies have shown that prolonged exposure of neoplastic cells to gemcitabine results in increased intracellular accumulation of the active metabolite of gemcitabine and that these higher intracellular levels result in increased cellular cytotoxity and improved therapeutic efficacy (3,6,7). The 1-hour infusion data were used to guide dosing in the multiple hour group. To evaluate the plasma effects of prolonged gemcitabine administration, cats were treated with gemcitabine at 1, 2.5, 5, or 10 mg/m2 per minute for 2, 4, or 6 h and the plasma levels of gemcitabine quantitated as described in analytical procedures. Some cats received more than 1 infusion, but always at different dose schedules and plasma was collected at each hour of infusion for analysis.
Plasma dFdU was quantitated in each sample assayed as described in analytical procedures.
Results were plotted as either EOI plasma gemcitabine and dFdU (μM) versus dose gemcitabine (mg/m2 per minute) or plasma gemcitabine and dFdU versus time. A non-linear regression line of the median plasma gemcitabine and dFdU with respect to both time and drug dose were plotted using least squares method and commercially available software (GraphPad Prism 6; GraphPad Software, La Jolla, California, USA).
Toxicity
If available, treatment-related adverse events were graded according to the Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events (VCOG-CTCAE) v1.1 (16).
Results
Cats (n = 15) with various malignancies (Table 1) received 61 infusions of gemcitabine as described below. The interval between infusions varied from once every 2 wk to once every 4 wk. Cats were either castrated males (n = 9) or spayed females (n = 6) with a median age of 12 y (range: 6 to 15 y), a median weight of 5.3 kg (range: 1.8 to 6.1 kg) and domestic short (n = 14) or long (n = 1) hair.
1-hour infusion
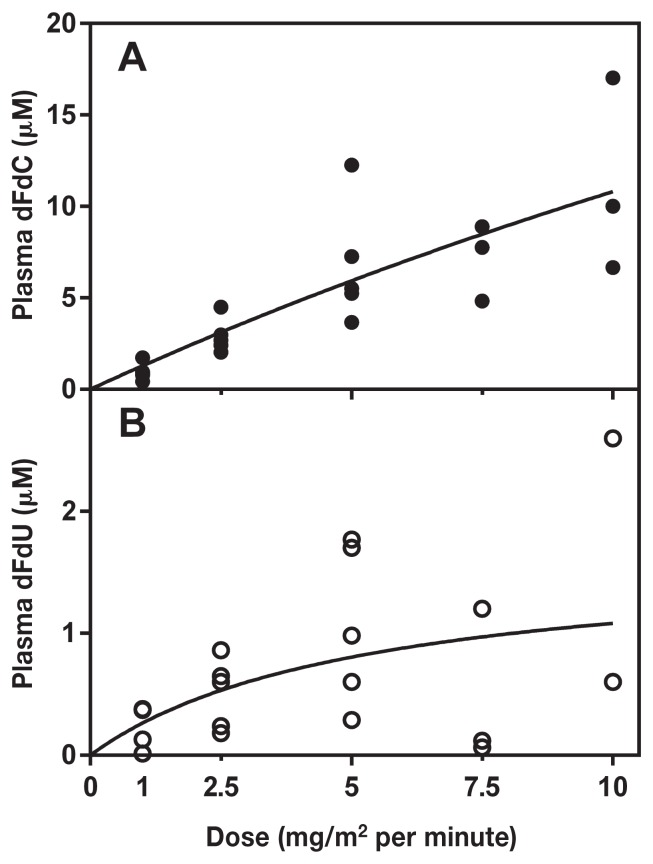
To determine the FDR that achieves the TPC, 5 cats were administered gemcitabine at 1 (n = 5), 2.5 (n = 5), 5 (n = 5), 7.5 (n = 3), and 10 (n = 3) mg/m2 per minute for 1 h (Figure 1A). Two cats were withdrawn from the study before completing all 5 infusion levels due to temperament. When administered at 1 mg/m2 per minute for 1 h, plasma concentration of gemcitabine ranged from < 0.4 μM to 1.7 μM. When administered at 2.5 mg/m2 per minute for 1 h, plasma concentration ranged from 2 to 4.5 μM. Infusions delivered at 5 mg/m2 per minute for 1 h resulted in plasma concentrations that ranged from 3.7 to 12.3 μM. The administration of gemcitabine at 7.5 mg/m2 per minute for 1 h resulted in plasma concentrations that ranged from 4.8 to 8.9 μM. When administered at 10 mg/m2 per minute for 1 h, plasma concentrations ranged from 6.7 to 17 μM. Only 1 out of the 3 cats in this group achieved TPC. The plasma concentration of dFdU (Figure 1B) remained less than 3 μM (range: 0.0 to 2.6 μM).
