Abstract
Objectives
To investigate the association of trimester-specific gestational weight gain with offspring fetal growth, obesity risk, and cardio-metabolic health outcomes from birth up to 4 years of age.
Study design
We conducted the present study in 977 mother-child pairs of the pregnancy cohort “Rhea” study in Crete, Greece. We measured birth weight, body mass index from 6 months to 4 years of age, waist circumference, skinfold thickness, blood pressure, and blood levels of lipids, C-reactive protein, and adipose tissue hormones at 4 years of age. We used multiple linear and log Poisson regression models to examine the association of exposure with continuous or binary outcomes respectively.
Results
Greater rate of gestational weight gain in the first trimester of pregnancy (per 200 g/week) was associated with increased risk of overweight/obesity from 2 years [RR: 1.25, (95% CI: 1.09, 1.42)] to 4 years of age [RR: 1.15, (95% CI: 1.05, 1.25)], but not with birth size. Each 200 gr/week of weight gain in the first trimester of pregnancy was also associated with greater risk of high waist circumference [RR: 1.13, (95% CI: 1.04, 1.23)], high sum of skinfold thickness [RR: 1.15 (95% CI: 1.02, 1.29)] and higher diastolic blood pressure at 4 years of age [β: 0.43 mmHg (95% CI: 0.00, 0.86)]. Greater rate of gestational weight gain during the second and third trimesters of pregnancy (per 200 gr/week) was associated with greater risk of large for gestational age neonates [RR: 1.22, (95% CI: 1.02, 1.45)] and higher levels of cord blood leptin [ratio of geometric means: 1.08 (95% CI: 1.00, 1.17)], but not with child anthropometry at later ages.
Conclusion
Timing of gestational weight gain may differentially influence childhood cardio-metabolic outcomes.
Keywords: pregnancy cohort, blood pressure, children, gestational weight gain, obesity
Introduction
Gestational diabetes, maternal obesity and excessive weight gain during pregnancy, each markers of fetal overnutrition, are considered among the most important modifiable early life risk factors of childhood obesity (1). From a public health perspective, gestational weight gain (GWG) has recently gained particular interest because interventions on GWG could benefit from the fact that they i) target women for the short duration of pregnancy, ii) can take advantage of the frequent visits of women to their obstetricians and if successful iii) they can reduce maternal postpartum weight retention and so the risk of maternal obesity that might complicate future pregnancies (2–4).
Excessive GWG has been associated with poor health outcomes for both mother and child over the short and long-term (5, 6). Two recent meta-analyses suggested that excessive GWG is associated with higher risk of offspring obesity throughout life (7, 8), while more debatable are the results when offspring adiposity is assessed by measures other than body mass index (BMI) (9–14). Some birth cohorts have attempted to disentangle the effect of GWG depending on the timing of gain and suggest that early pregnancy weight gain might be critical for the development of offspring obesity later in life (12, 13, 15–18). However, we are not aware of any studies that have examined the association of trimester-specific GWG with offspring obesity in the first 4 years of life.
Findings for the association between GWG and other offspring cardiovascular traits such as blood pressure or serum lipid profile have been less consistent mainly due to confounding by offspring adiposity (9, 13, 14, 19, 20). We are not aware of any studies that have examined the association of trimester-specific GWG with offspring blood pressure, lipid profile and adipose tissue hormones in children as young as 4 years of age in a population of substantial size.
In the present study, we examined the association of GWG (total and trimester-specific) with offspring birth weight, postnatal growth, obesity, and a range of cardio-metabolic risk factors at 4 years of age (waist circumference, skinfolds, blood pressure, lipids, adiponectin, leptin, and C-reactive protein) in the “Rhea” pregnancy cohort in Crete, Greece.
Materials and methods
We recruited mothers who became pregnant from February 2007 to January 2008, who were residents in the prefecture of Heraklion Crete, Greece (21). Research assistants invited women to provide blood and urine samples and to participate in a face-to-face interview at enrollment, before 15 weeks’ of gestation. The next contacts with the mothers were at 24 weeks of gestation, at birth, at 8–10 weeks after delivery and for child’s follow-up at 9th, 18th months, and at 4 years of age. The study was approved by the Ethical Committee of the University Hospital of Heraklion (Crete, Greece) and all participants provided written informed consent.
Of 1363 singleton live births, 977 had complete data on maternal GWG, offspring birth weight and BMI from 6 months to 4 years of age. Of these, 451 children had cord leptin measurements, 661 children had waist circumference and skinfold thickness measurements at 4 years of age, 518 had blood pressure measurements and 567 children provided blood samples at the 4 years of age follow-up. Trimester specific GWG data were available for 595 mother-child pairs. We excluded births prior to 34 weeks gestation (n = 20) to limit bias due to the dependence of GWG on gestational age (22).
Gestational weight gain
At enrollment research assistants measured mother’s height (cm) and weight (kg) in light clothing, without shoes and obtained information on pre-pregnancy weight. We calculated 1st trimester GWG rate, as the difference between self-reported pre-pregnancy weight and weight as measured at enrollment and divided it by the corresponding gestational age. We calculated 2nd&3rd trimesters GWG rate, as the difference between total and 1st trimester GWG. We decided to examine changes in 1st trimester and 2nd&3rd trimesters GWG per 200gr/week in an effort to use a common rate of change that would be reasonable for both periods of pregnancy (23). This weekly rate corresponds to a 2.8 kg more weight gain up to 14 weeks of gestation and to 5.2 kg more weight gain for the remaining period of pregnancy, which is close to the recommended weight gain for the first trimester of pregnancy, and corresponds to half of the recommended gain for the second and third trimester of pregnancy for normal weight women (23). We obtained information on total GWG postpartum (9±2 months), based on a phone-interview with the mother. We examined total GWG as continuous variable (per 2kg change) and as categorical (inadequate, adequate or excessive) (23).
