Abstract
Background
Neurological injuries or disease can impair the function of motor circuitry controlling forearm supination, and recovery is often limited. Preclinical animal models are essential tools for developing therapeutic interventions to improve motor function after neurological damage. Here we describe the supination assessment task, an automated measure of quantifying forelimb supination in the rat.
New Method
Animals were trained to reach out of a slot in a cage, grasp a spherical manipulandum, and supinate the forelimb. The angle of the manipulandum was measured using a rotary encoder. If the animal exceeded the predetermined turn angle, a reward pellet was delivered. This automated task provides a large, high-resolution dataset of turn angle over time. Multiple parameters can be measured including success rate, peak turn angle, turn velocity, area under the curve, and number of rotations per trial. The task provides a high degree of flexibility to the user, with both software and hardware parameters capable of being adjusted.
Results
We demonstrate the supination assessment task can effectively measure significant deficits in multiple parameters of rotational motor function for multiple weeks in two models of ischemic stroke.
Comparison with Existing Methods
Preexisting motor assays designed to measure forelimb supination in the rat require high-speed video analysis techniques. This operant task provides a high-resolution, quantitative end-point dataset of turn angle, which obviates the necessity of video analysis.
Conclusions
The supination assessment task represents a novel, efficient method of evaluating forelimb rotation and may help decrease the cost and time of running experiments.
Keywords: Supination, Forelimb, Stroke, Operant Behavior, Motor Function, Automated Task
1. Introduction
Many neurological injures or diseases impair the function of motor circuitry, which can lead to permanent physical disability (Jaquet et al. 2001; Anderson 2004; Walker and Pickett 2007; Langhorne et al. 2009). One common manifestation of motor dysfunction is impairment of forelimb supination, a fine motor skill critical for object manipulation (Braendvik et al. 2010; Lambercy et al. 2011; Klotz et al. 2013). Preclinical animal models are essential tools for developing therapeutic interventions to improve motor function after neurological damage. Given the prevalence of deficits in forearm rotation, it would be valuable to efficiently quantify this motor function in rats.
Motor assays designed to measure forelimb function in the rat, including pellet retrieval and pasta handling tasks, have provided important insight into motor learning and recovery after neurological damage (Whishaw et al. 1986; Montoya et al. 1991; Ballermann et al. 2001). While powerful, many of these tasks lack automation and require qualitative scoring of end-point measures. High-speed video analysis techniques provide valuable analysis of individual components of complex movements, including forelimb supination, that are too fast to measure in real-time (Whishaw et al. 1993; Alaverdashvili and Whishaw 2010; Carmel et al. 2010). However, video analysis greatly amplifies the cost and time to run experiments and precludes high-throughput testing.
We have developed a novel, automated method to quantitatively assess forelimb supination in rats. The task requires rats to reach through a narrow slot, grasp a spherical manipulandum similar to a doorknob, and rotate the manipulandum by supinating the forelimb to receive a reward pellet. The task is fully automated and allows testing of multiple animals simultaneously. Furthermore, this operant task provides a high-resolution, quantitative end-point dataset of turn angle, which obviates the necessity of video analysis. Assessment of various physical measures of forelimb rotational function can be calculated, including peak turn angle and rotational velocity.
Here we demonstrate that the supination assessment task can measure long-term deficits across multiple measures in two models of ischemic stroke. These results indicate that this task provides an efficient, sensitive measure of forelimb supination function, and may be useful to accelerate the preclinical development of therapies to improve complex aspects of motor function.
2. Methods
2.1 Subjects