Figure 1.
Plasma levels of gemcitabine and dFdU after a 1-hour infusion in cats. Gemcitabine was administered at 1, 2.5, and 5 mg/m2 per minute (n = 5 cats) and at 7.5 and 10 mg/m2 per minute (n = 3 cats) for 1 h. Plasma was obtained at the end of the infusion and levels of gemcitabine (A) and dFdU (B) in deproteinated plasma were quantitated by HPLC and plotted using a non-linear fit as described in Materials and methods.
Multiple hour infusions
Thirteen cats were treated in this arm, 3 of which had been previously included in the 1-hour group. A total of fourteen 2-hour infusions (n = 9 cats), a total of seventeen 4-hour infusions (n = 13 cats), and a total of thirteen 6-hour infusions (n = 8 cats) were delivered at 1 (n = 6 cats; 9 infusions), 2.5 (n = 4 cats; 12 infusions), 5 (n = 5 cats; 9 infusions), or 10 (n = 5 cats; 8 infusions) mg/m2 per minute. Data for 6 of the multiple-hour infusions were not collected during the infusion due to patient temperament.
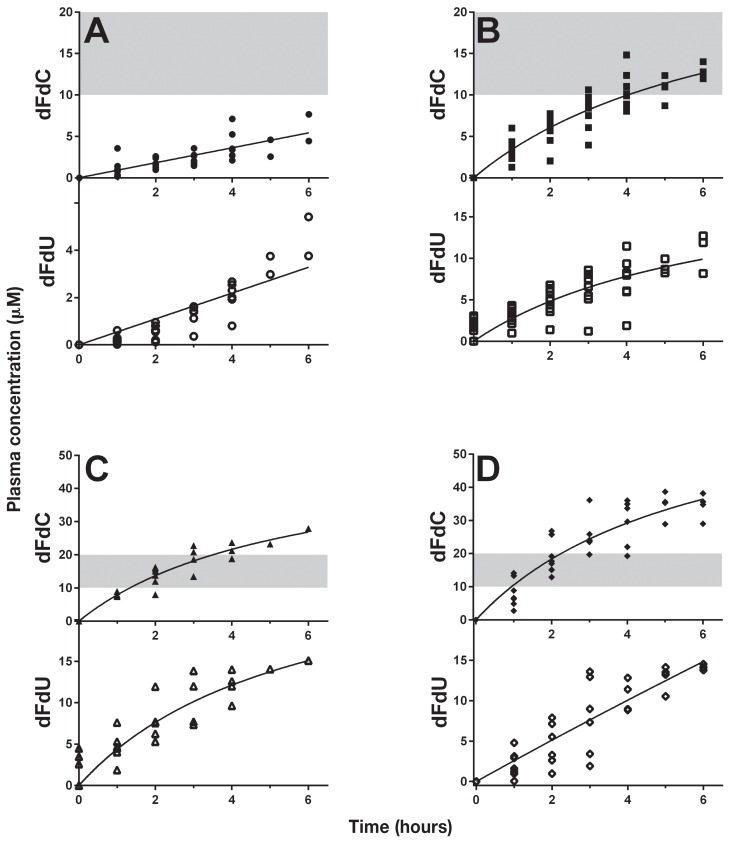
Cats treated with gemcitabine at 1 mg/m2 per minute for up to 6 h had low plasma dFdC concentrations (range: 0.18 to 7.7 μM) (Figure 2A) and the dFdU concentration ranged from 0 to 5.4 μM. Most cats treated with 2.5 mg/m2 per minute achieved TPC (range: 10.1 to 14.8 μM) between 3 and 5 h (Figure 2B). One cat did not achieve a plasma concentration within the TPC range even after a 5-hour infusion, but did so at 6 h. The dFdU concentration at this FDR for 0 to 6 h was a range of 0 to 12.7 μM. Those subjects treated with 5 mg/m2 per minute achieved TPC (range: 12 to 18.8 μM) after 2 h of infusion, began to exceed the TPC range after 3 h, and no cat remained within the TPC range when the infusion was continued beyond 4 h (Figure 2C). The plasma dFdU ranged from 0 to 15.1 μM. Finally, cats treated with 10 mg/m2 per minute (Figure 2D) began to reach TPC within 1 h of infusion (range: 2.0 to 14 μM), and the majority exceeded the TPC when infusions were continued for 2 h or longer (range: 23.8 to 38.2 μM). The dFdU concentration in cats treated at a FDR of 10 mg/m2 per minute for 0 to 6 h ranged from 0 to 14.5 μM. Four cats had detectable dFdU levels (range: 1.3 to 4.5 μM) at 0 h 2 wk after administration of dFdC (Figure 2B–C).
