Abstract
The antimicrobial susceptibility of canine urinary Escherichia coli (n = 113) isolated by a regional diagnostic laboratory over a 1-year period was determined. Antimicrobial minimum inhibitory concentrations were determined, and those isolates resistant to beta-lactams were screened for broad-spectrum beta-lactamases. Isolates were unexpectedly susceptible, 79.6% were susceptible to all drugs tested and no extended-spectrum beta-lactamases were identified. Our findings indicate that empiric treatment of canine urinary tract infections with first line drugs such as amoxicillin or trimethoprim + sulfamethoxazole is likely to be successful.
Résumé
Résistance antimicrobienne et production de bêta-lactamase par Escherichia coli causant des infections des voies urinaires canines : surveillance passive des isolats de laboratoire à Saskatoon, au Canada, en 2014. La susceptibilité antimicrobienne de la bactérie Escherichia coli (n = 113) d’origine urinaire canine isolée par un laboratoire de diagnostique régional pendant une période de 1 an a été déterminée. Les concentrations inhibitrices minimales d’antimicrobiens ont été déterminées et les isolats résistants aux bêta-lactamines ont été vérifiés pour la production de bêta-lactamases à large spectre. Fait inattendu, les isolats étaient sensibles et 79,6 % étaient sensibles à tous les médicaments testés et aucune bêta-lactamase à large spectre prolongé n’a été identifiée. Nos résultats indiquent que le traitement empirique des infections des voies urinaires canines avec des médicaments de première ligne, comme amoxicilline ou triméthoprime + sulfaméthoxazole, se traduira probablement par un succès du traitement.
(Traduit par Isabelle Vallières)
Escherichia coli is a ubiquitous colonizer of dogs and the most common cause of canine urinary tract infections (UTI) (1). Treatment of UTIs includes the use of antimicrobials, among which the β-lactams (penicillin type drugs) are the most commonly used in veterinary medicine (2). Historically E. coli from the Saskatoon region have been remarkably susceptible to antimicrobials, although a retrospective study identified increases in resistance among diagnostic isolates from 2002 to 2007 (1).
Emerging resistance to the β-lactam antimicrobials is troubling; this diverse antimicrobial class includes the penicillins, cephalosporins, cefamycins, carbapenems, and monobactams, many of which are classified by Health Canada as of very high importance to human health (3). In E. coli, resistance to the β-lactams most often results from the production of degradative enzymes, β-lactamases (4). Broad-spectrum β-lactamases, including the extended spectrum β-lactamases (ESBLs) and the AmpC type enzymes (such as CMY-2), are especially concerning due to their ability to degrade 3rd generation cephalosporins such as ceftriaxone and ceftiofur (5). Since the mid 2000s the emergence of these enzymes among human clinical isolates in Canada has been dramatic. Between 2007 and 2011 the frequency of ESBL producing E. coli among human clinical isolates rose significantly from 3.4% to 7.1% with the CTX-M type enzymes dominating (6). In contrast, almost nothing is known about the occurrence of ESBL-producing E. coli in dogs in Canada (5). The only published Canadian (Ontario) study to date which screened dogs and cats, or their isolates for ESBLs, failed to identify these genes (7).
The purpose of this investigation was to describe the antimicrobial susceptibility profiles of E. coli grown from canine urinary samples by a regional diagnostic laboratory. Additionally, this investigation aimed to describe the presence of genes for broad-spectrum β-lactamases among these isolates.