Child anthropometry
Weight and length at birth were obtained from the hospital delivery logs and medical records. Large for gestational age (LGA) neonates were defined as live-born infants above the 90th percentile of birth weight for gestational age in a referent population (24).
At child follow-up visits, trained research assistants measured weight and length (up to 2 years of age) or height (from 2 to 4 years of age) using validated scales (Seca 354 baby scale, Seca Bellisima 841) and stadiometers (Seca 210 measuring mat, Seca 213) according to standard operating procedures. Repeated measures of weight and length/height were also abstracted from the children’s health cards. We calculated BMI and converted raw values into sex and age specific standard deviation scores (SD-scores) by using internally generated growth reference curves. Because of variation in children’s age at measurement, we estimated BMI SD-scores at exactly 6 months, 1, 2, 3 and 4 years of age using a sex and age specific, multi-level (mixed) linear model fitted with fractional polynomials and random effects for child (25). To minimize the effect of children with implausible growth trajectories, we excluded children whose measurements were <−5 SD or >5 SD from the mean at any age.
We defined rapid BMI growth the first 6 months of life as a BMI SD-score gain greater than 0.67 while children with a BMI SD-score gain equal to or below 0.67 constituted the reference group (26). For defining childhood overweight/obesity at 2, 3 and 4 years of age we used the BMI cutoff points for sex and age proposed by the International Obesity Task Force (27).
Waist circumference was measured at 4 years of age in duplicate to the nearest 0.1 cm in a standing position, at the high point of the iliac crest at the end of a gentle expiration, using a measuring tape (Seca 201). Skinfold thickness, was measured at four anatomical sites (triceps, subscapular, suprailiac and thigh) on the right side of the body in triplicate to the nearest 0.1 mm, using a calibrated caliper (Harpenden HSK- BI, CE-0120). Intra-observer and inter-observer reliability assessments were undertaken following previous methodology (28). Intra-observer reliability was above 0.98 and inter-observer reliability was over 0.82 for all anthropometric measurements. We used the 75th percentile of the study cohort distribution as a cut-off point to denote high values on waist circumference (≥55.5 cm) and on skinfolds (≥49.7 mm for girls and ≥41.7 mm for boys) (29).
Child cardio-metabolic risk factors
At 4 years of age, trained research assistants measured systolic and diastolic blood pressure after 5 minutes rest in the seated position, at the child right arm with a cuff of appropriate size for arm circumference using a Dinamap Pro Care 400, which utilizes an oscillometric method. We used the average of five consecutive measurements, taken with 1 minute intervals (30). Non-fasting blood samples were collected from the children at the end of the 4th year follow-up visit. Blood samples were processed within 2 hours, with serum stored at −80°C until analysis. Total cholesterol (TC) and HDL-cholesterol (HDL-C) in serum were measured using standard enzymatic methods (Medicon, Greece). Leptin and adiponectin (Invitrogen, CA) were measured by an enzyme-linked immunosorbent assay and C-reactive protein was measured with a high-sensitivity homogenous immunoassay (ORS 6199, Beckman Coulter, USA). Cord leptin, was measured as described previously (31). Inter- and intra-assay coefficients of variation were <5%.
Statistical methods
We used linear regression models to estimate the β coefficients for the association between GWG and continuous outcomes and log-Poisson regression models to estimate the relative risks (RR) for the association between GWG and binary outcomes (32). We applied generalized additive models (GAMs) to explore the shape of the relationships between GWG and continuous outcomes, with adjustment for confounders. GAMs indicated that the associations between GWG (total or trimester specific) and the outcomes did not deviate from linearity. Leptin, adiponectin, and C-reactive protein were log transformed to normalize their distributions. The resultant regression coefficients were exponentiated to give a ratio of geometric means per change in exposure. To assess differences in BMI trajectories from birth to 4 years of age for each stratum of excessive, adequate and inadequate GWG, we constructed mixed effects linear regression models, using BMI for age percentiles of our population. Model parameters were estimated using restricted maximum likelihood (33).
We considered the following potential confounders in our models: maternal age at delivery, educational level at recruitment (low: ≤ 9 years, medium: > 9 years up to attending post-secondary school education and high: attending university or having a university/technical college degree), parity, smoking during pregnancy, pre-pregnancy BMI (kg/m2), paternal BMI (kg/m2), gestational length (for models using birth size as an outcome or total GWG as an exposure), duration of any breastfeeding (months), child’s sex, age at outcome assessment, energy intake at 4 years of age (kcals/day by a validated food frequency questionnaire) (34), TV watching at 4 years of age (“<30 minutes”, “1–2 hours”, “3 hours or more/day”) and child’s BMI at 4 years of age (for models using cardio-metabolic risk factors as outcomes). Level of completeness for covariates included in the main models was above 95%.
To assess the potential modifying effects of child sex (male, female), maternal pre-pregnancy BMI [as continuous variable, or as categorical (<25 kg/m2, ≥25 kg/m2 and as <25 kg/m2, 25–30 kg/m2, ≥30 kg/m2)], and breastfeeding duration (<1 month, 1–6 months, >6 months) we included appropriate interaction terms in regression models and stratified the sample. We also repeated analyses after excluding (1) children born preterm (< 37 gestational weeks), (2) children born small for gestational age, (3) pregnant women diagnosed with gestational diabetes mellitus and (4) pre-eclamptic pregnancies. We used Stata S.E. version 13 for all analyses (StataCorp, Texas, USA).
