Abstract
Introduction
Periodontal disease is an inflammatory condition caused by periodontal microorganisms. Viruses such as human cytomegalovirus (HCMV) and Epstein–Barr virus (EBV) are associated with certain types of periodontal disease, but their roles in promoting the disease are unclear. Because both viruses infect human macrophages, cells which play key roles in the clearance of pathogenic bacteria, it is likely that the viruses alter the functional capacity of macrophages by inhibiting their defense mechanisms against invading pathogens.
Methods
Macrophages preinfected with HCMV or EBV were evaluated following stimulation by selected oral bacteria. Bacteria-induced macrophage activation was assayed by measuring the levels of tumor necrosis factor-α (TNF-α) produced in the media, and phagocytic activity was analysed by a phagocytosis assay with fluorescein isothiocyanate-labeled bacteria. The virus-infected macrophages were also subjected to semi-quantitative polymerase chain reaction to measure the expression of toll-like receptor 9, which is involved in the activation of phagocytosis-related pathways.
Results
Both HCMV and EBV significantly diminished the TNF-α production typically induced by oral bacteria, inhibited the phagocytic activity of macrophages, and downregulated the expression of toll-like receptor 9.
Conclusion
Infection by HCMV or EBV inhibits the functional ability of macrophages to respond to bacterial challenge, thereby suggesting their pathogenic role in the development of periodontal disease.
Keywords: cytomegalovirus, Epstein-Barr virus, macrophages, phagocytosis
Periodontitis is a disease resulting from polymicrobial infections. Many oral bacteria, such as Porphyromonas gingivalis, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans, contribute to the pathogenesis of periodontitis. Macrophages are one of the key immune components that clear pathogenic bacteria in and around the gingival tissue. However, overly activated macrophages, along with other inflammatory cells, produce excessive amounts of proinflammatory cytokines that can lead to the destruction of gingival tissues and the tooth-supporting bone. In this way, the immune response of macrophages influences the development of periodontal disease.
Although bacteria play an essential etiological role in periodontal disease, two human herpesviruses, human cytomegalovirus (HCMV) and Epstein–Barr virus (EBV), are found to be associated with certain types of periodontitis. Coinfection by both viruses and bacteria apparently leads to a synergistic effect that exacerbates the progress of periodontal diseases (3–5). The DNA of both viruses has been detected in gingival tissue, gingival crevicular fluid and subgingival plaque from the periodontal lesions. HCMV is frequently detected at diseased sites of severe adult periodontitis, localized and generalized aggressive (juvenile) periodontitis, Papillon–Lefèvre syndrome periodontitis, acute necrotizing ulcerative gingivitis and periodontal abscesses (18, 21). In addition, active HCMV replication has been demonstrated in the periodontal lesions (2), and a recent study shows that HCMV infection leads to the increased adherence of A. actinomycetemcomitans to oral epithelial cells (26). EBV has also been increasingly detected in dental plaques and gingival tissues as a result of advances in polymerase chain reaction (PCR) technology (15, 16). The virus appears to be associated with aggressive, apical and marginal types of periodontitis (22, 23), and patients exhibiting recurrent periodontal disease with high subgingival EBV load respond well to antiviral treatment that decreases the presence of EBV, resulting in a dramatic improvement of their periodontal condition (24). Studies of clinical samples show that the prevalence of HCMV or EBV is strongly associated with the presence of periodontal pathogenic bacteria (7, 23).
HCMV infects monocytes/macrophages and establishes long-term latency in these cells (14). EBV primarily infects and establishes latent infection in B cells but also infects monocytes/macrophages (19). The EBV-infected monocytes/macrophages facilitate transmission of the virus to the oral epithelium, where EBV actively replicates and is released into the saliva (27). Both HCMV and EBV have the ability to manipulate host immune responses through a variety of mechanisms. HCMV induces the production of immune inhibitory cytokines, such as viral- and cell-derived interleukin-10 (13, 17, 28), and inhibits the differentiation of macrophages (12). EBV-infected macrophages exhibit downregulated tumor necrosis factor-α (TNF-α) induction by lipopolysaccharide (11), as well as inhibited phagocytic activity (25). In this study, we investigate whether infection with HCMV or EBV alters the functional ability of macrophages to respond to bacterial challenge. Our findings show that both HCMV and EBV downregulate oral bacteria-induced macrophage activation and inhibit their phagocytic activity.