Fifteen adult female Sprague-Dawley rats weighing approximately 250g throughout the study were used in this experiment. All rats were maintained above 85% of their ideal body weight for their specific age. The rats were housed in a 12:12 reversed light cycle environment and behavioral training was performed during the dark cycle to increase daytime activity levels. All handling, housing, surgical procedures, and behavioral training were approved by the University of Texas at Dallas Institutional Animal Care and Use Committee.
2.2 Behavioral apparatus
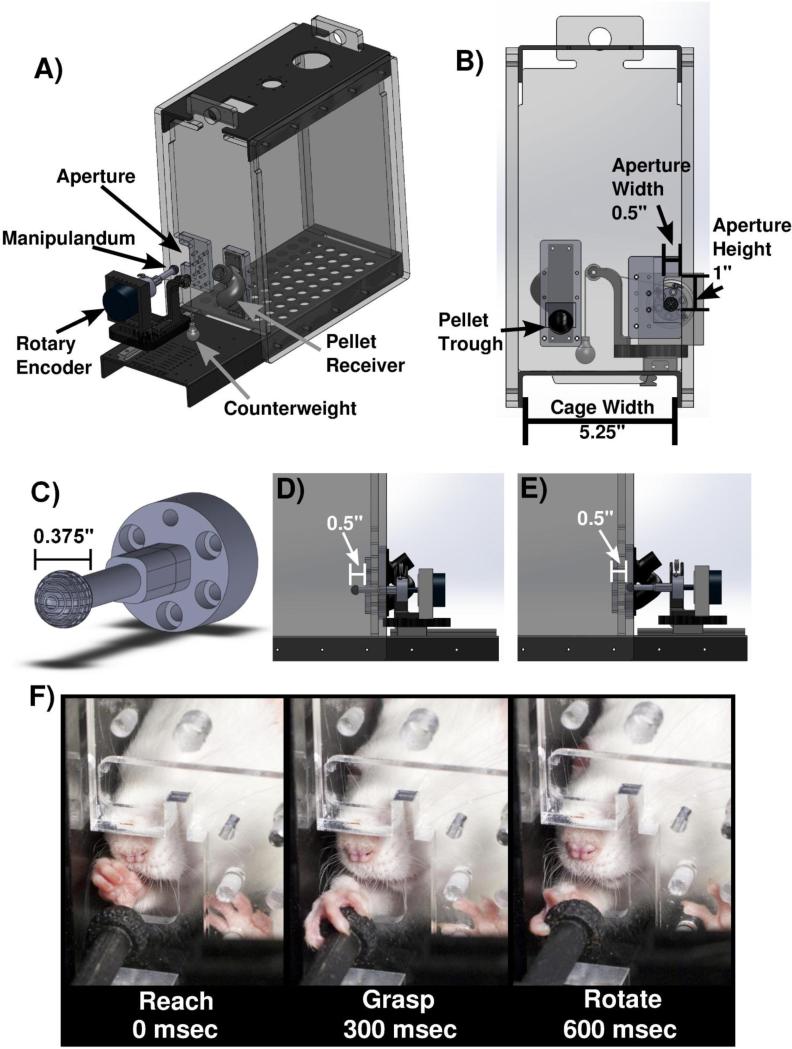
The behavioral chamber consists of a clear acrylic cage (12” × 4” × 10”) with a 0.5” wide slot on the right edge of the front wall (MotoTrak Base Cage Rat Model, Vulintus, Inc., Dallas, TX) (Fig 1A). The slot restricts use to the right forelimb while allowing full range of movement during interaction with the device (Fig. 1B). The spherical manipulandum is 0.375” in diameter and has miniature grooves to facilitate grip (Fig. 1C). An optical rotary encoder measures turn angle of the manipulandum with a 0.25 degree resolution. The encoder is mounted on a metal slide allowing the device to be placed at various fixed distances relative to the inside wall of the cage (Fig. 1D & 1E). A pulley provides counterweight to the manipulandum, limiting animals to clock-wise rotation (supination) while providing a constant torque and returning the manipulandum to the original location once released (Supplementary Video 1). Three pulley configurations with differing counterweights were used in this study: no counterweight, a 6-gram counterweight enacting 0.29mN*m of torque on the manipulandum, and a 7.5-gram counterweight enacting 0.37mN*m (MotoTrak Behavior Module, Vulintus, Inc., Dallas, TX).
Figure 1.
Behavioral apparatus. (A) Solidworks model of behavioral cage and device. (B) View from inside of the behavioral cage. Aperture dimensions and location restricts use to right forelimb only. Pellet trough is located on the left side of the front wall. (C) Close-up of manipulandum. (D) View of device located inside of the cage at the initial training position of −0.5” inside of cage wall. (E) Close-up of device fully retracted outside of the cage, at the final training location 0.5” from inside of cage wall. (F) Sequential illustration of animal reaching, grasping, and rotating the manipulandum.
2.3 Software and Behavioral Training
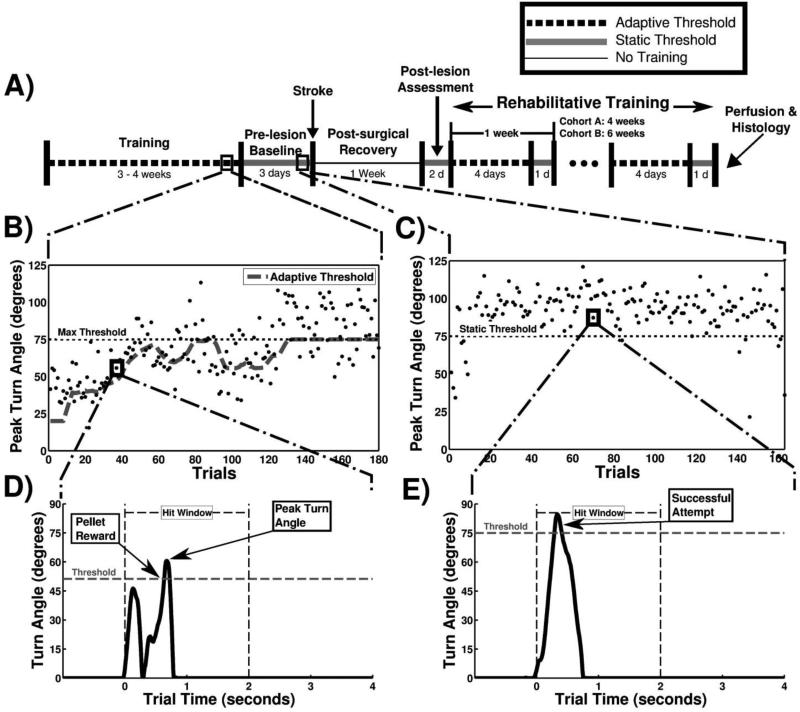
Custom MATLAB software was used to control the task (MotoTrak Software, Vulintus, Inc., Dallas, TX). The GUI displays real-time turn angle of the device in degrees with a 100 Hz sampling frequency and performance over the course of the behavioral session. Data was collected and stored on a trial-by-trial basis for each animal. Trial initiation occurred when the animal rotated the device a minimum of 5 degrees. Animals were required to rotate the pre-determined turn angle threshold within two seconds of trial initiation to receive a reward pellet and record a successful trial (Fig. 2D). If the turn angle did not exceed the threshold within the two seconds, the trial was recorded as a failure and no reward pellet was given. An additional two-second timeout window followed in which no pellet rewards were delivered to the animal. All activity one second prior and four seconds following trial initiation was recorded for analysis (Fig 2D & 2E). Reward pellets were delivered from pellet dispensers (Pellet Dispenser, Vulintus, Inc., Dallas, TX), delivering a 45mg pellet (dustless chocolate precision pellet, BioServ, Frenchtown, NJ) upon successful completion of a trial.
Figure 2.
Experimental timeline and behavioral training. (A) Timeline of experiment. Training was performed twice daily. One week represents 5 days of training or 10 behavioral sessions. (B) Peak turn angle of individual trials taken from one session during the adaptive training. Each point represents the peak turn angle of a single trial. The horizontal black dotted line at 75 degrees represents the maximum threshold for Cohort B, and the large gray dashed line indicates the adaptive threshold within one behavioral session. (C) Example session during static training with the horizontal black dotted line at 75 degrees indicating the static threshold. (D) A single, representative trial from a pre-lesion animal during an adaptive training session. The horizontal gray dashed line indicates the turn angle success threshold. The leftmost arrow indicates when a pellet reward is delivered once the turn angle crosses the success threshold, and the rightmost arrow shows the peak turn angle of the trial. (E) A single trial from a pre-lesion animal during a static training session, with the arrow indicating a successful trial attempt in which the turn angle exceeded the threshold.
