Abstract
Background & Aims
Uncontrolled studies show sitagliptin, an oral DPP-4 inhibitor, may improve alanine aminotransferase (ALT) and liver histology in nonalcoholic fatty liver disease (NAFLD) patients. We aimed to compare sitagliptin versus placebo's efficacy in reducing liver fat measured by MRI-derived proton density-fat fraction (MRI-PDFF).
Methods
This randomized, double-blind, allocation-concealed, placebo-controlled trial included 50 NAFLD patients with pre-diabetes or early diabetes randomized to sitagliptin orally 100 mg/day or placebo for 24 weeks. Primary outcome was liver fat change measured by MRI-PDFF in co-localized regions of interest within each of nine liver segments. Additional advanced assessments included MR spectroscopy (MRS) for internal validation of MRI-PDFF's accuracy, and MR elastography (MRE) and FIBROSpect® II to assess liver fibrosis.
Results
Sitagliptin was not significantly better than placebo in reducing liver fat measured by MRI-PDFF (mean difference between sitagliptin and placebo arms: −1.3%, p=0.4). Compared to baseline, there were no significant differences in end-of-treatment MRI-PDFF for sitagliptin (18.1% to 16.9%, p=0.27) or placebo (16.6% to 14.0%, p=0.07). The groups had no significant differences for changes in ALT, aspartate aminotransferase, low-density lipoprotein, homeostatic model assessment insulin resistance, and MRE-derived liver stiffness. In both groups at baseline and post-treatment, MRI-PDFF and MRS showed robust correlation coefficients ranging from r2=0.96 to r2=0.99 (p<0.0001), demonstrating the findings’ strong internal validity. FIBROSpect® II showed no changes in the sitagliptin group but was significantly increased in the placebo group (p=0.03).
Conclusions
Sitagliptin was safe but not better than placebo in reducing liver fat in prediabetic or diabetic patients with NAFLD.
Keywords: sitagliptin, placebo, fat mapping, MRI-proton-density-fat-fraction (PDFF), lipid lowering therapy, NAFLD, hepatic steatosis, nonalcoholic steatohepatitis, noninvasive assessment, magnetic resonance elastography, imaging, biomarker, fibrosis
Graphical Abstract
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease in the United States and the Western World.1-4 NAFLD is commonly associated with metabolic syndrome features, including obesity, dyslipidemia, and diabetes.5-7 The presence of pre-diabetes and diabetes is associated with the progressive form of NAFLD also termed as nonalcoholic steatohepatitis (NASH).8, 9 Several anti-diabetic therapies have been investigated in the treatment of NASH with varying success, including metformin,10-12 rosiglitazone,13, 14 pioglitazone,15-17 and liraglutide.18
Sitagliptin is an oral antihyperglycemic agent that competitively inhibits the enzyme dipeptidyl peptidase 4 (DPP-4), which inactivates the hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) released in response to meals. By blocking GLP-1 and GIP breakdown, sitagliptin increases insulin secretion and suppresses glucagon release in the pancreas,19 which lowers blood glucose levels and improves hemoglobin A1c (HbA1c). Improvement in hyperglycemia and hyperinsulinemia results in the downregulation of sterol regulatory element binding protein-1c (SREBP-1c) and the blockage of fatty acid synthase,20 which should lead to improvement in liver fat and NASH. This provides mechanistic justification to conduct human trials with sitagliptin in NAFLD patients.
In clinical trials conducted in patients with type 2 diabetes mellitus (T2DM), sitagliptin has been shown to be effective in improving glycemic control, cholesterol, and lipoproteins 21-23 compared to placebo. Recent studies have shown that sitagliptin may improve serum alanine (ALT) and aspartate (AST) aminotransferase levels and gamma- glutamyl transpeptidase (GGT) in Japanese patients with type 2 diabetes.24 In another uncontrolled pilot study, sitagliptin was shown to improve features of liver histology in 15 patients with type 2 diabetes over a 48 week period.25 However, human trials on sitagliptin have been limited to date because of lack of placebo-arm and allocation-concealment.