Materials and methods
Cells, viruses and bacteria
The THP-1 monocytic cell line was maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum. Human primary macrophages were separated from Ficoll-purified peripheral blood mononuclear cells (PBMC) by adherence to tissue culture plates. The purchase and use of human PBMC from a local blood bank were approved by the Institutional Review Board of the University of Kentucky. The HCMV AD169 strain was obtained from the National Institute of Health AIDS reference reagent program and amplified in MRC-5 cells. EBV was collected from the medium of B95.8 cells. The virus titers were determined by real-time PCR. Oral bacteria P. gingivalis, F. nucleatum, A. actinomycetemcomitans, and Streptococcus gordonii were grown in an anaerobic chamber with appropriate media.
TNF-α induction
THP-1 cells were induced to differentiate into macrophages by adding 12-O-tetradecanoylphorbol-13-acetate (TPA, 20 ng/ml) to the cultures for 24 h. The cells were then maintained in regular medium for 4 h. The virus infection was carried out by inoculation with HCMV or EBV for 2 h at a multiplicity of infection (MOI) of one. The virus-infected cells were incubated overnight before stimulation with oral bacteria: P. gingivalis, F. nucleatum, A. actinomycetemcomitans, or S. gordonii. Culture media were collected for enzyme-linked immunosorbent assay (ELISA) analysis of TNF-α concentration after 5 h of stimulation. ELISA was performed with a TNF-α detection kit (eBioscience, San Diego, CA) according to the manufacturer's instructions. For the primary macrophages, the isolated cells were incubated in complete RPMI-1640 medium for 24 h, after which viral infection and bacterial stimulation were performed similarly as described for the THP-1 cells.
Semi-quantitative PCR
Total RNA from virus-infected and non-infected THP-1-derived macrophages were prepared using Trizol reagent (Invitrogen, Carlsbad, CA), and first-strand complementary DNA (cDNA) was synthesized with the transcriptor high fidelity cDNA synthesis kit (Roche, Indianapolis, IN). Toll-like receptor 9 (TLR9) message was amplified by PCR with the forward primer 5′-CAA CAA CCT CAC TGT GGT GC-3′ and the reverse primer 5′-GAG TGA GCG GAA GAA GAT GC-3′ in 31 cycles. PCR amplification of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to show equivalent cDNA input.
Fluorescein isothiocyanate-labeling of Escherichia coli and phagocytosis assay
Fluorescein isothiocyanate (FITC) was purchased from Sigma-Aldrich Chemicals (St Louis, MO). Escherichia coli were washed once with phosphate-buffered saline (PBS), resuspended at 1 × 1010 bacteria in 1 ml FITC/NaHCO3 buffer (0.1 mg/ml FITC in 0.1 m NaHCO3, pH 9.0), and incubated at room temperature for 1 h. The unconjugated FITC was then removed by washing the bacteria five times with PBS. For the phagocytosis assays, FITC-labeled E. coli were added to THP-1-derived macrophages at MOI 100 and incubated together for 30 min in antibiotic-free medium. The cells were then fixed with formalin for 30 min. Ethidium bromide (5 μg/ml in PBS) was added to the sample to quench the FITC signals of the extracellular bacteria. Digital pictures were taken immediately using a fluorescence microscope. Eight randomly selected fields were photographed for each sample, and FITC-positive bacteria as well as the total cell number were enumerated using NIH image J software (National Institutes of Health, Bethesda, MD).
Statistic analysis
The results were obtained from three independent experiments performed in quadruplicate. A standard Student's two-tailed t-test was performed to compare two mean values, and data were presented as mean values ± standard deviation (SD). The results were considered statistically significant when P < 0.05.