Results
GWG (total and trimester-specific) by maternal and child characteristics are presented in Table 1. Women who were highly educated, those who were multiparous, with higher pre-pregnancy BMI, and those who developed gestational diabetes had lower GWG throughout pregnancy. Women of Greek origin and current smokers in pregnancy had higher 1st trimester GWG. According to the 2009 Institute of Medicine recommendations, 45% of women gained excessive weight, 32% gained adequate weight, and 23% gained inadequate weight during their pregnancy. The prevalence of LGA neonates was 17%, while 31% of infants had rapid BMI growth the first 6 months of life. The prevalence of overweight and obesity was 10% and 1% at 2 years of age, 16% and 3% at 3 years and 21% and 4% at 4 years of age respectively.
Table 1.
Gestational weight gain (total and trimester specific) by maternal and child characteristics in “Rhea” pregnancy cohort.
| Characteristic | Subjects% | Total GWG (kg) n=977 |
1st trimester GWG (gr/week) n=595 |
2nd&3rd trimesters GWG (gr/week) n=595 |
|---|---|---|---|---|
| Study population (median IQR) | 100% | 14 (8) | 153 (260) | 449 (260) |
| Maternal characteristics | ||||
| Maternal origin | ||||
| Greek | 92% | 14.1 | 184 (265) | 451 (205) |
| Other | 8% | 13.3 | 60 (241) | 450 (200) |
| Maternal educational level | ||||
| Low | 17% | 13.2 | 206 (292) | 405 (218) |
| Medium | 51% | 14.4 | 178 (276) | 470 (213) |
| High | 32% | 13.8 | 162 (232) | 439 (183) |
| Marital status | ||||
| Married | 89% | 13.9 | 168 (257) | 445 (208) |
| Other than married | 11% | 15.3 | 233 (304) | 490 (175) |
| Maternal age at delivery | ||||
| <30 years | 47% | 14.7 | 197 (291) | 472 (215) |
| ≥30 years | 53% | 13.4 | 159 (236) | 431 (193) |
| Parity | ||||
| Primiparous | 43% | 14.8 | 183 (300) | 469 (203) |
| Multiparous | 57% | 13.4 | 165 (233) | 438 (207) |
| Maternal smoking in pregnancy | ||||
| Smoker | 26% | 15.3 | 224 (230) | 480 (207) |
| Non-smoker | 74% | 13.5 | 156 (270) | 442 (204) |
| Pre-pregnancy BMI | ||||
| Obese | 11% | 10.9 | 127 (318) | 372 (249) |
| Overweight | 21% | 13.4 | 166 (274) | 447 (193) |
| Normal | 63% | 14.6 | 184 (250) | 464 (195) |
| Underweight | 5% | 15.3 | 222 (263) | 453 (242) |
| IoM categories for total GWG | ||||
| Excessive | 45% | 18.6 | 239 (270) | 585 (170) |
| Adequate | 32% | 11.9 | 123 (236) | 407 (122) |
| Inadequate | 23% | 7.9 | 128 (264) | 245 (161) |
| Physically active in pregnancy | ||||
| Yes | 8% | 13.5 | 135 (196) | 462 (172) |
| No | 92% | 14.0 | 181 (269) | 449 (208) |
| Energy intake in pregnancy | ||||
| ≥2000 kcals/day | 45% | 14.5 | 204 (302) | 458 (232) |
| <2000 kcals/day | 55% | 13.5 | 161 (245) | 445 (193) |
| Gestational diabetes | ||||
| Yes | 9% | 12.0 | 111 (363) | 353 (242) |
| No | 91% | 14.1 | 144 (267) | 460 (197) |
| Mode of delivery | ||||
| Caesarian | 51% | 13.8 | 191 (253) | 443 (202) |
| Vaginal | 49% | 14.2 | 158 (274) | 459 (207) |
| Child characteristics | ||||
| Preterm (34–<37 weeks) | ||||
| Yes | 9% | 13.2 | 173 (235) | 437 (196) |
| No | 91% | 14.1 | 176 (266) | 452 (205) |
| Gender | ||||
| Male | 50% | 14.3 | 190 (269) | 456 (208) |
| Female | 50% | 13.7 | 162 (258) | 445 (201) |
| LGA neonates | ||||
| Yes | 17% | 15.6 | 209 (304) | 492 (216) |
| No | 83% | 13.7 | 169 (248) | 443 (202) |
GWG, gestational weight gain; BMI, body mass index; IoM, institute of medicine; IQR, interquartile range; LGA, large for gestational age. Values are mean (standard deviation) unless indicated otherwise.
Bold indicates statistically significant at p<0.05 for comparisons by Man-Whitney U-test, Kruskal-Wallis.