Figure 2.
Fixed-dose-rate gemcitabine in the cat. Cats (n = 15) with various malignancies received gemcitabine (dFdC) delivered as a fixed-dose rate infusion of 1 mg/m2 per minute (A), 2.5 mg/m2 per minute (B), 5 mg/m2 per minute (C), and 10 mg/m2 per minute (D) for 1 to 6 h. Plasma dFdU was quantitated in parallel. Prior to and at each hour during infusion, plasma was collected, deproteinated and then frozen at −80°C until analysis by HPLC. The data was plotted using a non-linear regression line as described in Materials and methods. The shaded area represents the target plasma concentration of gemcitabine in this study. Individual cats may be represented more than once and in more than one graph.
Toxicity
Evaluation of toxicity was not an endpoint of this trial and therefore adverse events were not recorded consistently. However, 4 of the 5 cats treated with 1-hour infusions of various FDR had CBCs and chemistry panels performed 1 wk after infusion. No hematological or biochemical toxicities were observed. Additionally, there were 3 cats (patient numbers 1, 5, 10) treated at 10 mg/m2 per minute (1 treated at 2 h, 2 treated at 6 h) that developed significant bone marrow toxicity. Two out of 3 cats developed a fever, and 2 out of 3 had documented grade IV neutropenia. One of the febrile cats was treated supportively at the referring veterinarian but a record of the grade of neutropenia was unavailable for review. All 3 cats recovered uneventfully after treatment with intravenous fluids and antibiotics. Two out of the 3 cats (patient numbers 1 and 10) went on to receive additional doses of gemcitabine chemotherapy.
Discussion
Gemcitabine is a nucleoside analog with activity against various malignancies in human oncology (1–6). Efficacy in veterinary patients has been limited, and evaluation of an optimal dosing regimen is lacking in small animal patients (12–15,17–21). This is the first in vivo study evaluating plasma concentration of gemcitabine and its metabolite, dFdU, in the cat. In the last 10 y, gemcitabine administration in humans has changed from a high dose for a short period of time to a lower dose for longer, continuous infusions. A study comparing intracellular dFdCTP in PBMCs from patients with pancreatic adenocarcinoma treated with gemcitabine weekly on days 1, 8, and 15 of a 4-week cycle at 2200 mg/m2 over 30 min (73 mg/m2 per minute) versus 1500 mg/m2 over 150 min (10 mg/m2 per minute) (8) proved the utility of this transition. Patients who received the FDR infusion had a 2-fold increase in intracellular dFdCTP concentration in PBMCs used as surrogate tissue, and importantly experienced a survival benefit over those patients treated with the standard 30-minute infusion.
The optimal dose or administration schedule of gemcitabine for cats has not been established. The veterinary literature reports treatments in cats with various malignancies (12–15) using gemcitabine as a 30-minute infusion. These reports are reminiscent of the early dosing in humans, and were in fact extrapolated from human studies (9). In these feline studies when gemcitabine was adminstered weekly or twice weekly, bone marrow and GI toxicity were dose-limiting. Consequently, in order to allow adequate recovery of normal tissues without the potential of compounding toxicity in cats that received multiple infusions, an interval of a minimum of every 2 wk was chosen.