Results
Inhibition of oral bacteria-induced TNF-α production
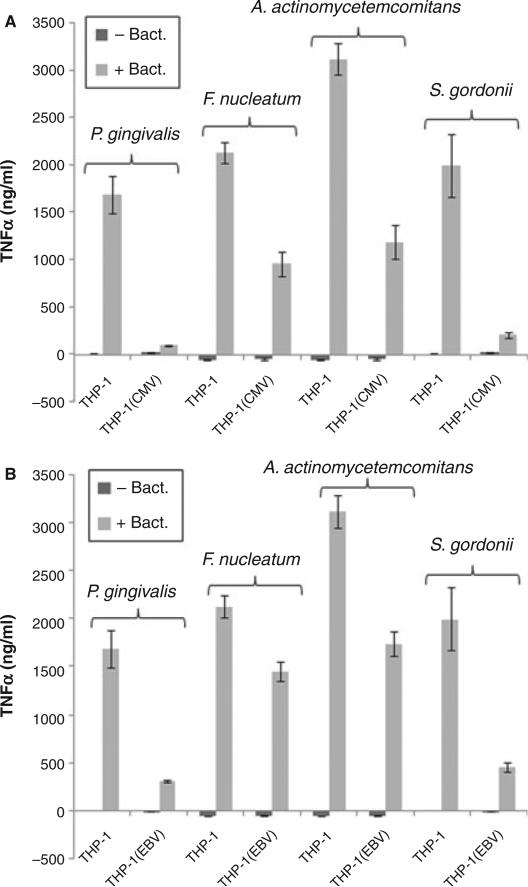
Widely used to study macrophage differentiation and functions, THP-1 cells are also used as a model for HCMV latent infection. Using THP-1-derived macrophages, we first determined whether or not HCMV infection could alter their activation in response to stimulation by oral bacteria. Measuring TNF-α as an indicator of macrophage activation, we tested the effects of four oral bacteria, P. gingivalis, F. nucleatum, A. actinomycetemcomitans, and S. gordonii. THP-1 cells were first induced to differentiate into macrophages with TPA and then infected with HCMV. As shown in Fig. 1A, all four oral bacteria tested in the experiment were able to induce THP-1-derived macrophages to produce varying levels of TNF-α. F. nucleatum and A. actinomycetemcomitans were able to induce high levels of TNF-α at a bacteria-to-cell ratio of 10 : 1, while much higher bacterial inputs were needed to induce a similar level of TNF-α for P. gingivalis (ratio of 100 : 1) and S. gordonii (ratio of 1000 : 1). HCMV by itself did not induce TNF-α production in the macrophages, but the viral infection significantly inhibited bacteria-induced TNF-α production for each type of bacteria tested. Similar outcomes were obtained when using EBV as the infectious agent (Fig. 1B). These results indicate that HCMV and EBV infections inhibit the activation of macrophages.
Fig. 1.
Inhibition of oral bacteria-induced tumor necrosis factor-α (TNF-α) production by human cytomegalovirus (HCMV) and Epstein–Barr virus (EBV). THP-1-derived macrophages were infected with HCMV (A) or EBV (B). Twenty-four hours after the infection, cells were challenged with the indicated bacteria for 5 h, and the TNF-α concentration was measured by enzyme-linked immunosorbent assay. The mean and standard deviation are shown as column and bar, respectively.
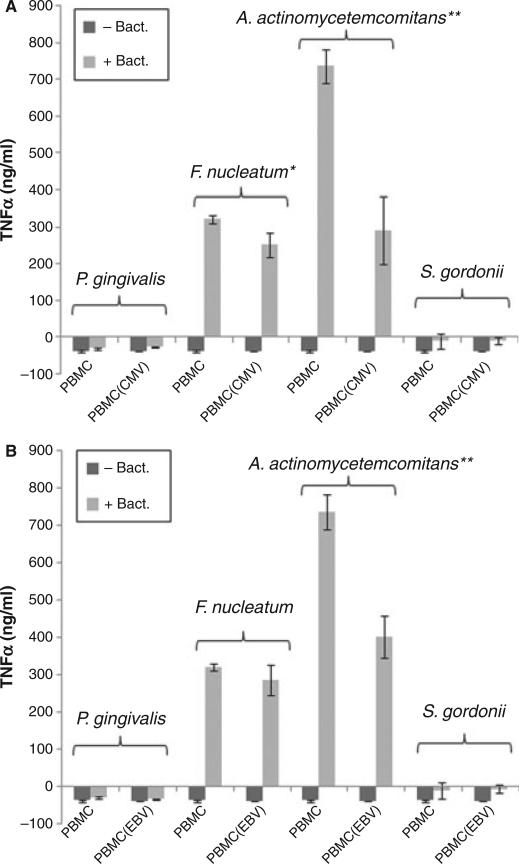
To confirm that HCMV and EBV can inhibit bacterially associated TNF-α production in macrophages, we repeated the experiments using human primary macrophages. Human primary macrophages were isolated from Ficoll-purified PBMC by attachment to tissue culture plates. Twenty-four hours after attachment, the macrophages were infected with HCMV or EBV for 2 h. The virus-infected cells were then incubated in regular culture media overnight for recovery before stimulation by oral bacteria. As shown in Fig. 2, F. nucleatum and A. actinomycetemcomitans induced TNF-α production in the primary macrophages, while P. gingivalis and S. gordonii were unable to do so. In addition, the levels of F. nucleatum-induced and A. actinomycetemcomitans-induced TNF-α in these cells were significantly lower than that in THP-1-derived macrophages. Furthermore, F. nucleatum-induced TNF-α production was only modestly inhibited by HCMV or EBV, whereas A. actinomycetemcomitans-induced TNF-α production in the primary macrophages was significantly inhibited by the viruses.