GWG and childhood obesity
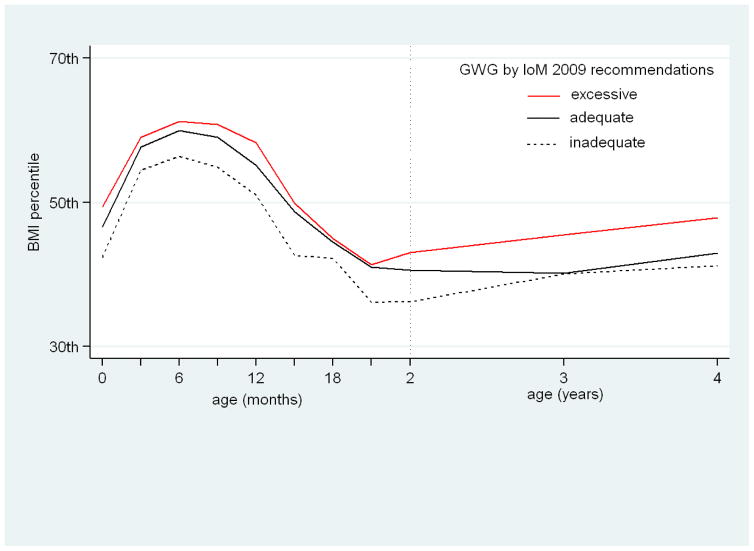
Table 2, presents the associations of total and trimester-specific GWG with offspring size up to 4 years of age and the risks of LGA neonates, rapid BMI growth in the first 6 months of life, and child obesity/adiposity from 2 to 4 years of age. Figure 1, presents the modeled BMI for age percentiles growth trajectory from birth to 4 years of age by strata of excessive, adequate or inadequate GWG. Figure 2, presents the predicted probability and 95% CIs of childhood overweight/obesity at 4 years of age, by trimester specific GWG rate.
Table 2.
Adjusted associations of gestational weight gain (total and trimester-specific) with offspring size at birth, and adiposity outcomes from infancy to early childhood.
| Outcome | Measure of association | Total GWG (per 2kg) | 1st trimester GWG (per 200gr/week) | 2nd&3rd trimesters GWG (per 200gr/week) |
|---|---|---|---|---|
| Birth and infancy | ||||
|
| ||||
| Birth Weight SD-score | β (95% CI) | 0.05 (0.03, 0.07) | 0.04 (−0.01, 0.10) | 0.07 (0.00, 0.14) |
| BMI SD-score, 6 months | β (95% CI) | 0.04 (0.01, 0.06) | 0.07 (0.01, 0.13) | 0.03 (−0.03, 0.10) |
| LGA neonates | RR (95% CI) | 1.12 (1.07, 1.17) | 1.10 (0.97, 1.24) | 1.22 (1.02, 1.45) |
| Rapid BMI growth in the first 6 monthsa | RR (95% CI) | 1.05 (1.02, 1.08) | 1.05 (0.98, 1.13) | 1.10 (0.99, 1.23) |
|
| ||||
| Early childhood (1–4 years) | ||||
|
| ||||
| BMI SD-score, 1 year | β (95% CI) | 0.03 (0.01, 0.06) | 0.07 (0.01, 0.13) | 0.03 (−0.04, 0.10) |
| BMI SD-score, 2 years | β (95% CI) | 0.03 (0.01, 0.06) | 0.07 (0.01, 0.13) | 0.03 (−0.04, 0.10) |
| BMI SD-score, 3 years | β (95% CI) | 0.03 (0.01, 0.06) | 0.07 (0.01, 0.13) | 0.03 (−0.04, 0.10) |
| BMI SD-score, 4 years | β (95% CI) | 0.03 (0.01, 0.06) | 0.07 (0.01, 0.13) | 0.03 (−0.04, 0.10) |
| Overweight/obese 2 yearsb | RR (95% CI) | 1.11 (1.05, 1.17) | 1.25 (1.09, 1.42) | 1.08 (0.82, 1.40) |
| Overweight/obese 3 yearsb | RR (95% CI) | 1.07 (1.02, 1.11) | 1.18 (1.07, 1.31) | 0.97 (0.83, 1.15) |
| Overweight/obese 4 yearsb | RR (95% CI) | 1.05 (1.01, 1.08) | 1.15 (1.05, 1.25) | 1.00 (0.87, 1.14) |
|
| ||||
| Adiposity measurements, 4 years | ||||
|
| ||||
| Waist circumference (cm) | β (95% CI) | 0.13 (−0.001, 0.28) | 0.35 (0.01, 0.69) | 0.03 (−0.38, 0.46) |
| Sum of skinfolds (mm) | β (95% CI) | 0.42 (0.00, 0.85) | 0.72 (−0.33, 1.78) | 0.21 (−1.09, 1.53) |
| Waist circumference≥75th percentile | RR (95% CI) | 1.08 (1.04, 1.13) | 1.13 (1.04, 1.23) | 1.12 (0.94, 1.33) |
| Sum of skinfold≥75th percentile | RR (95% CI) | 1.04 (0.98, 1.09) | 1.15 (1.02, 1.29) | 0.98 (0.82, 1.17) |
GWG, gestational weight gain; BMI, body mass index; LGA, large for gestational age; RR, relative risk.
Rapid BMI growth defined as a gain in SD score for BMI > 0.67 SD (reference category ≤ 0.67).
Overweight/obese defined using the BMI cut-off point for sex and age proposed by the International Obesity Task Force.
All models were adjusted for maternal age, education, parity, smoking in pregnancy, pre-pregnancy BMI, paternal BMI, gestational length (for models using birth size as an outcome or total GWG as an exposure), breastfeeding duration up to the corresponding age (except for models using birth size as an outcome), child’s sex, and age at outcome assessment. Bold indicates statistically significant at the 5% level coefficients.
Figure 1.
BMI percentile trajectories, from birth to 4 years of age, for offspring of mothers with excessive, adequate or inadequate gestational weight gain according to IoM 2009 recommendations, adjusted for maternal age, education, parity, smoking in pregnancy, pre-pregnancy BMI, paternal BMI, gestational length and breastfeeding duration.
Figure 2.
Predicted probability and 95% CIs for childhood overweight or obesity by trimester specific gestational weight gain rate (kg/week) after adjusting for maternal age, education, parity, smoking in pregnancy, pre-pregnancy BMI, paternal BMI and breastfeeding duration. The bars on the x-axis illustrate the observed gestational weight gain rate values. Similar patterns were observed at 2 and 3 years of age (not shown).