Although evaluation of toxicity was not a goal of this study and limited data were available, when administered once as a 1-hour infusion, there was minimal bone marrow toxicity. We don’t know if administration at more frequent intervals would result in cumulative toxicity. Our data suggest an FDR of 10 mg/m2 per minute for multiple hours may lead to untoward toxicity. In addition, when evaluating Figure 2D, an FDR of 10 mg/m2 per minute at 5 and 6 h exceeded the high end of the TPC; therefore, there would be limited justification for treating cats at this FDR. There were not enough data to draw conclusions about the potential for toxicity in cats treated at 10 mg/m2 per minute for 2 and 4 h. In future studies, complete toxicity evaluation of FDR at various doses (for any length of time) will help guide the determination of the maximally tolerated dose and dose limiting toxicities.
Evaluation of tumor response was also not a primary goal of this study. As designed, the study allowed cats to receive gemcitabine infusions at different dosages for different lengths of time. The time interval between infusions also varied from once every 2 to once very 4 wk. Thus, ascribing response to a specific gemcitabine FDR was difficult. Where response was mentioned in the medical record, 2 cats had subjective responses (patient numbers 1 and 2). In order to achieve the goal of our study, doses of 1, 2.5, 5, 7.5, and 10 mg/m2 per minute delivered over 60 min were initially evaluated to assess for the first time, the range of plasma gemcitabine accumulation that could be achieved. An upper limit of 10 mg/m2 per minute was chosen because this dose is the standard used in humans. Analysis of the 1-hour infusion data revealed that an FDR of 10 mg/m2 per minute provided data points within the TPC in the 3 cats evaluated (Figure 1A). Thus, the first cohort of multiple-hour infusions began at this level. The plasma gemcitabine concentrations from most of the cats treated with multiple hours of an FDR of 10 mg/m2 per minute exceeded the target range by 3 h (Figure 1D). We therefore began the next cohort of cats at 1 mg/m2 per minute (Figure 2A) and all of these cats failed to achieve the TPC even after 6 h. Cats treated with an FDR 2.5 mg/m2 per minute (Figure 2B) did achieve TPC, however, only after 3 h and did so at the low end of the range. When treated at 5 mg/m2 per minute (Figure 2C) the cats in this study remained in TPC for the longest period; however, several data points still exceeded the upper limit. We elected not to treat cats at 7.5 mg/m2 per minute because we anticipated that the levels would be higher than those obtained at 5 mg/m2 per minute.
Prolonged exposure of cells to the active metabolite, dFdCTP, improves efficacy of the drug and may provide clinical benefit to the patients treated (8). It has also been shown that the rate-limiting metabolic activation step requiring the enzyme dCK is saturated at an infusion rate of 10 mg/m2 per minute IV in humans (7). Our data demonstrated that a FDR of 5 mg/m2 per minute provided the longest duration of plasma gemcitabine concentrations in the proposed target range.
For proof of concept, measurement of dFdCTP in tumor cells or incorporation of gemcitabine into DNA would be required. Repeat biopsies of tumor tissue for evaluation of gemcitabine levels in the target cells would further strengthen the findings of this study; however, morbidity to the patient precluded this action.
The breakdown of gemcitabine produces a metabolite, dFdU, which was successfully measured in the cats in this study (Figure 1, 2A–D). When gemcitabine was administered for 1 h at any FDR (Figure 1B) or at a FDR of 1 mg/m2 per minute for up to 6 h (Figure 2A lower graph), the levels of dFdU remained low. Above a FDR of 2.5 mg/m2 per minute, dFdU concentrations rose with increasing duration of infusion, but did not surpass 15.1 μM, despite increasing concentrations of plasma dFdC. This suggests that the degradation of dFdC by feline CDA is a saturable process. While a study evaluating human CDA utilizing dFdC as a substrate has been performed (22), no such study exists for feline CDA. Interestingly, detectable levels of dFdU were found repeatedly in several cats receiving multiple infusions 2 wk after the previous gemcitabine administration (Figure 2B–C, refer to 0 h time-point on the dFdU graphs). While some cats may have dFdU persist in circulation for at least 2 wk, correlation to the FDR level or the duration remains to be determined. This finding may be due to the decreased degradation of gemcitabine observed in the cat (11). This slower degradation may explain the unexpected toxicity observed in cats treated with gemcitabine twice weekly as a radiosensitizer (14) because dFdU itself has been shown to be a radiosensitizer under hypoxic conditions (23).