Animals underwent two 30-minute behavioral training sessions daily, five days per week, with at least a 2-hour interval between training periods (Fig 2A). During the initial phases of shaping, the manipulandum was placed 0.5” inside from the cage wall, a position that allows animals to easily interact with it (Fig. 1D). The reward threshold was set to 5 degrees and no counterweight was attached, allowing the device to freely spin. During the initial phases of training, the experimenter encouraged animal interaction with the manipulandum by using ground pellet dust. When association between device rotation and pellet rewards was made, the device was retracted outside of the cage in 0.25” increments to a final location of 0.50” outside of the inner cage wall (Fig. 1E). The counterweight was then added and animals began the adaptive training program.
A training algorithm that uses adaptive success thresholds was utilized throughout this study. The algorithm uses the median of the peak turn angle of the previous 10 trials to calculate the current trial success threshold, with programmable minimum and maximum adaptive threshold bounds (Fig. 2B). The present study consisted of two cohorts (Cohort A and B), with both cohorts training with a minimum adaptive threshold bound of 15 degrees (i.e., the success threshold was never lower than 15 degrees). Cohort A was trained with a 7.5-gram counterweight and a 60-degree maximum adaptive threshold, and Cohort B was trained with a 6-gram counterweight and a 75-degree maximum adaptive threshold. Success rate on adaptive stages is defined as the percentage of trials greater than the maximum threshold (Fig. 2B). In both cohorts, once animals recorded four consecutive behavioral sessions with at least a 50% success rate, animals progressed to the prelesion baseline phase of training. Pre-lesion baseline was conducted on a static (i.e., non-adaptive) threshold stage, with the threshold fixed at the maximum adaptive threshold (Fig. 2C; Cohort A: 60 degrees; Cohort B: 75 degrees). Training continued until animals achieved a 75% success rate or greater averaged across six consecutive training sessions. Data from these six sessions was used for the “PRE” time point in all analyses. At this point, animals were considered proficient at the task and unilateral ischemic lesions were administered.
No behavioral testing was conducted for the 7 days following lesion. Following this seven day recovery period, animals were re-assessed on the static stage for four sessions, with this data being used for the “POST” time point in all analyses. Behavioral testing then continued twice daily for either 4 or 6 weeks depending on the cohort. Testing and analyses was split in to 1 week blocks, each consisting of ten consecutive sessions. The first eight sessions of testing for each week were conducted on the adaptive stage, and the ninth and tenth weekly sessions were on the static stage (Fig. 2A).
2.4 Unilateral motor cortex ischemic lesion
Unilateral motor cortex ischemic lesions were administered similar to previously described (Hays et al. 2013a, 2013b, 2014; Khodaparast et al. 2013, 2014, 2015; Sloan et al. 2015). Rats were anesthetized with ketamine hydrochloride (80mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and given supplemental doses as needed. Rats were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) and a craniotomy was performed to expose the forelimb area of motor cortex contralateral to the trained limb. The dura mater was delicately removed. A 26-gauge Hamilton syringe affixed to the stereotaxic frame was used to inject endothelin-1 (Bachem, Torrence, CA, 1mg/mL in saline) at eight locations: anteriorposterior 2.5mm, 1.5mm, 0.5mm, −0.5mm, and mediolateral 2.5mm and 3.5mm relative to bregma, at a depth of 1.8mm. In Cohort B, an additional ninth injection site at 3.0mm lateral and 0mm anteriorposterior from bregma at a depth of 6mm was administered to target the dorsolateral striatum. All injections consisted of 0.2uL of endothelin-1 every 30 seconds for a total of 2 minutes and 1uL total volume. The Hamilton syringe was left in place for an additional 3 minutes after injection to minimize backflow. After the final injection, Kwik-Cast Sealant (World Precision Instruments, Sarasota, FL) was used to cover the craniotomy and coated with a thin layer of acrylic. The head incision was then sutured and treated with antibiotic ointment.
2.5 Histology
Within one week of the conclusion of behavioral testing, a subset of animals from Cohort A (n = 5) and Cohort B (n = 5) were transcardially perfused with 4% paraformaldehyde. Brains were removed and fixed in 4% paraformaldehyde overnight, and then cryoprotected in a 30% sucrose solution. Tissue was sectioned in 50-μm slices and processed with Nissl and myelin stains for lesion identification.
2.6 Statistics
All data is represented as mean ± SEM. All comparisons were planned in the experimental design a priori, and significant differences were determined using one-way repeated measures ANOVA, and two-tailed t-tests where appropriate. Alpha level was set to 0.05 for single comparisons and a Bonferroni-corrected alpha of 0.01 for Experiment 1 and an alpha of 0.007 for Experiment 2 for multiple comparisons were used where appropriate. Statistical tests for each comparison are noted in the text. Differences of p<0.05 are indicated with an asterisk (*) and error bars are ± SEM in all figures.
3. Results
3.1 Experiment 1: Motor cortex lesion impairs forelimb rotation
A cohort of rats (Cohort A: n = 7) were trained on the supination assessment task. Rats began to form an operant association between device manipulation and pellet reward within 5 ± 2 sessions, and completed adaptive training within 21 ± 2 sessions. Animals continued to train for an additional 31 ± 7 sessions on the final device configuration of 7.5-gram counterweight and 60 degree static threshold until they reached the criteria for lesion, defined as a 75% success rate or greater across six consecutive sessions. In total, animals completed training within 56 ± 11 sessions.