Total GWG
Higher total GWG was associated with higher birth weight SD-score [β: 0.05 (95% CI: 0.03, 0.07)] and greater risk of LGA neonates [RR: 1.12, (95% CI: 1.07, 1.17)] in the fully adjusted models (Table 2). Total GWG was consistently positively associated with child BMI SD-score across the study period and correspondingly with greater risk of rapid BMI growth in the first 6 months of life, and of overweight/obesity at 2, 3, and 4 years of age (Table 2). It was also associated with greater risk of high waist circumference [RR: 1.08 (95% CI: 1.04, 1.13)] and high skinfold thickness measurements at 4 years of age [β: 0.42 mm (95% CI: 0.00, 0.85)].
Institute of Medicine categories of GWG
Offspring of mothers with excessive GWG had 60% increased risk of being LGA neonates (95% CI: 1.14, 2.23) (Table 1S in the Supplementary Appendix) and were in higher BMI percentiles across the study period compared to children of mothers with adequate GWG (Figure 1). Offspring of mothers who gained inadequate GWG compared to the reference group, had lower birth weight SD score [β: −0.16 (95% CI: −0.32, −0.001)] (Table 1S in the Supplementary Appendix), and remained in lower BMI percentiles up to 4 years of age compared to the reference group of adequate GWG (Figure 1)
Trimester-specific GWG
Greater 1st trimester GWG rate was associated with higher child BMI SD-score at 6 months, 1 year, 2, 3 and 4 years of age (Table 2). Each 200 gr/week increase in 1st trimester GWG was associated with higher risk of overweight/obesity at 2 years [RR: 1.25 (95% CI: 1.09, 1.42)], at 3 years [RR: 1.18, (95% CI: 1.07, 1.31)] and at 4 years of age [RR: 1.15, (95% CI: 1.05, 1.25)]. Overall, 1st trimester GWG rate showed a positive linear relationship with the probability of overweight/obesity at 2, 3 and 4 years of age (Figure 2). Higher 1st trimester GWG was also associated with increased risk of high waist circumference [RR: 1.13, (95% CI: 1.04, 1.23)], and skinfold thickness measurements at 4 years of age [RR: 1.15, (95% CI: 1.02, 1.29)] (Table 2). Greater 2nd&3rd trimesters GWG rate, was associated with increased birth weight SD-score [β: 0.07, (95% CI: 0.00, 0.14)] and risk of LGA neonates [RR: 1.22, (95% CI: 1.02, 1.45)], but not with child BMI SD-scores at later ages (Table 2). Rate of 2nd&3rd trimester GWG showed no association with the probability of overweight/obesity at 2, 3 and 4 years of age (Figure 2, Table 2).
Cardio-metabolic risk factors
Total and trimester-specific GWG were generally not associated with lipids, adiponectin or C-reactive protein at 4 years of age (Table 3). We observed a positive association of 1st trimester GWG with diastolic blood pressure at 4 years of age, even with adjustment for child’s BMI [β: 0.43 mmHg, (95% CI: 0.00, 0.86)] (Table 3). Each 200 gr/week increase in 2nd&3rd trimesters GWG was associated with higher cord leptin levels [ratio of geometric means: 1.08, (95% CI: 1.00, 1.17)], but with lower leptin levels at 4 years of age [ratio of geometric means: 0.89, (95% CI: 0.83, 0.97)] (Table 3).
Table 3.
Adjusted associations of gestational weight gain (total and trimester-specific) with offspring cardio-metabolic risk factors.
| Cardiometabolic outcome | Total GWG (per 2kg) | 1st trimester GWG (per 200gr/week) | 2nd&3rd trimesters GWG (per 200gr/week) | |
|---|---|---|---|---|
| β (95% CI) | ||||
|
| ||||
| Systolic blood pressure, mmHg | −0.15 (−0.38, 0.07) | 0.09 (−0.68, 0.48) | 0.05 (−0.68, 0.78) | |
| Diastolic blood pressure, mmHg | 0.00 (−0.15, 0.17) | 0.43 (0.00, 0.86) | −0.059 (−0.61, 0.49) | |
| Total cholesterol, mg/dL | −0.57 (−1.46, 0.32) | −0.04 (−2.42, 2.33) | −1.91 (−4.88, 1.06) | |
| HDL cholesterol, mg/dL | −0.10 (−0.43, 0.23) | 0.31 (−0.57, 1.19) | −0.89 (−1.99, 0.20) | |
| CRP, ratio GM | 1.01 (0.96, 1.05) | 1.01 (0.90, 1.14) | 1.03 (0.89, 1.20) | |
| Adiponectin, ratio GM | 0.99 (0.97, 1.01) | 0.98 (0.93, 1.03) | 0.95 (0.89, 1.02) | |
|
| ||||
| Leptin | cord blood, ratio GM | 1.02 (1.00, 1.05) | 0.99 (0.93, 1.05) | 1.08 (1.00, 1.17) |
|
| ||||
| 4 years, ratio GM | 0.97 (0.95, 1.00) | 1.01 (0.95, 1.08) | 0.89 (0.83, 0.97) | |
GWG, gestational weight gain; LDL, low density lipoprotein; HDL, high density lipoprotein; CRP, C-reactive protein; ratio GM, ratio of geometric mean (the null value for these ratios is 1).
All models were adjusted for maternal age, education, parity, smoking in pregnancy, pre-pregnancy BMI, paternal BMI, gestational length (for models using total GWG as an exposure), child’s sex, age at outcome assessment and child’s BMI. Models using cord blood leptin as an outcome were adjusted for birth weight for gestational age instead of child’s BMI.