Fig. 2.
Inhibition of oral bacteria-induced tumor necrosis factor-α (TNF-α) production by human cytomegalovirus (HCMV) and Epstein–Barr virus (EBV) in human primary macrophages. Human macrophages were isolated from PBMC and infected with HCMV (A) or EBV (B). Twenty-four hours after the infection, cells were challenged with the indicated bacteria for 5 h, and the TNF-α concentration was measured by enzyme-linked immunosorbent assay. The mean and standard deviation are shown as column and bar, respectively. A Student's t-test was performed, whereby * and ** indicate values of P < 0.05 and P < 0.005, respectively.
Inhibition of phagocytic activity of macrophages
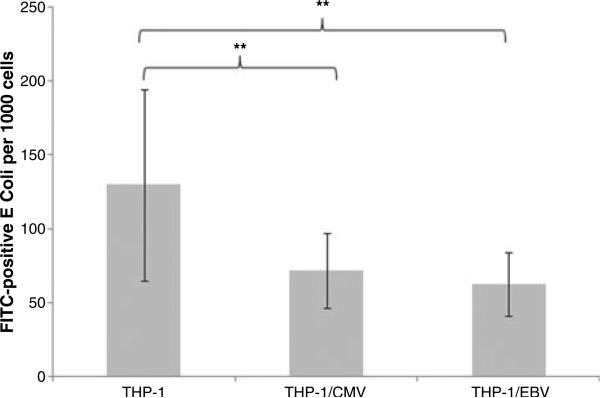
To determine whether or not HCMV and EBV infections could alter the phagocytic activity of macrophages, we performed phagocytosis assays in which THP-1-derived macrophages were incubated with FITC-labeled E. coli. Non-specific FITC signals from extracellular (non-phagocytosed) E. coli were quenched by ethidium bromide, while fluorescence signals from internalized bacteria remained unaffected. Consequently, the FITC-positive bacteria and total cell number in each microscopic field were enumerated. As shown in Fig. 3, HCMV and EBV infections significantly inhibited the phagocytic activity of the macrophages.
Fig. 3.
Inhibition of phagocytic activity by human cytomegalovirus (HCMV) and Epstein–Barr virus (EBV). THP-1-derived macrophages were infected with HCMV or EBVas previously described and then incubated with fluorescein isothiocyanate (FITC)-labeled bacteria for 30 min. The internalized bacteria and the macrophages were counted from eight randomly selected microscopic fields. The number of bacteria per 1000 cells was calculated. The mean and standard deviation are shown as column and bar, respectively. A Student's t-test was performed, whereby ** indicates values of P < 0.005.
Downregulation of TLR9
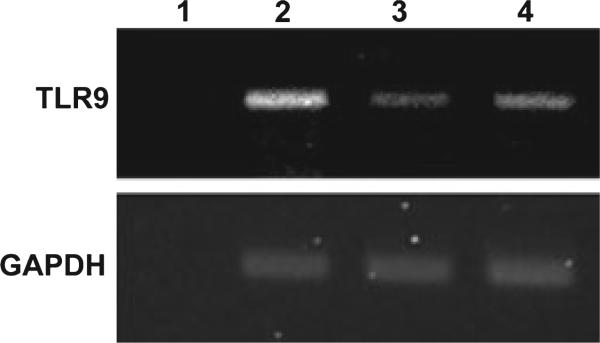
Toll-like receptors function as biological sensors that recognize a wide variety of microbes, including bacteria, viruses, and fungi. TLR9 was recently found to play an important role in triggering the signals for phagocytosis in macrophages (8). In this study, we investigated whether or not the expression of TLR9 was altered after HCMV and EBV infection. We performed semi-quantitative PCR to compare the expression levels of TLR9 between non-infected macrophages, HCMV-infected cells, and EBV-infected cells. The GAPDH gene was used as an internal control to verify equivalent cDNA input between samples. As shown in Fig. 4, TLR9 expression was downregulated in both HCMV-infected and EBV-infected macrophages.