The aim of this study was to evaluate the efficacy of sitagliptin versus placebo in high risk patients with well-characterized, imaging quantified, NAFLD in reducing liver fat as measured by an accurate and well-validated, robust, quantitative magnetic resonance (MR) imaging (MRI)-based biomarker: proton density-fat fraction (MRI-PDFF). Additionally, we evaluated the efficacy of sitagliptin versus placebo in reducing liver fibrosis over a 24-week period using both advanced MR elastography (MRE) techniques and biomarker-based FIBROSpect® II testing. MRE has been shown to be effective in the noninvasive measurement of hepatic stiffness as a surrogate for hepatic fibrosis in NAFLD patients. 26-31 FIBROSpect® II has also been shown to be a highly accurate, noninvasive test to diagnose hepatic fibrosis.32, 33
MATERIALS AND METHODS
Study Design and Patient Population
We conducted an investigator-initiated, randomized, double-blind, allocation-concealed, placebo-controlled clinical trial to examine the efficacy of sitagliptin at 100 mg/day orally versus identical placebo given over 24 weeks to improve hepatic steatosis as measured by MRI-PDFF, a validated, accurate, and quantitative biomarker for hepatic steatosis. The trial was conducted in strict accordance with CONSORT guidelines (see supplementary materials for CONSORT checklist). The patient population for the trial was derived from the San Diego Integrated NAFLD Research Consortium – a city-wide network established by the principal investigator (RL) that includes four sites: UCSD Medical Center, Balboa Naval Medical Center, Kaiser Permanente Medical Center, and Sharp Health System. Patients deemed eligible were referred to the UCSD NAFLD Research Center 28, 34-38 for screening into the trial. The trial was conducted at the UCSD Clinical and Translational Research Institute and all imaging was performed at the UCSD Liver Imaging Group MRI laboratory. FIBROSpect® II testing was performed by PROMETHEUS® Therapeutics & Diagnostics (San Diego, USA). The trial was registered at clinicaltrials.gov (registration number: NCT01963845) and the trial protocol received FDA approval under an Investigational New Drug application held by RL. This clinical trial protocol was approved by the human subjects Institutional Review Board at UCSD, and all patients provided a written informed consent at the initial visit.
Inclusion Criteria
Patients were included if they were ≥18 years of age, had ALT above upper limits of normal (19 U/L for women, 30 U/L for men), had documented hepatic steatosis (≥5% on MRI-PDFF), were either pre-diabetic or controlled diabetic patients (HbA1c 5.7% – 8.0%), and provided written informed consent.
Exclusion Criteria
Patients were excluded if they met any of the following exclusion criteria: uncontrolled diabetes (HbA1c >8.0), alcohol intake >30 grams/day (3 drinks per day) within the previous 10 years or >10 grams/day within the previous year; evidence of other forms of liver disease, including hepatitis B (positive serum hepatitis B surface antigen), hepatitis C (positive hepatitis C viral RNA), autoimmune hepatitis (positive autoimmune serology and consistent biopsy), alpha-1 antitrypsin deficiency (low alpha-1 antitrypsin levels and consistent biopsy), hemochromatosis (homozygosity or heterozygosity on genetic analysis and 3+ or 4+ iron staining on biopsy), Wilson's disease (ceruloplasmin with consistent biopsy), drug-induced liver disease based on exposure and history, and biliary duct obstruction based on imaging studies; evidence of decompensated cirrhosis (Child-Pugh score >7 points); advanced liver disease (platelet count <75,000 mm2, prothrombin time >16 seconds, or a history of bleeding disorders); history of gastrointestinal bypass or use of drugs known to cause hepatic steatosis; recent initiation or change of anti-diabetic drugs, including insulin, sulfonylureas, or thiazolidinediones, or recent initiation of sitagliptin (or other drugs in the same class), within 90 days of randomization; history of acute pancreatitis within 5 years (except gallstone pancreatitis); evidence of hepatocellular carcinoma; positive human immunodeficiency virus test; active substance abuse; pregnant or trying to become pregnant; renal insufficiency; significant systemic illnesses; contraindications to sitagliptin use; and inability to undergo MRI.
Baseline Assessment at Screening
All patients underwent a baseline assessment before randomization, including detailed medical history and physical exam (see supplementary material for details).