Over a 12-month period from October 2013 to 2014, all urinary E. coli isolated from dogs by Prairie Diagnostic Services in Saskatoon, Saskatchewan were saved for analysis. Urine samples submitted for analysis are routinely cultured on sheep’s blood and MacConkey agars; E. coli are identified based on colony morphology, lactose fermentation and the spot indole test. A total of 113 isolates were frozen at −80°C in tryptic soy broth plus 10% glycerol for future analysis. Antimicrobial minimum inhibitory concentrations were determined by broth micro-dilution using the Sensititre system (Trek Diagnostics, Cleveland Ohio, USA). Ampicillin (AMP), amoxicillin plus clavulanate (AUG), cefoxitin (FOX), ceftiofur (TIO), ceftriaxone (AXO), tetracycline (TET), trimethoprim plus sulfamethoxazole (SXT), sulfisoxazole (SOX), chloramphenicol (CHL), nalidixic acid (NAL), ciprofloxacin (CIP), and gentamicin (GEN) were included on test panels. Testing was performed and interpreted in accordance with the manufacturer’s instructions and the Clinical and Laboratory Standards Institute guidelines (8,9). For quality control, E. coli ATCC 25922 and S. aureus 29213 were tested in parallel. Isolates resistant to amoxicillin plus clavulanic acid, ceftiofur or ceftriaxone were screened for CTX-M, SHV and TEM type ESBLs and group 2 CMY type β-lactamases by polymerase chain reaction (PCR) using previously published primers (10–13). Positive controls (organisms previously identified to possess each gene) and no template controls were included with each set of PCR reactions for quality control. Following PCR amplification, products were sent for sequencing to a commercial laboratory (Macrogen, Seoul, Korea) to identify the allele amplified.
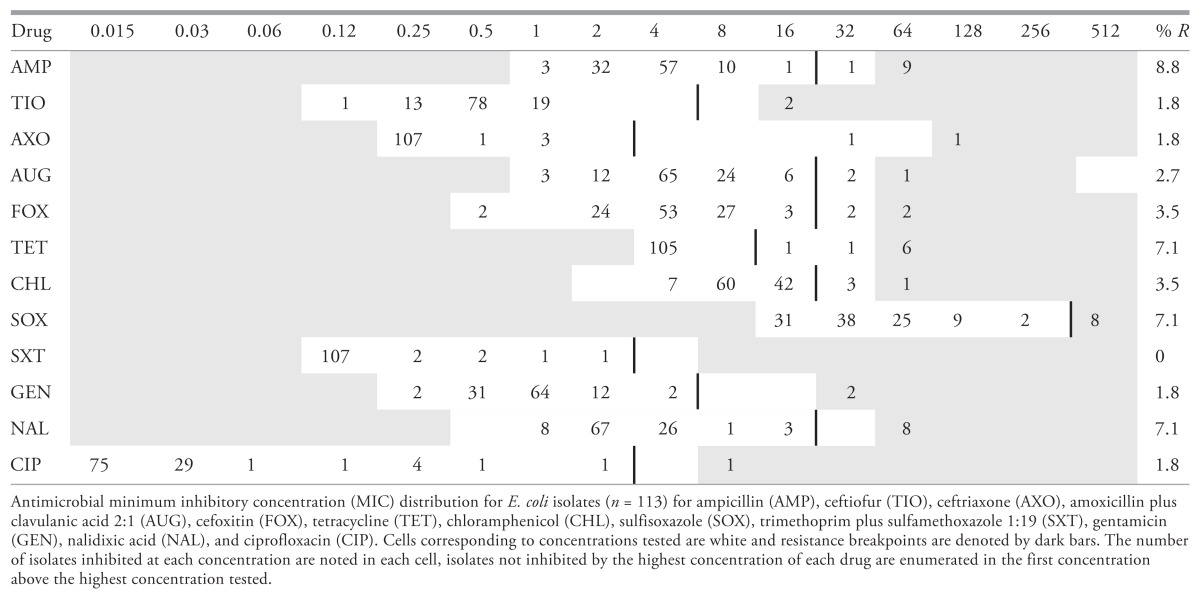
Isolates were remarkably susceptible; the most common profile identified was pan-susceptibility detected in 90 (79.6%) isolates (Table 1). Resistance to ampicillin was most common, occurring in 10 (8.8%) isolates, followed by tetracycline, sulfisoxazole, and nalidixic acid which were each identified in 8 (7.1%) isolates (Table 2). Resistance to trimethoprim plus sulfamethoxazole was not detected. Resistance to all other drugs occurred in fewer than 5% of isolates. Six multidrug resistant isolates (resistant to 3 or more classes) were identified, including 1 resistant to the β-lactams, tetracycline, nalidixic acid, and ciprofloxacin and another resistant to the β-lactams, tetracycline, nalidixic acid, sulfisoxazole, and gentamicin. Both of these isolates carried the CMY-2 gene, an AmpC type β-lactamase which confers resistance to 3rd generation cephalosporins, potentiated penicillins, and cefoxitin (5). No ESBLs were detected.