Bold indicates statistically significant at the 5% level coefficients.
Stratified and sensitivity analyses
When we repeated analyses adjusting also for birth weight and child lifestyle factors (TV watching and child’s energy intake), results were very similar to those presented in the main analyses (Table 2S in the Supplementary Appendix). We saw no evidence for a multiplicative interaction of GWG with child sex, pre-pregnancy BMI, or breastfeeding duration (Table 3S in the Supplementary Appendix). Finally, we repeated analyses after excluding i) children born preterm (n=90), ii) children born small for gestational age (n=48), iii) women diagnosed with gestational diabetes mellitus (n=37) and iv) pre-eclamptic pregnancies (n=7). Results did not differ substantially from those derived from the main analyses (data not shown).
Comment
In this analysis from a pregnancy cohort, we have shown that 1st trimester GWG was consistently positively associated with offspring’s BMI from infancy to early childhood. Greater 1st trimester GWG was also associated with higher adiposity measurements, and diastolic blood pressure at 4 years of age. Greater 2nd&3rd trimester GWG was associated with excess fetal growth and higher cord blood leptin, but not with child anthropometry at later ages.
Four previous cohort studies examined early pregnancy GWG with offspring obesity risk in mid childhood, reporting positive associations (13, 15, 16, 18). Fraser et al, reported that 1st trimester GWG was incrementally associated not just with BMI, but also, with waist circumference and fat mass as assessed in children 9 years of age (13). Estampador et al, examined fat mass by air displacement plethysmography in a small sample of 31 infants at 4 months of age and showed a strong association of weight gain in mid-pregnancy with infant fat mass (12). We extend previous knowledge by showing a positive association of 1st trimester GWG with offspring adiposity measures (BMI, waist circumference and skinfolds measurements) in early childhood.
Excessive early pregnancy GWG represents greater maternal fat deposition and possibly a state of maternal dysmetabolism (35, 36). This altered maternal environment may interact with placental factors leading to increased supply of fuels to the fetus (37–39). Animal models suggest that offspring of mothers who were overfed from conception, exhibit malprogramming of the appetite-regulating system in the hypothalamus towards hyperphagia (40). Rattanatray et al, showed that overnutrition in early gestation upregulates genes involved in adipogenesis and lipogenesis in lambs (41). Epidemiologic evidence from the ALSPAC cohort, shows that greater GWG in early pregnancy is related to higher DNA methylation in offspring cord blood, suggesting a possible epigenetic mechanism for the adverse health outcomes of early GWG (42).
GWG from 2nd trimester onwards, coincident with the period of maximal fetal growth, was associated with birth weight and LGA but not later size in our study. Our results are in line with those reported recently from two birth cohorts showing that 2nd or 3rd trimester GWG mostly predicted the risk for LGA and not the later risk for obesity (16, 18). The positive association of 2nd&3rd trimesters GWG with cord blood leptin may suggest that overnutrition in late pregnancy might determine fetal leptin synthesis, as has been shown in animal models (43, 44).
Among a range of cardio-metabolic risk factors examined at 4 years of age, we observed a positive association of 1st trimester GWG with diastolic blood pressure. We have shown that this association was not attributable to the association of GWG with offspring obesity, contrary to results from other studies (9, 13, 19, 20). Animal models support a direct mechanism, linking overnutrition in fetal life to endothelial dysfunction and raised blood pressure in offspring through an altered autonomic control (45). We found that offspring leptin levels at 4 years of age were inversely associated to 2nd&3rd trimesters GWG. It is difficult to interpret the exact nature of this relation, as offspring blood leptin in early childhood might reflect lean rather than fat mass and to date there are no studies examining the association between trimester specific GWG and circulating leptin levels in early childhood.
Strengths of the present study include the population-based prospective design, the opportunity to examine GWG as trimester specific, the repeated measures of offspring BMI and the detailed cardio-metabolic measurements in early childhood. We were able to assess a number of confounding factors including both maternal and paternal BMI, several socio-demographic as well as child lifestyle characteristics. A limitation is that maternal pre-pregnancy weight and total GWG were self-reported, which might have led to misclassification. However, studies have shown that recall of pre-pregnancy BMI and GWG is reproducible and valid (46) and underreporting of GWG, which tends to be more frequent than over-reporting, would most likely bias estimates toward the null (47, 48). We were not able to disentangle the effects of 2nd and 3rd trimester GWG, as we had no data on maternal weight at the end of the 2nd trimester of pregnancy. However, studies assessing the association of trimester-specific GWG with offspring obesity suggest that the effects of 2nd and 3rd trimester GWG are comparable in terms of direction and size of association (16, 18), while IoM recommendations are the same for GWG rate in 2nd and 3rd trimester of pregnancy. Child’s BMI values were imputed for specific ages based on growth curve trajectories, as children were not measured at exactly the same time intervals. However, the model fits were excellent, and the residuals showed no important trend across age, or heteroscedasticity.
Our findings suggest that the effect of GWG on childhood obesity outcomes depends on the timing of gestation, highlighting the importance of 1st trimester GWG. Future studies are needed to replicate these findings and to understand the complex underlying mechanisms so as to determine the critical windows in early life when interventions on GWG may be more effective in preventing childhood obesity and its associated comorbidities.
Supplementary Material
Acknowledgments
Funding: The Rhea project was financially supported by European projects (EU FP6-2003-Food-3-NewGeneris, EU FP6. STREP Hiwate, EU FP7 ENV.2007.1.2.2.2. Project No 211250 Escape, EU FP7-2008-ENV-1.2.1.4 Envirogenomarkers, EU FP7-HEALTH-2009- single stage CHICOS, EU FP7 ENV.2008.1.2.1.6. Proposal No 226285 ENRIECO, EU- FP7- HEALTH-2012 Proposal No 308333 HELIX), MeDALL (FP7 European Union project, No. 264357) and the Greek Ministry of Health (Program of Prevention of obesity and neurodevelopmental disorders in preschool children, in Heraklion district, Crete, Greece: 2011–2014; “Rhea Plus”: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012–15).