Fig. 4.
Inhibition of toll-like receptor-9 (TLR9) expression by human cytomegalovirus (HCMV) and Epstein–Barr virus (EBV). THP-1-derived macrophages infected with HCMVor EBV were collected for RNA extraction 24 h postinfection. Complemetary DNA (cDNA) were synthesized, and semi-quantitative polymerase chain reaction was performed. Lane 1, no cDNA control; lane 2, non-infected macrophages; lane 3, HCMV-infected macrophages; and lane 4, EBV-infected macrophages. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was used as a control to show equivalent inputs of cDNA in all the samples.
Discussion
In recent years, accumulated data have shown that HCMV and EBV are associated with the development of certain types of periodontitis. These viruses presumably play a role in promoting the disease. It is not well understood how periodontal HCMV and EBV influence host immune responses to the challenge of oral pathogens. Both viruses are able to infect monocytes/macrophages, indicating their ability to potentially limit the inherent function of macrophages to eradicate invading microbial pathogens.
In this study, we first investigated whether or not HCMV and EBV can inhibit the activation of macrophages when challenged by four different strains of oral bacteria, P. gingivalis, F. nucleatum, A. actinomycetemcomitans, and S. gordonii, some of which typically cause periodontal disease. Using TNF-α as a marker for macrophage activation, our data showed that the tested oral bacteria differentially activated THP-1-derived macrophages. Periodontal pathogens P. gingivalis, F. nucleatum and A. actinomycetemcomitans were able to induce high levels of TNF-α production at low or medium bacteria : cell ratios. The non-pathogenic S. gordonii induced TNF-α only when a much higher bacteria : cell ratio was used, suggesting that macrophages were relatively unresponsive to commensal bacteria. Both HCMV and EBV downregulated bacteria-induced TNF-α production, indicating that this could be a mechanism used by the viruses to inhibit the activation of macrophages when challenged by microorganisms. When the experiment was performed with primary macrophages, we observed reduced TNF-α induction by the bacteria. We found that F. nucleatum and A. actinomycetemcomitans, but not P. gingivalis and S. gordonii, were able to induce TNF-α. In addition, the levels of the induced TNF-α were several-fold lower than what was observed in THP-1 cells. The discrepancy between THP-1-derived macrophages and the primary macrophages was probably the result of the heterogeneous population of the primary macrophages responding to oral bacteria differently.
In this study, we showed that HCMV and EBV could inhibit the phagocytic ability of macrophages. In assessing the feasibility of using FITC-labeled oral bacteria for the phagocytosis assay, we found that the labeling condition was not optimal for P. gingivalis and F. nucleatum: poor labeling for P. gingivalis and aggregation for F. nucleatum (data not shown). However, given that P. gingivalis, F. nucleatum, and A. actinomycetemcomitans are able to actively invade cells, the definitive cause for internalization of labeled bacteria – phagocytosis or invasion – may need to be resolved using a combination of alternative labeling conditions and killed bacteria.
The inhibition of phagocytosis by HCMV and EBV may be involved in multiple ways. It has been reported that HCMV downregulates complement receptors (CRs) that mediate phagocytosis. Because HCMV suppresses the expression of CR3 and CR4, it inhibits CR-mediated phagocytosis in macrophages (10). In addition, HCMV inhibits cytokine-induced macrophage differentiation (12). EBV inhibits the activation of monocytes by suppressing the biosynthesis of prostaglandin E2 (20). Furthermore, EBV infects monocytes and reduces the phagocytic ability of these cells by blocking protein kinase C activity (19, 25). In this study, we found that HCMV and EBV downregulated the expression of TLR9. TLR9 is part of the innate immune system and functions as a sensor for microbial DNA sequences containing unmethylated CpG dinucleotides. A recent study showed that activation of the TLR9 signaling pathway not only promotes phagocytosis, but also induces the expression of genes involved in the phagocytic program (8). Consequently, downregulation of TLR9 expression may contribute to the reduced phagocytic activity of macrophages caused by HCMV and EBV infection.