Table 1.
Antimicrobial susceptibility profiles and β-lactamases identified from canine urinary E. coli isolates (n = 113) collected over 12 months (beginning in November, 2013) by a regional diagnostic laboratory in Saskatoon, Canada
| Phenotype | Number (%) isolates |
|---|---|
| Pan-susceptible | 90 (79.6%) |
| AMP | 5 (4.4%) |
| SOX | 3 (2.7%) |
| TET | 3 (2.7%) |
| CHL | 1 (0.9%) |
| NAL | 1 (0.9%) |
| CHL + NAL | 1 (0.9%) |
| NAL + SOX | 1 (0.9%) |
| TET + NAL | 1 (0.9%) |
| AMP + AUG | 1 (0.9%) |
| AMP + NAL + GEN | 1 (0.9%) |
| FOX + CHL + SOX | 1 (0.9%) |
| AMP + TET + NAL + SOX | 1 (0.9%) |
| FOX + CHL + TET + SOX | 1 (0.9%) |
| AMP + FOX + AUG + AXO + TIO + TET + NAL + CIP | 1 (0.9%)a |
| AMP + FOX + AUG + AXO + TIO + TET + NAL + SOX + GEN | 1 (0.9%)a |
Isolate carried the CMY-2 type β-lactamase gene.
Table 2.
Minimum inhibitory concentration distribution of canine urinary E. coli isolates isolated over 12 months (beginning in November, 2013) by a regional diagnostic laboratory in Saskatoon, Canada
The findings of this study indicate that antimicrobial resistance is not common among canine E. coli in our region, and that first line drugs such as amoxicillin are likely to be effective empiric therapeutic options. Although relatively few data are available, the frequency of resistance identified in this investigation is very low compared to other regions (5,14). A recent, multi-regional study in the United States conducted between 2008 and 2013 identified higher rates of resistance to ampicillin, amoxicillin plus clavulanate, trimethoprim plus sulfamethoxazole, chloramphenicol, and ciprofloxacin than were found in the present investigation (14). Globally, companion animal infections with E. coli producing ESBLs and CMY-2 type β-lactamases have been reported from similar studies of diagnostic isolates in Europe and the United States (5). Perhaps most alarmingly was the recent description of E. coli producing the NDM-1 carbapenemase isolated from 5 dogs and 1 cat (including 4 urinary isolates) in the United States (15).
We aim to continue our surveillance activities to detect the emergence of resistance, and provide timely local data to practitioners to facilitate the evidence-based use of antimicrobials. Furthermore, we believe there would be great value in the development of a harmonized resistance surveillance strategy targeting companion animal pathogens including urinary E. coli. We encourage the veterinary diagnostic community to pursue such collaborative efforts. Consistent with descriptions of increasing resistance among human E. coli isolates, we anticipate identifying an increasing frequency of broad-spectrum β-lactamases among canine urinary isolates. Although resistance was infrequently identified, we stress the importance of culture and susceptibility testing to ensure that antimicrobial therapy is evidence-based in line with diagnostic and therapeutic best practices (16).