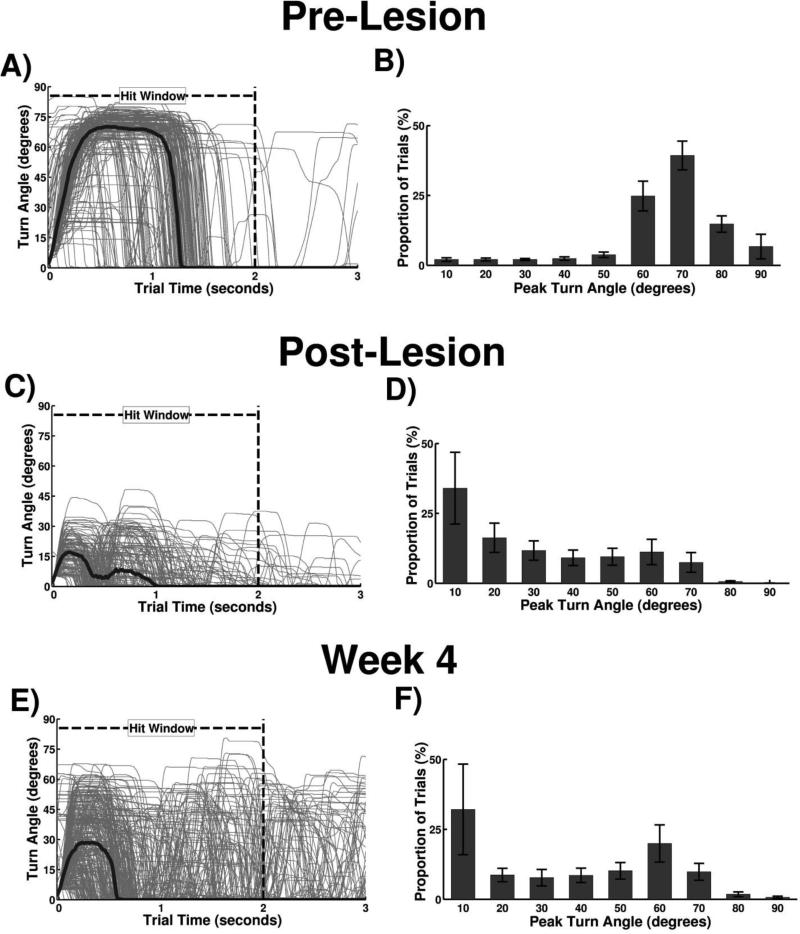
Animals were highly skilled at the task prior to injury, rotating the manipulandum a minimum of 60 degrees within 2 seconds on 84.0 ± 1.6% of trials (Fig. 3B). Forelimb kinematics of a trained animal performing a single trial are illustrated in Figure 1F. Individual trial attempts demonstrate a narrow distribution with the majority of attempts exceeding 60 degrees, indicative of consistent performance from trial to trial (Fig. 4A). The average pre-lesion turn angle histogram across all animals in Cohort A exhibits a strong left skew greater than 60 degrees, indicating that most trials exceed the performance threshold (Fig. 4B).
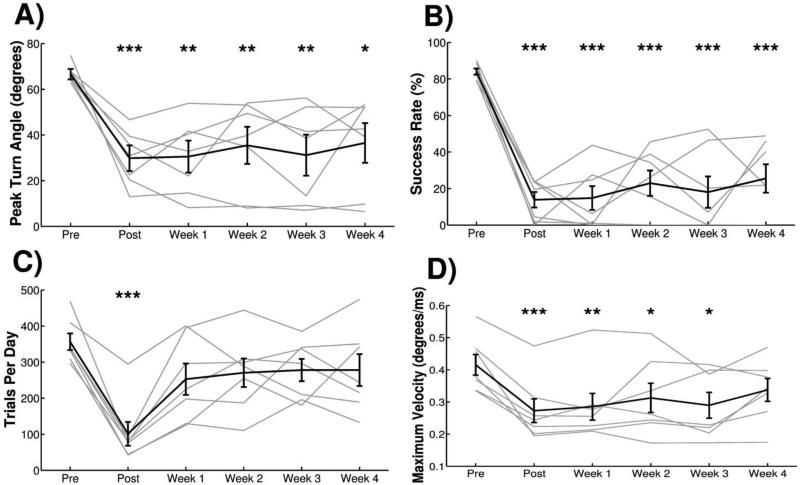
Figure 3.
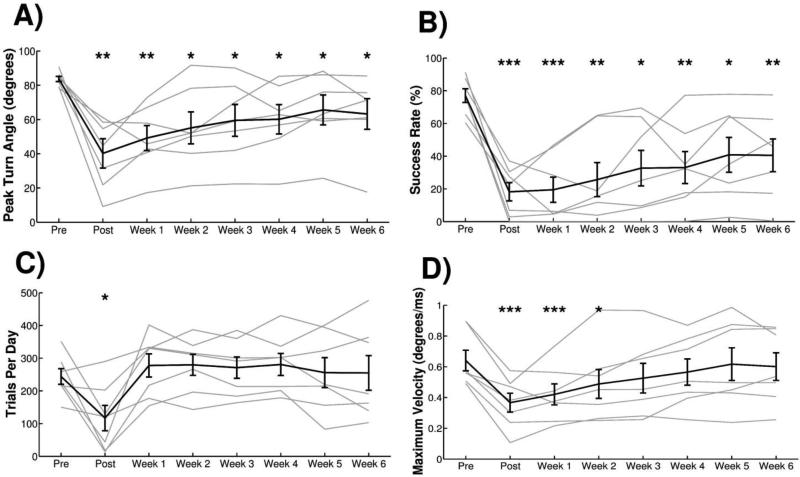
Experiment 1: Ischemic lesion of motor cortex impairs multiple measures of task performance. (A) Peak turn angle and (B) success rate of animals in Cohort A was significantly reduced compared to pre-lesion at all time points following lesion. (C) Number of trials performed per day showed a transient reduction during Post, but returned to pre-lesion levels during Weeks 1-4. (D) The maximum turn velocity, calculated as the maximum of the derivative of the turn angle, exhibited a transient reduction following injury but did not reach significance at Week 4. All plots show group averages (N=7) in black lines and light gray lines represent individual animals. Error bars indicate SEM. Significant differences were determined by paired t-tests and are noted as *p<0.05, **p<0.01, ***p<0.001.