In summary, our data showed that HCMV and EBV inhibited macrophage activation induced by oral bacteria and inhibited the phagocytic ability of these cells. The reduced function of macrophages was associated with the downregulation of TLR9 expression by the viruses. The function of macrophages can be altered by viral infection in various ways. For example, human immunodeficiency virus-infected macrophages show impaired phagocytosis and killing of Toxoplasma gondii and Candida albicans (1, 6), while macrophages infected by respiratory syncytial virus show increased phagocytic activity (9). The development of periodontal disease is determined by the outcome of interactions between host and a variety of periodontal microorganisms. Although functions of periodontal macrophages have been known to be influenced by factors associated with bacterial infection, they can also be affected by viral infection, as indicated by the current study. While there are overlapping abilities among viruses in affecting macrophage functions, each virus exhibits its own unique property as well. Our data indicate that HCMV and EBV may contribute to the development of certain types of periodontal disease by inhibiting phagocytic clearance of pathogenic bacteria and by reducing host immune response via TNF-α production.
Acknowledgments
This project was supported by Grant RFA-RR-03-014 from the National Center for Research Resources (NCRR), a component of National Institutes of Health (NIH), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- 1.Biggs BA, Hewish M, Kent S, Hayes K, Crowe SM. HIV-1 infection of human macrophages impairs phagocytosis and killing of Toxoplasma gondii. J Immunol. 1995;154:6132–6139. [PubMed] [Google Scholar]
- 2.Cappuyns I, Gugerli P, Mombelli A. Viruses in periodontal disease – a review. Oral Dis. 2005;11:219–229. doi: 10.1111/j.1601-0825.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- 3.Contreras A, Slots J. Herpesviruses in human periodontal disease. J Periodontal Res. 2000;35:3–16. doi: 10.1034/j.1600-0765.2000.035001003.x. [DOI] [PubMed] [Google Scholar]
- 4.Contreras A, Umeda M, Chen C, Bakker I, Morrison JL, Slots J. Relationship between herpesviruses and adult periodontitis and periodontopathic bacteria. J Periodontol. 1999;70:478–484. doi: 10.1902/jop.1999.70.5.478. [DOI] [PubMed] [Google Scholar]
- 5.Contreras A, Zadeh HH, Nowzari H, Slots J. Herpesvirus infection of inflammatory cells in human periodontitis. Oral Microbiol Immunol. 1999;14:206–212. doi: 10.1034/j.1399-302x.1999.140402.x. [DOI] [PubMed] [Google Scholar]
- 6.Crowe SM, Vardaxis NJ, Kent SJ, et al. HIV infection of monocyte-derived macrophages in vitro reduces phagocytosis of Candida albicans. J Leukoc Biol. 1994;56:318–327. doi: 10.1002/jlb.56.3.318. [DOI] [PubMed] [Google Scholar]
- 7.Ding F, Feng XH, Meng HX, et al. Relationship between herpesviruses and periodontal pathogenic bacteria in subgingival plaque. Beijing Da Xue Xue Bao. 2008;40:318–322. [PubMed] [Google Scholar]
- 8.Doyle SE, O'Connell RM, Miranda GA, et al. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke-Ullmann G, Pfortner C, Walter P, et al. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J Immunol. 1995;154:268–280. [PubMed] [Google Scholar]
- 10.Gafa V, Manches O, Pastor A, et al. Human cytomegalovirus downregulates complement receptors (CR3, CR4) and decreases phagocytosis by macrophages. J Med Virol. 2005;76:361–366. doi: 10.1002/jmv.20358. [DOI] [PubMed] [Google Scholar]
- 11.Gosselin J, Menezes J, Addario M, et al. Inhibition of tumor necrosis factor-alpha transcription by Epstein–Barr virus. Eur J Immunol. 1991;21:203–208. doi: 10.1002/eji.1830210130. [DOI] [PubMed] [Google Scholar]