Randomization and Allocation Concealment
The UCSD Investigational Drug Services randomized the patients into either sitagliptin or placebo groups in blocks of four in a 1:1 ratio using computer-generated numbers. Blinding and allocation concealment was rigorously maintained by independent pharmacists at the UCSD Investigational Drug Services who dispensed active or placebo treatment pills that were identical in appearance to one another. The pills were prepackaged in identical bottles and also labeled based on the computer-generated randomization numbers. The allocation sequence remained concealed throughout the trial from all study investigators. Un-blinding of treatment allocation was done only after all study procedures were completed in all study patients. The trial dataset was locked and directly analyzed by the study statistician using pre-specified data analysis plan.
Study Visits
After careful assessment at the baseline visit, patients meeting all inclusion and exclusion criteria were randomized to receive sitagliptin 100 mg orally daily or placebo for 24 weeks. Patients returned to the research clinic for follow-up visits at weeks 4, 12, and 24 (see supplementary material for details).
Primary and Secondary Outcomes
The primary outcome was change in liver fat quantified by MRI-PDFF in co-localized regions of interest (ROI) within each of the nine liver segments. The secondary outcomes were insulin sensitivity improvement as determined by HOMA-IR, change in serum AST and ALT values, and change in LDL. Changes in liver stiffness as quantified by MRE and FIBROSpect® II were exploratory end-points.
MRI and MRE Protocols
MRI-PDFF for Fat Quantification
MRI-PDFF is a non-invasive, objective, and quantitative MR imaging-based biomarker that can accurately estimate liver fat content.39, 40 PDFF measurements are independent of confounders that frequently confound fat quantification via conventional MRI techniques, including scanner manufacturer, scanner platform, and field strength.41, 42 Previously, we have shown the utility of MRI-PDFF for assessing treatment response in NASH trials.34, 37 In this study, the mean (±SD) time interval from obtaining the baseline MRI-PDFF to initiating the study drug/placebo was 27.7 (±82.1) days.
MRI-PDFF for Detailed Fat Mapping of the Entire Liver
All MR examinations were done by experienced research MR technologists in the UCSD Liver Imaging Group MRI Laboratory under the direction of the radiology investigator (CS). A trained image analyst blinded to the patients’ treatment group allocation, clinical and biochemical data, and order of scans (baseline and follow-up), performed the images analyses (see supplementary material for details).
ROI Colocalization Before and After Treatment
Colocalized ROIs were used to assess longitudinal fat changes over time. One colocalized ROI was placed in each of the nine liver segments, which were represented by nine different ROIs, on the MR exams at baseline and follow-up.
Internal Validation of MRI-PDFF using MR Spectroscopy (MRS)
MRI-PDFF and MRS were performed for each patient in a single location (voxel) in the right hepatic lobe.41, 43 The PDFF measured by MRS served as the reference for MRI-PDFF, thus allowing for internal validation of the accuracy of MRI-PDFF measurements. To ensure the colocalization of MRI-PDFF with the MRS voxel, three ROIs were placed on the MRI-PDFF maps through the top, middle, and bottle thirds of the voxel and the averages of the three MRI-PDFF estimates were obtained.
MR Elastography
MRE has been shown to be a robust, accurate biomarker for the quantitative, noninvasive evaluation of liver stiffness as a surrogate for liver fibrosis26, 27, 44, 45 and may also be helpful for the early NASH detection.46 MRE acquisitions was obtained using previously published parameters.27, 46 A direct inversion algorithm was used to convert the collected data into images (called elastograms) showing the shear stiffness of the liver at different locations (see supplementary material for details).
Colocalization for Assessing Longitudinal MRE Changes
During baseline and follow-up MRE exams, multi-slice, colocalized ROIs were manually specified in order to assess for longitudinal changes in the mechanical properties of liver stiffness and fibrosis. For all ROIs, only liver parenchyma was included, and the excluded regions were (1) regions where the magnitude or shear wave amplitude were inadequate and (2) the five top and bottom slices to avoid boundary effect.
FIBROSpect® II
FIBROSpect® II (PROMETHEUS® Therapeutics & Diagnostics, San Diego, United States) was used to assess changes in hepatic fibrosis in the sitagliptin and placebo groups at weeks 0 and 24. The FIBROSpect® II score utilizes a proprietary algorithm calculated using the biomarkers alpha-2 macroglobulin, hyaluronic acid, and tissue inhibitor metalloproteinase I (TIMP1), which were measured from blood samples collected at weeks 0 and 24. The FIBROSpect® II score then estimates the patients’ risk of having fibrosis.