Figure 4.
Turn angle measurements from a single animal and group turn angle histograms. (A) Trial signals overlaid from one pre-lesion animal during a behavioral session. Gray lines represent individual trial signals, and thick black line indicates average turn angle at each sample. (B) Pre-lesion group histogram of peak turn angle from Cohort A. (C) Trial signals and average overlaid from one post-lesion behavioral session. (D) Post-lesion group histogram of peak turn angle from Cohort A. (E) Trial signals and average overlaid from one session during the fourth week of post-injury training. (F) Group histogram from the fourth week of post-injury training.
Ischemic lesions were administered targeting the forelimb area of the left motor cortex to impair motor function of the trained forelimb (Figure 5). All observed metrics of task performance were substantially reduced by ischemic lesion. A repeated measures one-way ANOVA showed a significant effect of lesion on peak turn angle (Fig. 3A, F[5,30] = 8.56, p<0.001) and success rate (Fig. 3B, F[5,30] = 26.649, p<0.001). Peak turn angle was significantly decreased relative to pre-lesion during POST and Week 1, and hit rate was significantly decreased relative to pre-lesion at all post-lesion time points (Pre-lesion v. each week, paired t-test, p < 0.01 for all time points). The number of trials attempted per day showed a significant reduction following lesion at the POST time point, but returned to pre-lesion levels for the following weeks (Fig. 3C, Repeated measures one-way ANOVA, F[5,30] = 7.78, p=0.003; Pre-lesion v. each week, paired t-test, p < 0.01 at POST). Analysis of the maximum turn velocity exhibited a transient reduction during POST and Week 1 time points (Fig. 3D, Repeated measures one-way ANOVA, F[5,30] = 8.87, p<0.001; Pre-lesion v. each week, paired t-test, p < 0.01 at POST and Week 1). This suggests that the speed with which the animals turn the manipulandum is transiently slowed after injury, consistent with forelimb bradykinesia.
Figure 5.
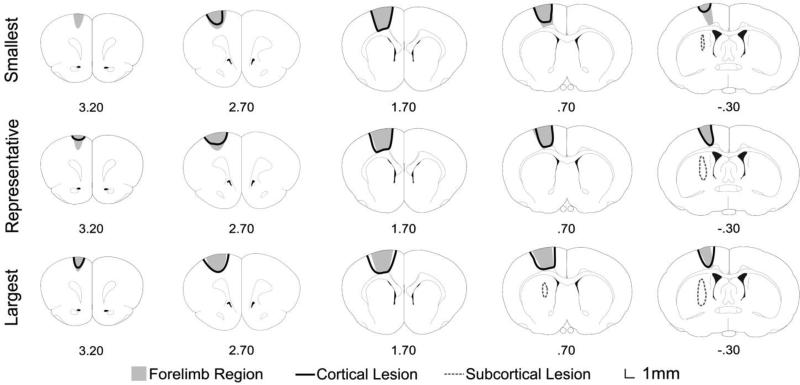
Reconstructions detailing the extent of ischemic damage from the smallest, representative, and largest lesions observed. Numbers denote distance from bregma in mm.
Examination of performance on individual trials highlights impairments in rotational function (Supplementary Video 2: POST; Supplementary Video 3: Week 4). Turn attempts became lower and more dispersed following injury, with substantially fewer attempts exceeding 60 degrees even on the fourth week of training (Fig. 4C & 4E). The group turn angle histogram shows a prominent leftward shift following injury, with a slight shift right after four weeks of training indicating partial recovery of function (Fig. 3D & 3F). Together, these findings demonstrate that the supination assessment task can measure lasting impairments in forelimb rotation after cortical ischemia.
3.2 Experiment 2: Combined cortical and subcortical lesion impairs forelimb rotation
A second experiment was conducted to replicate the results above with different task parameters and lesion. Animals (Cohort B: n = 7) were trained with a final device configuration of 6-gram counterweight and 75-degree maximum threshold. All other training procedures were conducted identically to Experiment 1. Operant association between device interaction and pellet rewards was achieved within 6 ± 1 sessions. Animals continued on adaptive training for 18 ± 5 sessions and completed pre-lesion baselines within 24 ± 5 sessions. Training was completed within 48 ± 8 sessions. Animals became highly proficient at the task preceding injury, successfully completing trials 77.0 ± 1.5% % of the time.
Consistent with the results in Experiment 1, combined cortical and subcortical lesions resulted in significant reductions in multiple measures of performance (Figure 6). A repeated measures ANOVA showed a significant effect of lesion on peak turn angle (Fig. 6A, F[7,42] = 9.43, p<0.001) and success rate (Fig. 6B, F[7,42] = 11.17, p<0.001). Peak turn angle was significantly decreased relative to pre-lesion for POST and Week 1 time points, and hit rate was significantly decreased relative to pre-lesion during POST and Weeks 1-3 (Pre-lesion v. each week, paired t-test, p < 0.007 for POST and Weeks 1-3). A repeated measures ANOVA revealed a significant effect of lesion on trials per day, however paired t-tests at each time point post-lesion compared to pre-lesion do not show any significant reductions (Fig. 6C, Repeated measures one-way ANOVA, F[7,42] = 5.34, p=0.01; Pre-lesion v. each week, paired t-test, p > 0.007 for all time points). Maximum turn velocity showed a transient significant reduction following lesion up to Week 2 (Fig. 6D, Repeated measures one-way ANOVA, F[7,42] = 7.78, p=0.003; Pre-lesion v. each week, paired t-test, p < 0.007 for POST and Weeks 1-2). These findings confirm that the supination assessment task is capable of detecting long-lasting impairments in forelimb rotation after neurological injury.