- 12.Gredmark S, Tilburgs T, Soderberg-Naucler C. Human cytomegalovirus inhibits cytokine-induced macrophage differentiation. J Virol. 2004;78:10378–10389. doi: 10.1128/JVI.78.19.10378-10389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins C, Garcia W, Godwin MJ, et al. Immunomodulatory properties of a viral homolog of human interleukin-10 expressed by human cytomegalovirus during the latent phase of infection. J Virol. 2008;82:3736–3750. doi: 10.1128/JVI.02173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo K. Persistent/latent infection of human beta-herpesvirus. Nippon Rinsho. 1998;56:83–89. [PubMed] [Google Scholar]
- 15.Konstantinidis A, Sakellari D, Papa A, Antoniadis A. Real-time polymerase chain reaction quantification of Epstein–Barr virus in chronic periodontitis patients. J Periodontal Res. 2005;40:294–298. doi: 10.1111/j.1600-0765.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 16.Kubar A, Saygun I, Ozdemir A, Yapar M, Slots J. Real-time polymerase chain reaction quantification of human cytomegalo-virus and Epstein–Barr virus in periodontal pockets and the adjacent gingiva of periodontitis lesions. J Periodontal Res. 2005;40:97–104. doi: 10.1111/j.1600-0765.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin YL, Chang PC, Wang Y, Li M. Identification of novel viral interleukin-10 isoforms of human cytomegalovirus AD169. Virus Res. 2008;131:213–223. doi: 10.1016/j.virusres.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalowicz BS, Ronderos M, Camara-Silva R, Contreras A, Slots J. Human herpesviruses and Porphyromonas gingivalis are associated with juvenile periodontitis. J Periodontol. 2000;71:981–988. doi: 10.1902/jop.2000.71.6.981. [DOI] [PubMed] [Google Scholar]
- 19.Savard M, Belanger C, Tardif M, Gourde P, Flamand L, Gosselin J. Infection of primary human monocytes by Epstein–Barr virus. J Virol. 2000;74:2612–2619. doi: 10.1128/jvi.74.6.2612-2619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savard M, Belanger C, Tremblay MJ, et al. EBV suppresses prostaglandin E2 biosyn-thesis in human monocytes. J Immunol. 2000;164:6467–6473. doi: 10.4049/jimmunol.164.12.6467. [DOI] [PubMed] [Google Scholar]
- 21.Slots J. Update on human cytomegalovirus in destructive periodontal disease. Oral Microbiol Immunol. 2004;19:217–223. doi: 10.1111/j.1399-302X.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- 22.Slots J, Saygun I, Sabeti M, Kubar A. Epstein–Barr virus in oral diseases. J Periodontal Res. 2006;41:235–244. doi: 10.1111/j.1600-0765.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 23.Sunde PT, Olsen I, Enersen M, Beiske K, Grinde B. Human cytomegalovirus and Epstein–Barr virus in apical and marginal periodontitis: a role in pathology? J Med Virol. 2008;80:1007–1011. doi: 10.1002/jmv.21180. [DOI] [PubMed] [Google Scholar]
- 24.Sunde PT, Olsen I, Enersen M, Grinde B. Patient with severe periodontitis and subgingival Epstein–Barr virus treated with anti-viral therapy. J Clin Virol. 2008;42:176–178. doi: 10.1016/j.jcv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Tardif M, Savard M, Flamand L, Gosselin J. Impaired protein kinase C activation/trans-location in Epstein–Barr virus-infected monocytes. J Biol Chem. 2002;277:24148–24154. doi: 10.1074/jbc.M109036200. [DOI] [PubMed] [Google Scholar]
- 26.Teughels W, Sliepen I, Quirynen M, et al. Human cytomegalovirus enhances A. actinomycetemcomitans adherence to cells. J Dent Res. 2007;86:175–180. doi: 10.1177/154405910708600213. [DOI] [PubMed] [Google Scholar]
- 27.Tugizov S, Herrera R, Veluppillai P, Greenspan J, Greenspan D, Palefsky JM. Epstein–Barr virus (EBV)-infected monocytes facilitate dissemination of EBV within the oral mucosal epithelium. J Virol. 2007;81:5484–5496. doi: 10.1128/JVI.00171-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zedtwitz-Liebenstein K, Jaksch P, Wulkersdorfer B, et al. Usefulness of interleukin-10 detection in lung transplant patients with human cytomegalovirus infection with respect to virus persistence. Transplantation. 2007;84:268–271. doi: 10.1097/01.tp.0000267157.78945.9d. [DOI] [PubMed] [Google Scholar]