Dr Chatzi received a Fulbright grant to conduct research at the Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA, USA, from May 1st to July 31, 2014.
Dr. Oken was supported by the US National Institutes of Health (K24 HD069408).
The authors would like to thank all the cohort participants for their generous collaboration.
Footnotes
Disclosure: The authors report no conflict of interest.
Paper presentation: A preliminary analysis of this paper was presented as a poster presentation at “The Power of Programming 2014 - Developmental Origins of Adiposity and Long-term Health” March 13–15, 2014, Munich, Germany.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition--an old hypothesis with new importance? Int J Epidemiol. 2013;42(1):7–29. doi: 10.1093/ije/dys209. [DOI] [PubMed] [Google Scholar]
- 2.Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. American Journal of Obstetrics and Gynecology. 2010;202(2) doi: 10.1016/j.ajog.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herring SJ, Rose MZ, Skouteris H, Oken E. Optimizing weight gain in pregnancy to prevent obesity in women and children. Diabetes Obes Metab. 2012;14(3):195–203. doi: 10.1111/j.1463-1326.2011.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM Southampton Women’s Study Group. Do women change their health behaviours in pregnancy? Findings from the Southampton Women’s Survey. Journal of Epidemiology and Community Health. 2008;62:A10–A1. doi: 10.1111/j.1365-3016.2009.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poston L. Gestational weight gain: influences on the long-term health of the child. Curr Opin Clin Nutr Metab Care. 2012;15(3):252–7. doi: 10.1097/MCO.0b013e3283527cf2. [DOI] [PubMed] [Google Scholar]
- 6.Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol. 2009;170(2):173–80. doi: 10.1093/aje/kwp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamun AA, Mannan M, Doi SA. Gestational weight gain in relation to offspring obesity over the life course: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2014;15(4):338–47. doi: 10.1111/obr.12132. [DOI] [PubMed] [Google Scholar]
- 8.Nehring I, Lehmann S, von Kries R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: a meta-analysis. Pediatr Obes. 2013;8(3):218–24. doi: 10.1111/j.2047-6310.2012.00110.x. [DOI] [PubMed] [Google Scholar]
- 9.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. American Journal of Obstetrics and Gynecology. 2007;196(4) doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2010;91(6):1745–51. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensenauer R, Chmitorz A, Riedel C, Fenske N, Hauner H, Nennstiel-Ratzel U, et al. Effects of suboptimal or excessive gestational weight gain on childhood overweight and abdominal adiposity: results from a retrospective cohort study. Int J Obes (Lond) 2013;37(4):505–12. doi: 10.1038/ijo.2012.226. [DOI] [PubMed] [Google Scholar]
- 12.Estampador AC, Pomeroy J, Renstrom F, Nelson SM, Mogren I, Persson M, et al. Infant body composition and adipokine concentrations in relation to maternal gestational weight gain. Diabetes Care. 2014;37(5):1432–8. doi: 10.2337/dc13-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L, et al. Association of Maternal Weight Gain in Pregnancy With Offspring Obesity and Metabolic and Vascular Traits in Childhood. Circulation. 2010;121(23):2557–U48. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dello Russo M, Ahrens W, De Vriendt T, Marild S, Molnar D, Moreno LA, et al. Gestational weight gain and adiposity, fat distribution, metabolic profile, and blood pressure in offspring: the IDEFICS project. Int J Obes (Lond) 2013;37(7):914–9. doi: 10.1038/ijo.2013.35. [DOI] [PubMed] [Google Scholar]
- 15.Andersen CS, Gamborg M, Sorensen TIA, Nohr EA. Weight gain in different periods of pregnancy and offspring’s body mass index at 7 years of age. International Journal of Pediatric Obesity. 2011;6(2-2):E179–E86. doi: 10.3109/17477166.2010.521560. [DOI] [PubMed] [Google Scholar]
- 16.Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF. Trimester of Maternal Gestational Weight Gain and Offspring Body Weight at Birth and Age Five. Maternal and Child Health Journal. 2012;16(6):1215–23. doi: 10.1007/s10995-011-0846-1. [DOI] [PubMed] [Google Scholar]
- 17.Laitinen J, Jaaskelainen A, Hartikainen AL, Sovio U, Vaarasmaki M, Pouta A, et al. Maternal weight gain during the first half of pregnancy and offspring obesity at 16 years: a prospective cohort study. Bjog-an International Journal of Obstetrics and Gynaecology. 2012;119(6):716–23. doi: 10.1111/j.1471-0528.2012.03319.x. [DOI] [PubMed] [Google Scholar]
- 18.Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013;21(5):1046–55. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 19.Hochner H, Friedlander Y, Calderon-Margalit R, Meiner V, Sagy Y, Avgil-Tsadok M, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation. 2012;125(11):1381–9. doi: 10.1161/CIRCULATIONAHA.111.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamun AA, O’Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119(13):1720–7. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- 21.Chatzi L, Plana E, Daraki V, Karakosta P, Alegkakis D, Tsatsanis C, et al. Metabolic syndrome in early pregnancy and risk of preterm birth. Am J Epidemiol. 2009;170(7):829–36. doi: 10.1093/aje/kwp211. [DOI] [PubMed] [Google Scholar]