Figure 6.
Experiment 2: Combined cortical and subcortical lesion impairs multiple measures of task performance. (A) Peak turn angle and (B) success rate was significantly reduced at all time points following injury. (C) Number of trials performed per day showed a transient reduction but returned to pre-lesion levels during Weeks 1-4. (D) The maximum turn velocity exhibited a transient reduction following injury through Week 2. All plots show group averages (N=7) in black lines and light gray lines represent individual animals. Error bars indicate SEM. Significant differences were determined by paired t-test and are noted as * p<0.05, ** p<0.01, *** p<0.001.
3.3 Adaptive training provides equivalent measures of forelimb rotational function and increased trial counts compared to static thresholds
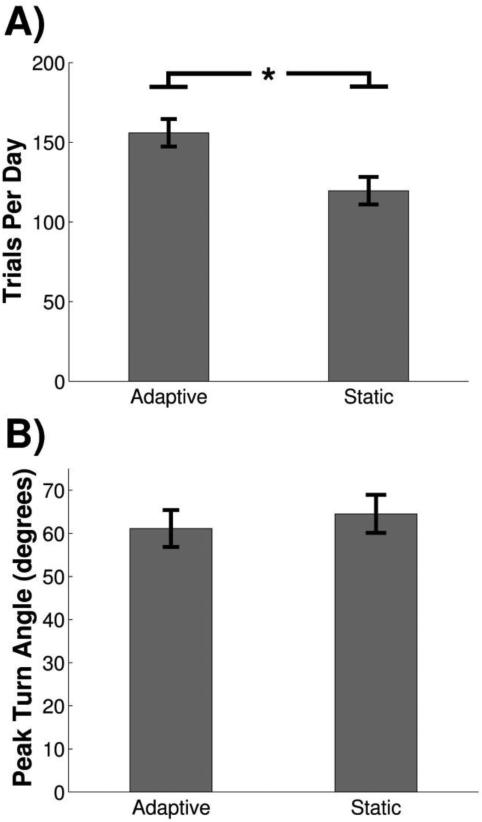
Two different thresholding paradigms were used in this study in order to account for variations in performance after injury. Adaptive thresholding calculates the median peak turn angle of the previous 10 trials to set the current trial success threshold within a training session (Fig. 2B & 2D). Static thresholding uses a fixed, user-defined threshold throughout an entire training session (Fig. 2C & 2E). We tested whether the different thresholding paradigms altered performance. To investigate this, animals in Cohort B were tested each week on eight sessions of adaptive training and two sessions with a static threshold. No significant difference in peak turn angle was detected between adaptive and static thresholds (Fig. 7A; Adaptive, 61.14 ± 4.29; Static, 64.53 ± 4.42; paired t-test, p=0.15). However, animals performed significantly more trials on adaptive than static (Fig. 7B; Adaptive, 155.95 ± 9.91; Static, 119.59 ± 8.64; paired t-test, p<0.01). These results demonstrate that the adaptive thresholding algorithm provides an equivalent measure of performance and increases trial counts compared to static thresholding.
Figure 7.
Adaptive training increases trial counts while providing an equivalent measure of performance compared to static thresholds. (A) Animals perform significantly more trials per day during adaptive training compared to static training. (B) Peak turn angle was not significantly different between adaptive and static thresholding sessions.
4. Discussion
In this study we describe a novel, automated task to quantitatively assess volitional forelimb rotation in rats. The task requires animals to reach, grasp, and supinate their forelimb to turn a spherical manipulandum attached to a rotary encoder measuring turn angle. Various hardware and software parameters can easily be modified, such as reach distance and turn angle thresholds. Two models of ischemic stroke result in significant, chronic impairments in multiple performance metrics, indicating that this task may be useful in the preclinical development and evaluation of therapies focused on improving forelimb function.
Many forms of neurological injuries and disease impair precise arm and hand motor function (Duncan et al. 1994; Noble et al. 1998; Snoek et al. 2004). Forelimb rotation is often severely diminished, and recovery is minimal (Vergara-Aragon et al. 2003; MacLellan et al. 2006; Lambercy et al. 2011). The prevalence of rotational deficits highlights the need for an efficient preclinical test to evaluate this motor function in rodents. Here, we demonstrate that the supination assessment task can effectively measure deficits in multiple parameters of rotational motor function in two models of ischemic stroke. Additionally, the animals in the present study underwent intensive training over multiple weeks, performing 7851 ± 651 trials after injury. Despite substantial task-oriented training after injury, several metrics remained significantly impaired weeks after injury. These results are consistent with the lasting forearm supination impairments in patients with neurological injury and disease and provide a framework for testing therapeutic interventions to restore rotational function.
Recently, several automated methods of evaluating motor function in the rat have been developed (Hays et al. 2013a, 2013b; Poddar et al. 2013; Jarrahi et al. 2015; Kawai et al. 2015; Sloan et al. 2015; Wong et al. 2015; Becker et al. 2016). Automation of motor assessments obviates many limitations of traditional assessments such as single pellet retrieval, permitting high-throughput testing and potentially decreasing the cost and time of running experiments. While these automated methods provide valuable insight in to complex learned behaviors, motor coordination, and forelimb strength, no current methods provide specific measurement of forelimb supination. Current methods to evaluate forelimb supination necessitate the use of video analysis, which is laborious and requires supervision by skilled personnel. The supination task eliminates the need of scoring and reduces experimenter oversight, providing high-throughput, unbiased measures of performance. Additional measures of task performance, such as turn velocity, acceleration, total turn time, and latency to reach threshold can be calculated. Examination of these secondary metrics may lend valuable insight in to motor function and recovery in various injury models. The efficiency of this system has the potential to decrease the cost and time of running experiments while providing accurate measures of forelimb rotational function.