Statistical Analysis
The SAS statistical software package, version 9.4 (SAS, Cary, United States), was used to perform all statistical analyses in this study. To compare between the sitagliptin and placebo groups, the Chi-squared test or Fisher's exact test were used for categorical variables, and the independent samples t-test or Wilcoxon-Mann-Whitney test were used for the differences between continuous variables. An intention-to-treat analysis was performed as the primary analysis. Additional analyses of primary and secondary outcomes within treatment groups were performed by using two-tailed independent sample t-tests, paired t-tests, or non-parametric tests, when indicated. The associations between variables in different groups were evaluated using Spearman rank correlations (rs). Given the skewed distribution of ALT, AST, and GGT, we used the natural logarithm of each of the three variables in separate multiple linear regression models to compare the slopes of each variables in the sitagliptin and placebo groups. A two-tailed significance level p≤0.05 was considered statistically significant. All study authors had access to study data and approved the final data analysis and submission.
A priori, we expected that a 5% (net effect) difference between the sitagliptin and placebo arms would be the minimally appreciable and clinically relevant difference between the two groups. Based on the results of our previous clinical trial involving colesevelam,34 we expected the sitagliptin group to have a liver fat reduction of ≥6% compared to baseline, the placebo group to have <1% reduction in liver fat compared to baseline, and a dropout rate of <10%.34 We needed ≥19 patients in each arm to achieve a power of at least 80% with a β of 0.05. Therefore, we planned to enroll 50 patients in the study and randomize at least 44 of them to the sitagliptin or placebo groups to ensure study adequate study power even with dropouts.
Additionally, Power analysis indicated an 84.5% chance of detecting a difference of 12 U/L ALT between the groups as significant (two-tailed alpha=0.05). This assumed a total of 22 patients in each group, allowing a 10% sample size reduction to adjust for the skewed distribution of ALT.
RESULTS
From January 2014 to March 2015, 50 NAFLD patients were randomized to receive sitagliptin 100 mg/day orally or placebo. 84 total patients were screened for the study, (see Supplementary Figure 1 for the derivation of cohort). In the sitagliptin arm, all 25 patients completed the study. In the placebo arm, 22 out of 25 patients completed the study, with two patients lost to follow-up and one patient discontinuing due to work schedule. 58% of the study population were women, 32% were non-Hispanic whites, and 36% were Hispanic. The two groups had similar baseline characteristics (Table 1).
Table 1.
Baseline Demographic, Biochemical, and Histologic Characteristics of Subjects
| Sitagliptin (n=25) | Placebo (n=25) | P-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 52.9 ± 11.9 | 54.9 ± 11.3 | .5358 |
| Female patients | 12 (48%) | 17 (68%) | .1520 |
| Weight (kg) | 92.8 ± 18.7 | 86.3 ± 20.6 | .2483 |
| BMI (kg/m2) | 31.9 ± 5.4 | 31.7 ± 4.7 | .8614 |
| Non-Hispanic Whites | 6 (24%) | 10 (40%) | .3582 |
| Hispanics | 9 (36%) | 9 (36%) | 1.000 |
| Diabetes | 12 (48%) | 13 (52%) | .7773 |
| Biochemical profile | |||
| ALT (IU/L) | 43.0 (26.0) | 40.0 (26.0) | .5437 |
| AST (IU/L) | 28.0 (15.0) | 29.0 (19.0) | .4877 |
| AST:ALT | 0.7 (0.3) | 0.8 (0.4) | .3713 |
| Alk Phos (U/L) | 73.0 (29.0) | 79.0 (18.0) | .3271 |
| GGT (U/L) | 34.0 (38.0) | 32.0 (36.0) | .7958 |
| Total bilirubin (mg/dL) | 0.4 (0.1) | 0.5 (0.4) | .5921 |
| Glucose (mg/dL) | 104.0 (27.0) | 106.0 (38.5) | .6690 |
| Insulin (μU/mL) | 21.0 (14.0) | 23.0 (8.5) | .8904 |
| Hgb A1C (%) | 6.1 (0.5) | 6.2 (0.8) | .6765 |
| FFA (mmol/L) | 0.5 (0.4) | 0.5 (0.3) | .5211 |
| HOMA-IR | 5.9 (4.8) | 5.4 (3.0) | .4993 |
| Triglycerides (mg/dL) | 185.0 (52.0) | 150.0 (96.0) | .4276 |
| Total cholesterol (mg/dL) | 191.0 (48.0) | 188.0 (49.0) | .8113 |
| LDL (mg/dL) | 100.0 (42.0) | 89.5 (46.0) | .4851 |
Mean ± standard deviation is presented above for normally distributed variables with associated p-values from t-test, Median (interquartile range) for non-normally distributed variables with associated p-values from Wilcoxon-Mann-Whitney performed and chi-square or Fisher's exact test on all categorical variables.