Acknowledgments
The authors thank Zoetis Canada for providing a grant in support of this research and for stipend support for RC. We also thank Prairie Diagnostic Services for supplying diagnostic isolates. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Ball KR, Rubin JE, Chirino-Trejo M, Dowling PM. Antimicrobial resistance and prevalence of canine uropathogens at the Western College of Veterinary Medicine Veterinary Teaching Hospital, 2002–2007. Can Vet J. 2008;49:985–990. [PMC free article] [PubMed] [Google Scholar]
- 2.Prescott JF, Hanna WJ, Reid-Smith R, Drost K. Antimicrobial drug use and resistance in dogs. Can Vet J. 2002;43:107–116. [PMC free article] [PubMed] [Google Scholar]
- 3.Categorization of Antimicrobial Drugs Based on Importance in Human Medicine. Health Canada. 2009. [Last accessed August 24, 2016]. Available from: http://www.hc-sc.gc.ca/dhp-mps/vet/antimicrob/amr_ram_hum-med-rev-eng.php.
- 4.Livermore DM, Woodford N. The beta-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 2006;14:413–420. doi: 10.1016/j.tim.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Rubin JE, Pitout JDD. Extended-spectrum β-lactamase, carbapenemase and AmpC producing Enterobacteriaceae in companion animals. Vet Microbiol. 2014;170:10–18. doi: 10.1016/j.vetmic.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Denisuik AJ, Lagace-Wiens PR, Pitout JD, et al. Molecular epidemiology of extended-spectrum beta-lactamase-, AmpC beta-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007–11. J Antimicrob Chemother. 2013;68( Suppl 1):i57–65. doi: 10.1093/jac/dkt027. [DOI] [PubMed] [Google Scholar]
- 7.Murphy C, Reid-Smith RJ, Prescott JF, et al. Occurrence of antimicrobial resistant bacteria in healthy dogs and cats presented to private veterinary hospitals in southern Ontario: A preliminary study. Can Vet J. 2009;50:1047–1053. [PMC free article] [PubMed] [Google Scholar]
- 8.CLSI. VET01-S2 Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; second informational supplement. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 9.CLSI. M100-S24 Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2014. [Google Scholar]
- 10.Pitout JD, Hossain A, Hanson ND. Phenotypic and molecular detection of CTX-M-beta-lactamases produced by Escherichia coli and Klebsiella spp. J Clin Microbiol. 2004;42:5715–5721. doi: 10.1128/JCM.42.12.5715-5721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasman H, Mevius D, Veldman K, Olesen I, Aarestrup FM. beta-lactamases among extended-spectrum beta-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J Antimicrob Chemother. 2005;56:115–121. doi: 10.1093/jac/dki190. [DOI] [PubMed] [Google Scholar]
- 12.Arlet G, Rouveau M, Philippon A. Substitution of alanine for aspartate at position 179 in the SHV-6 extended-spectrum beta-lactamase. FEMS Microbiol Lett. 1997;152:163–167. doi: 10.1016/s0378-1097(97)00196-1. [DOI] [PubMed] [Google Scholar]
- 13.List of primers for detection of antimicrobial resistance genes version 15.09.2011. Technical University of Denmark ERLfAR; Lyngby, Denmark: [Last accessed August 24, 2016]. Available from: www.crl-ar.eu/201-resources.htm. [Google Scholar]
- 14.Thungrat K, Price SB, Carpenter DM, Boothe DM. Antimicrobial susceptibility patterns of clinical Escherichia coli isolates from dogs and cats in the United States: January 2008 through January 2013. Vet Microbiol. 2015;179:287–295. doi: 10.1016/j.vetmic.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Shaheen BW, Nayak R, Boothe DM. Emergence of a New Delhi metallo-beta-lactamase (NDM-1)-encoding gene in clinical Escherichia coli isolates recovered from companion animals in the United States. Antimicrob Agents Chemother. 2013;57:2902–2903. doi: 10.1128/AAC.02028-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olin SJ, Bartges JW. Urinary tract infections: Treatment/comparative therapeutics. Vet Clin North Am Small Anim Pract. 2015;45:721–746. doi: 10.1016/j.cvsm.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]