In this study adaptive thresholds were utilized during the pre and post-lesion phases. Adaptive thresholding allows software control of success thresholds, which streamlines training progression. Following injury, adaptive thresholds allow the software to precisely track performance across a range of behavioral deficits. In the current study, no animals were excluded due to variability in motor impairment, which may be due in part to the adaptive thresholding paradigm. Additionally, trial counts remain consistent across subjects, potentially making comparisons across groups more reliable. Independent of injury, custom training regimes may also be employed to evaluate other specific aspects of motor learning.
This task provides a high degree of flexibility to the user, with multiple dimensions of the task capable of being adjusted. Hardware parameters, including counterweight and reach distance, were adjusted throughout this study to facilitate training. Software parameters, such as turn angle criterion, adaptive thresholding, and hit-window length, can be modified before or online during training sessions. This flexibility may allow the task to be applied to a wide range of injury models with variable impairments, such as spinal cord injury or Parkinson's disease. Additionally, this task could also be adapted for mice to capitalize on the diverse set of genetic models. Although not utilized in this study, our system has the hardware and software required to implement infrared slot detectors to measure reach attempts. This sensor could be used to monitor initiation of reaches, quantify the number of reach attempts, and correlate the number of reaches with the number of rotational attempts.
In summary, here we provide a description of the supination assessment task, an automated measure of forelimb rotation. The task can detect long-lasting impairments in models of ischemic stroke and provides automated collection and analysis of a rich, quantitative dataset of forelimb rotation. Various aspects of the task are easily modified, which may allow it to be adapted for various lesion types and rodent models. The supination assessment task represents a novel, efficient method of evaluating forelimb rotation and may help accelerate the development of motor therapies.
Supplementary Material
Highlights.
Here we describe the supination assessment task, a novel automated method of quantifying forelimb supination in the rat.
Animals are trained to reach out of a cage, grasp a spherical manipulandum, and supinate the forelimb.
A rotary encoder provides a high-resolution, quantitative dataset of turn angle over time.
Ischemic lesions of primary motor cortex significantly impair multiple measures of task performance.
The supination assessment task represents a novel, efficient method of evaluating forelimb rotation and may help decrease the cost and time of running experiments.
Acknowledgements
This project was supported in part by funding from the Defense Advanced Research Projects Agency, NIH NINDS R01 NS085167, NIH NIDCD R01 DC010433, NIH NINDS R44NS086344, NIH NINDS R03NS091737 and the Texas Biomedical Device Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: AMS and RLR own shares in Vulintus, Inc., which is developing products based on this research. Vulintus, Inc. did not have any role in data collection, analysis, or the decision to publish.
References
- Alaverdashvili M, Whishaw IQ. Neuroscience [Internet] 1. Vol. 167. Elsevier Inc.; May 28, 2010. [2014 Oct 31]. Compensation aids skilled reaching in aging and in recovery from forelimb motor cortex stroke in the rat. pp. 21–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20149844. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Ballermann M, Metz GA, McKenna JE, Klassen F, Whishaw IQ. The pasta matrix reaching task: a simple test for measuring skilled reaching distance, direction, and dexterity in rats. [2014 Oct 18];J Neurosci Methods [Internet] 2001 Mar;106(1):39–45. doi: 10.1016/s0165-0270(01)00326-0. Available from: http://www.sciencedirect.com/science/article/pii/S0165027001003260. [DOI] [PubMed] [Google Scholar]
- Becker AM, Meyers E, Sloan A, Rennaker R, Kilgard M, Goldberg MP. J Neurosci Methods [Internet] Vol. 258. Elsevier B.V.; 2016. An automated task for the training and assessment of distal forelimb function in a mouse model of ischemic stroke. pp. 16–23. Available from: http://dx.doi.org/10.1016/j.jneumeth.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braendvik SM, Elvrum A-KG, Vereijken B, Roeleveld K. Relationship between neuromuscular body functions and upper extremity activity in children with cerebral palsy. [2014 Oct 31];Dev Med Child Neurol [Internet] 2010 Mar;52(2):e29–34. doi: 10.1111/j.1469-8749.2009.03490.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19811515. [DOI] [PubMed] [Google Scholar]
- Carmel JB, Kim S, Brus-Ramer M, Martin JH. Feed-forward control of preshaping in the rat is mediated by the corticospinal tract. [2014 May 1];Eur J Neurosci [Internet] 2010 Nov;32(10):1678–85. doi: 10.1111/j.1460-9568.2010.07440.x. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3058632&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Goldstein LB, Horner RD, Landsman PB, Samsa GP, Matchar DB. Similar motor recovery of upper and lower extremities after stroke. Stroke. 1994;25(6):1181–8. doi: 10.1161/01.str.25.6.1181. [DOI] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, et al. The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. [2014 Oct 3];Neuroreport [Internet] 2014 Jun 18;25(9):682–8. doi: 10.1097/WNR.0000000000000154. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24818637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Sloan AM, Fayyaz T, Hulsey DR, Ruiz AD, et al. The bradykinesia assessment task: An automated method to measure forelimb speed in rodents. [2014 Aug 11];J Neurosci Methods [Internet] 2013a Mar 30;214(1):52–61. doi: 10.1016/j.jneumeth.2012.12.022. Available from: http://www.sciencedirect.com/science/article/pii/S016502701200489X. [DOI] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Sloan AM, Hulsey DR, Pantoja M, Ruiz AD, et al. The isometric pull task: A novel automated method for quantifying forelimb force generation in rats. [2014 Aug 10];J Neurosci Methods [Internet] 2013b Jan 30;212(2):329–37. doi: 10.1016/j.jneumeth.2012.11.007. Available from: http://www.sciencedirect.com/science/article/pii/S0165027012004633. [DOI] [PubMed] [Google Scholar]