BMI, Body Mass Index; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; Hgb A1C, hemoglobin A1C; LDL, Low-Density Lipoprotein; FFA, Free Fatty Acids; Alk Phos, Alkaline Phosphatase; GGT, Gamma-Glutamyl Transferase; HOMA-IR, homeostatic model assessment of insulin resistance.
Primary Outcome: Effect of Sitagliptin on Liver Fat Assessed using MRI-PDFF
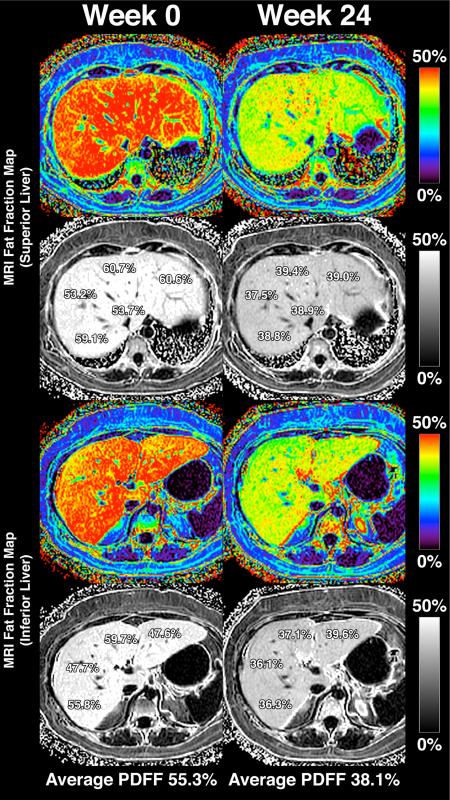
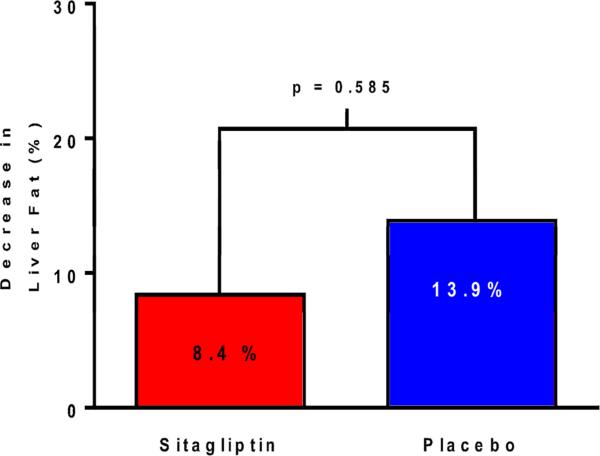
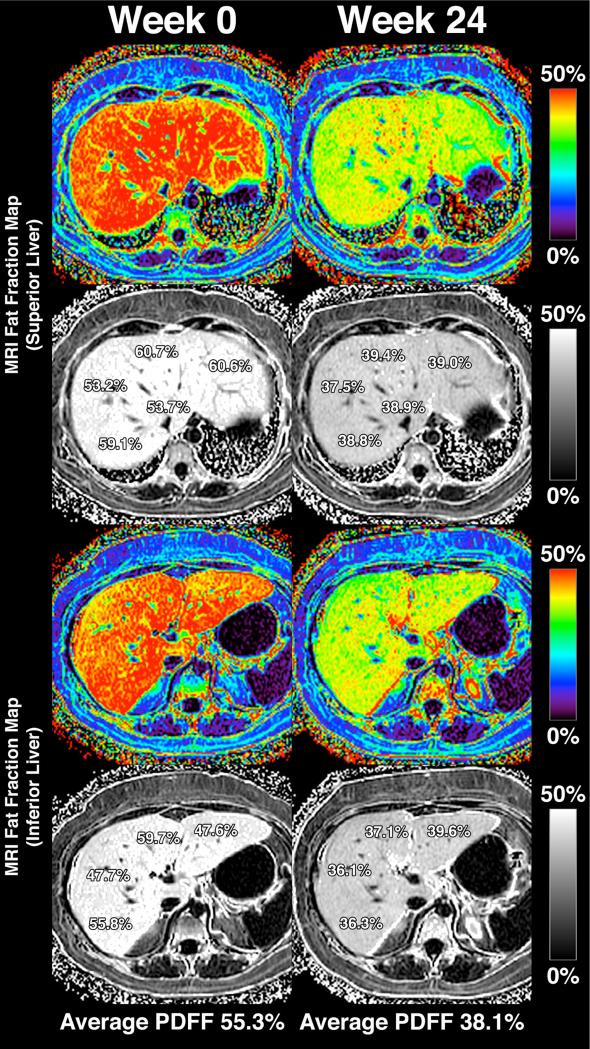
Liver fat, as measured by MRI-PDFF, was not significantly reduced in the sitagliptin group compared to the placebo group. The mean difference in liver fat change between the sitagliptin and placebo groups was −1.3%, p=0.4096 (Table 2A). The percent change between baseline and end-of-treatment MRI-PDFF between the two groups are shown in Figure 1. Compared to baseline, there was no significant difference in end-of-treatment MRI-PDFF in the sitagliptin group (18.1% to 16.9%, p=0.2673) or the placebo group (16.6% to 14.0%, p=0.0729) (Table 2A). Figure 2 shows MRI-PDFF fat mapping from all nine liver segments before and after treatment in one representative patient.
Table 2.