There were several limitations of this study including small patient number, and lack of complete pharmacokinetic data and evaluation of the intracellular active metabolite. There was also incomplete toxicity and tumor response data, as well as incomplete data collection due to patient compliance in both the 1-hour and multiple-hour studies. The 1-hour infusion cohort was small (3 to 5 cats) and data from this group were meant to guide the remainder of the study. However, there was overlap of the plasma dFdC concentrations at FDR of 5, 7.5, and 10 mg/m2 per minute (Figure 1A). Armed with these data, we anticipated that the FDR that achieved TPC in the cat would be similar to that of humans (i.e., 10 mg/m2 per minute). When we extended infusions to multiple hours, there was great interpatient variability in the plasma dFdC and dFdU at all FDR tested. This interpatient variability has also been seen in human studies (3). These findings highlight the need for quantitation of plasma dFdC in future clinical trials. Lack of complete pharmacokinetic data prevented us from defining an area under the curve that would maximize the time at the TPC. The quantitation of the active intracellular metabolite, dFdCTP or the quantity of gemcitabine incorporated into DNA would provide further evidence that the optimal plasma gemcitabine concentration of 10 to 20 μM in humans is also ideal in cats. Finally, complete toxicity profile and tumor response data were not primary goals of this study and thus were not consistently obtained. Gathering such data in future trials in which a larger cohort of cats are treated with the same FDR for a defined period of time would allow complete evaluation of both adverse events and tumor responses. Additionally, administering a higher dose rate of gemcitabine for a short period of time in order to achieve TPC rapidly followed by a lower FDR over a longer period of time to maintain steady state is a considered goal of future studies.
In conclusion, the FDR to achieve and maintain TPC levels in the cat was found to range between 2.5 and 5 mg/m2 per minute. The optimal dose remains to be determined. Our findings warrant investigation of gemcitabine administered as a FDR to additional cats with neoplasia in order to evaluate therapeutic benefit and treatment-related toxicities. A Phase II study of FDR gemcitabine in cats with cancer is currently underway.
Acknowledgment
This work was funded by a grant from the Center for Companion Animal Health, University of California, Davis, California 95616, USA. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V. Gemcitabine: Metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22:3–10. [PubMed] [Google Scholar]
- 2.Abbruzzese JL. New applications of gemcitabine and future directions in the management of pancreatic cancer. Cancer. 2002;15:941–945. doi: 10.1002/cncr.10753. [DOI] [PubMed] [Google Scholar]
- 3.Abbruzzese JL, Grunewald R, Weeks EA, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 4.Storniolo AM, Allerheiligen SR, Pearce HL. Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol. 1997;24:S7-2–S7-7. [PubMed] [Google Scholar]
- 5.Nabhan C, Krett N, Gandhi V, Rosen S. Gemcitabine in hematologic malignancies. Curr Opin Oncol. 2001;13:514–521. doi: 10.1097/00001622-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Grunewald R, Kantarjian H, Du M, Faucher K, Tarassoff P, Plunkett W. Gemcitabine in leukemia: A phase I clinical, plasma, and cellular pharmacology study. J Clin Oncol. 1992;10:406–413. doi: 10.1200/JCO.1992.10.3.406. [DOI] [PubMed] [Google Scholar]
- 7.Grunewald R, Abbruzzese JL, Tarassoff P, Plunkett W. Saturation of 2′,2′-difluorodeoxycytidine 5′-triphosphate accumulation by mono-nuclear cells during a phase I trial of gemcitabine. Cancer Chemother Pharmacol. 1991;27:258–262. doi: 10.1007/BF00685109. [DOI] [PubMed] [Google Scholar]