- 22.Hutcheon JA, Bodnar LM, Joseph KS, Abrams B, Simhan HN, Platt RW. The bias in current measures of gestational weight gain. Paediatr Perinat Epidemiol. 2012;26(2):109–16. doi: 10.1111/j.1365-3016.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen KM, ALY . Weight gain during pregnancy: Reexamining the guidelines. Washington DC: US Institute of Medicine and National Research Counsil: Institute of Medicine and National Research Counsil of the National Academies; 2009. [PubMed] [Google Scholar]
- 24.Carrascosa A, Yeste D, Copil A, Audi L, Gusinye M, Vicens-Calvet E, et al. Fetal growth regulation and intrauterine growth retardation. Journal of Pediatric Endocrinology & Metabolism. 2004;17:435–43. [PubMed] [Google Scholar]
- 25.Royston P, Wright EM. A method for estimating age-specific reference intervals (‘normal ranges’) based on fractional polynomials and exponential transformation. Journal of the Royal Statistical Society Series a-Statistics in Society. 1998;161:79–101. [Google Scholar]
- 26.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev. 2005;6(2):143–54. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 27.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284–94. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 28.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82:165. doi: 10.1017/s0007114599001348. [DOI] [PubMed] [Google Scholar]
- 29.Ehrlich SF, Rosas LG, Ferrara A, King JC, Abrams B, Harley KG, et al. Pregnancy Glucose Levels in Women without Diabetes or Gestational Diabetes and Childhood Cardiometabolic Risk at 7 Years of Age. J Pediatr-Us. 2012;161(6):1016–21. doi: 10.1016/j.jpeds.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92(4):1049–57. doi: 10.1161/01.cir.92.4.1049. [DOI] [PubMed] [Google Scholar]
- 31.Karakosta P, Georgiou V, Fthenou E, Papadopoulou E, Roumeliotaki T, Margioris A, et al. Maternal weight status, cord blood leptin and fetal growth: a prospective mother-child cohort study (Rhea study) Paediatr Perinat Epidemiol. 2013;27(5):461–71. doi: 10.1111/ppe.12074. [DOI] [PubMed] [Google Scholar]
- 32.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 33.Ware JH. Linear models for the analysis of longitudinal studies. The American Statistician. 1985;39(2):95–101. [Google Scholar]
- 34.Leventakou V, Georgiou V, Chatzi L, Sarri K. Relative validity of an FFQ for pre-school children in the mother-child ‘Rhea’ birth cohort in Crete, Greece. Public Health Nutr. 2014:1–7. doi: 10.1017/S1368980014000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol. 1976;19(3):489–513. doi: 10.1097/00003081-197609000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update. 2010;16(3):255–75. doi: 10.1093/humupd/dmp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007;113(1):1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 38.Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet. 2011;2:27. doi: 10.3389/fgene.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–80. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breton C. The hypothalamus-adipose axis is a key target of developmental programming by maternal nutritional manipulation. J Endocrinol. 2013;216(2):R19–R31. doi: 10.1530/JOE-12-0157. [DOI] [PubMed] [Google Scholar]
- 41.Rattanatray L, MacLaughlin SM, Kleemann DO, Walker SK, Muhlhausler BS, McMillen IC. Impact of maternal periconceptional overnutrition on fat mass and expression of adipogenic and lipogenic genes in visceral and subcutaneous fat depots in the postnatal lamb. Endocrinology. 2010;151(11):5195–205. doi: 10.1210/en.2010-0501. [DOI] [PubMed] [Google Scholar]
- 42.Morales E, Groom A, Lawlor DA, Relton CL. DNA methylation signatures in cord blood associated with maternal gestational weight gain: results from the ALSPAC cohort. BMC Res Notes. 2014;7(1):278. doi: 10.1186/1756-0500-7-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devaskar SU, Anthony R, Hay W., Jr Ontogeny and insulin regulation of fetal ovine white adipose tissue leptin expression. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):R431–8. doi: 10.1152/ajpregu.2002.282.2.R431. [DOI] [PubMed] [Google Scholar]
- 44.Muhlhausler BS, Roberts CT, Yuen BS, Marrocco E, Budge H, Symonds ME, et al. Determinants of fetal leptin synthesis, fat mass, and circulating leptin concentrations in well-nourished ewes in late pregnancy. Endocrinology. 2003;144(11):4947–54. doi: 10.1210/en.2003-0555. [DOI] [PubMed] [Google Scholar]
- 45.Poston L. Influence of maternal nutritional status on vascular function in the offspring. Microcirculation. 2011;18(4):256–62. doi: 10.1111/j.1549-8719.2011.00086.x. [DOI] [PubMed] [Google Scholar]
- 46.Wright CS, Weiner M, Localio R, Song L, Chen P, Rubin D. Misreport of gestational weight gain (GWG) in birth certificate data. Matern Child Health J. 2012;16(1):197–202. doi: 10.1007/s10995-010-0724-2. [DOI] [PubMed] [Google Scholar]
- 47.Schieve LA, Perry GS, Cogswell ME, Scanion KS, Rosenberg D, Carmichael S, et al. Validity of self-reported pregnancy delivery weight: an analysis of the 1988 National Maternal and Infant Health Survey. NMIHS Collaborative Working Group. Am J Epidemiol. 1999;150(9):947–56. doi: 10.1093/oxfordjournals.aje.a010103. [DOI] [PubMed] [Google Scholar]
- 48.McClure CK, Bodnar LM, Ness R, Catov JM. Accuracy of maternal recall of gestational weight gain 4 to 12 years after delivery. Obesity (Silver Spring) 2011;19(5):1047–53. doi: 10.1038/oby.2010.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.