- Jaquet JB, Luijsterburg AJ, Kalmijn S, Kuypers PD, Hofman A, Hovius SE. Median, ulnar, and combined median-ulnar nerve injuries: functional outcome and return to productivity. [2014 Oct 4];J Trauma [Internet] 2001 Oct;51(4):687–92. doi: 10.1097/00005373-200110000-00011. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11586160. [DOI] [PubMed] [Google Scholar]
- Jarrahi M, Sedighi Moghadam B, Torkmandi H. J Neurosci Methods [Internet] Vol. 251. Elsevier B.V.; Aug, 2015. An experimental evaluation of a new designed apparatus (NDA) for the rapid measurement of impaired motor function in rats. pp. 138–42. Available from: http://www.sciencedirect.com/science/article/pii/S0165027015002149. [DOI] [PubMed] [Google Scholar]
- Kawai R, Markman T, Poddar R, Ko R, Fantana AL, Dhawale AK, et al. Motor Cortex Is Required for Learning but Not for Executing a Motor Skill. Neuron [Internet] 2015:800–12. doi: 10.1016/j.neuron.2015.03.024. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0896627315002202. [DOI] [PMC free article] [PubMed]
- Khodaparast N, Hays S a, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, et al. Vagus Nerve Stimulation Delivered During Motor Rehabilitation Improves Recovery in a Rat Model of Stroke. [2014 Sep 5];Neurorehabil Neural Repair [Internet] 2014 Sep 1;28(7):698–706. doi: 10.1177/1545968314521006. Available from: http://nnr.sagepub.com/content/early/2014/02/14/1545968314521006.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, et al. Neurobiol Dis [Internet] Vol. 60. Elsevier Inc.; Dec, 2013. [2014 Aug 27]. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. pp. 80–8. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0969996113002234. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, et al. Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats. Neurorehabil Neural Repair [Internet] 2015 Nov 4; doi: 10.1177/1545968315616494. Available from: http://nnr.sagepub.com/cgi/doi/10.1177/1545968315616494. [DOI] [PMC free article] [PubMed]
- Klotz MCM, Kost L, Braatz F, Ewerbeck V, Heitzmann D, Gantz S, et al. Gait Posture [Internet] 1. Vol. 38. Elsevier B.V.; May, 2013. [2014 Oct 30]. Motion capture of the upper extremity during activities of daily living in patients with spastic hemiplegic cerebral palsy. pp. 148–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23218727. [DOI] [PubMed] [Google Scholar]
- Lambercy O, Dovat L, Yun H, Wee SK, Kuah CWK, Chua KSG, et al. J Neuroeng Rehabil [Internet] 1. Vol. 8. BioMed Central Ltd; Jan, 2011. [2014 May 1]. Effects of a robot-assisted training of grasp and pronation/supination in chronic stroke: a pilot study. p. 63. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3280186&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. [2014 Jul 10];Lancet Neurol [Internet] 2009 Aug;8(8):741–54. doi: 10.1016/S1474-4422(09)70150-4. Available from: http://www.sciencedirect.com/science/article/pii/S1474442209701504. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Gyawali S, Colbourne F. Skilled reaching impairments follow intrastriatal hemorrhagic stroke in rats. [2014 Oct 31];Behav Brain Res [Internet] 2006 Dec 25;175(1):82–9. doi: 10.1016/j.bbr.2006.08.001. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16956678. [DOI] [PubMed] [Google Scholar]
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36(2- 3):219–28. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. [2014 Oct 4];J Trauma [Internet] 1998 Jul;45(1):116–22. doi: 10.1097/00005373-199807000-00025. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9680023. [DOI] [PubMed] [Google Scholar]
- Poddar R, Kawai R, Ölveczky BP. Schaefer A, editor. A Fully Automated High-Throughput Training System for Rodents. PLoS One [Internet] 2013 Dec 6;8(12):e83171. doi: 10.1371/journal.pone.0083171. Available from: http://dx.plos.org/10.1371/journal.pone.0083171. [DOI] [PMC free article] [PubMed]
- Sloan AM, Fink MK, Rodriguez AJ, Lovitz AM, Khodaparast N, Rennaker RL, et al. Sutherland R, editor. A Within-Animal Comparison of Skilled Forelimb Assessments in Rats. PLoS One [Internet] 2015 Oct 27;10(10):e0141254. doi: 10.1371/journal.pone.0141254. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26506434. [DOI] [PMC free article] [PubMed]
- Snoek GJ, IJzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord [Internet] 2004;42(9):526–32. doi: 10.1038/sj.sc.3101638. Available from: http://www.nature.com/doifinder/10.1038/sj.sc.3101638. [DOI] [PubMed] [Google Scholar]
- Vergara-Aragon P, Gonzalez CLR, Whishaw IQ. A Novel Skilled-Reaching Impairment in Paw Supination on the “Good” Side of the Hemi-Parkinson Rat Improved with Rehabilitation. [2014 Sep 4];J Neurosci [Internet] 2003 Jan 15;23(2):579–86. doi: 10.1523/JNEUROSCI.23-02-00579.2003. Available from: http://www.jneurosci.org/content/23/2/579.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WC, Pickett TC. Motor impairment after severe traumatic brain injury: A longitudinal multicenter study. J Rehabil Res Dev. 2007;44(7):975–82. doi: 10.1682/jrrd.2006.12.0158. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, O'Connor WT, Dunnett SB. The contributions of motor cortex, nigrostriatal dopamine and caudate-putamen to skilled forelimb use in the rat. Brain. 1986;109(Pt 5):805–43. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny B, Kolb B, Tetzlaff W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav Brain Res [Internet] 1993 Jul;56(1):59–76. doi: 10.1016/0166-4328(93)90022-i. Available from: http://linkinghub.elsevier.com/retrieve/pii/016643289390022I. [DOI] [PubMed] [Google Scholar]
- Wong CC, Ramanathan DS, Gulati T, Won SJ, Ganguly K. J Neurosci Methods [Internet] Vol. 246. Elsevier B.V.; May, 2015. An automated behavioral box to assess forelimb function in rats. pp. 30–7. Available from: http://dx.doi.org/10.1016/j.jneumeth.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.