- 8.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: Thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 9.Toschi L, Finocchiaro G, Bartolini S, Gioia V, Cappuzzo F. Role of gemcitabine in cancer therapy. Future Oncol. 2005;1:7–17. doi: 10.1517/14796694.1.1.7. [DOI] [PubMed] [Google Scholar]
- 10.Heinemann V, Xu YZ, Chubb S, et al. Cellular elimination of 2′,2′-difluorodeoxycytidine 5′-triphosphate: A mechanism of self-potentiation. Cancer Res. 1992;52:533–539. [PubMed] [Google Scholar]
- 11.O’Brien DR, Guerrero TA, Frazier SA, Rebhun RB, Skorupski KA, Rodriguez CO. Gemcitabine degradation in whole blood from humans, dogs, cats, and horses. J Vet Sci Technol. 2012;3:119. [Google Scholar]
- 12.Martinez-Ruzafa I, Dominguez PA, Dervisis NG, et al. Tolerability of gemcitabine and carboplatin doublet therapy in cats with carcinomas. J Vet Intern Med. 2009;23:570–577. doi: 10.1111/j.1939-1676.2009.0279.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones PD, de Lorimier LP, Kitchell BE, Losonsky JM. Gemcitabine as a radiosensitizer for nonresectable feline oral squamous cell carcinoma. J Am Anim Hosp Assoc. 2003;39:463–467. doi: 10.5326/0390463. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc AK, LaDue TA, Turrel JM, Klein MK. Unexpected toxicity following use of gemcitabine as a radiosensitizer in head and neck carcinomas: A veterinary radiation therapy oncology group pilot study. Vet Radiol Ultrasound. 2004;45:466–470. doi: 10.1111/j.1740-8261.2004.04080.x. [DOI] [PubMed] [Google Scholar]
- 15.Linderman MJ, Brodsky EM, de Lorimier LP, Clifford CA, Post GS. Feline exocrine pancreatic carcinoma: A retrospective study of 34 cases. Vet Comp Oncol. 2013;11:208–218. doi: 10.1111/j.1476-5829.2012.00320.x. [DOI] [PubMed] [Google Scholar]
- 16.Veterinary Cooperative Oncology Group (VCOG) Veterinary cooperative oncology group — Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biologic antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2011:1–30. doi: 10.1111/j.1476-5829.2011.00283.x. [DOI] [PubMed] [Google Scholar]
- 17.Freise KJ, Martin-Jimenez T. Pharmacokinetics of gemcitabine and its primary metabolite in dogs after intravenous bolus dosing and its in vitro pharmacodynamics. J Vet Pharmacol Ther. 2006;29:137–145. doi: 10.1111/j.1365-2885.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 18.Kosarek CE, Kisseberth WC, Gallant SL, Couto CG. Clinical evaluation of gemcitabine in dogs with spontaneously occurring malignancies. J Vet Intern Med. 2005;19:81–86. doi: 10.1892/0891-6640(2005)19<81:ceogid>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Freise KJ, Martin-Jimenez T. Pharmacokinetics of gemcitabine and its primary metabolite in dogs after intravenous infusion. J Vet Pharmacol Ther. 2006;29:147–152. doi: 10.1111/j.1365-2885.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- 20.Marconato L, Lorenzo RM, Abramo F, Couto CG. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet Comp Oncol. 2008;6:90–101. doi: 10.1111/j.1476-5829.2007.00143.x. [DOI] [PubMed] [Google Scholar]
- 21.Turner AI, Hahn KA, Rusk A, et al. Single agent gemcitabine chemotherapy in dogs with spontaneously occurring lymphoma. J Vet Intern Med. 2006;20:1384–1388. doi: 10.1892/0891-6640(2006)20[1384:sagcid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Bouffard DY, Laliberté J, Momparler RL. Kinetic studies on 2′,2′-difluorodeoxycytidine (gemcitabine) with purified human deoxycytidine kinase and cytidine deaminase. Biochem Pharmacol. 1993;45:1857–1861. doi: 10.1016/0006-2952(93)90444-2. [DOI] [PubMed] [Google Scholar]
- 23.Wouters A, Pauwels B, Burrows N, et al. The radiosensitising effect of gemcitabine and its main metabolite dFdU under low oxygen conditions is in vitro not dependent on functional HIF-1 protein. BMC Cancer. 2014;14:594. doi: 10.1186/1471-2407-14-594. [DOI] [PMC free article] [PubMed] [Google Scholar]