Sitagliptin versus Placebo: Longitudinal Full Liver Fat Mapping Using Magnetic Resonance Imaging Proton Density Fat-Fraction (MRI PDFF) and Magnetic Resonance Spectroscopy (MRS) With Co-localized MRI Measurements
| 2A | Sitagliptin (n=24) | Placebo (n=17) | Difference | ||||
|---|---|---|---|---|---|---|---|
| Liver Segments | Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | (P-Value) |
| 1 | 17.4 (12.7) | 17.0 (11.1) | .6830 | 16.4 (10.5) | 14.2 (8.8) | .1127 | −1.8 (.2739) |
| 2 | 17.1 (13.8) | 16.2 (11.7) | .4663 | 15.7 (9.9) | 13.5 (8.6) | .1171 | −1.3 (.4549) |
| 3 | 18.6 (12.3) | 17.7 (12.4) | .7810 | 16.3 (10.3) | 14.2 (8.3) | .1458 | −1.8 (.3234) |
| 4a | 18.5 (13.5) | 17.2 (11.9) | .3026 | 16.9 (9.6) | 14.3 (8.2) | .0664 | −1.4 (.4538) |
| 4b | 18.8 (14.0) | 16.9 (11.9) | .1330 | 16.4 (10.8) | 14.0 (8.4) | .0996 | −0.5 (.7837) |
| 5 | 17.8 (11.7) | 16.4 (11.0) | .1617 | 16.6 (10.7) | 13.6 (9.0) | .0618 | −1.6 (.3576) |
| 6 | 17.4 (12.4) | 16.1 (11.6) | .2695 | 16.3 (10.0) | 13.4 (8.3) | .0728 | −1.6 (.4150) |
| 7 | 18.6 (12.9) | 17.2 (11.8) | .2494 | 17.6 (10.3) | 14.7 (8.5) | .0697 | −1.5 (.4275) |
| 8 | 19.1 (12.8) | 17.3 (11.6) | .0940 | 17.4 (10.4) | 14.2 (8.4) | .0371 | −1.4 (.4073) |
| MRI PDFF Average | 18.1 (12.7) | 16.9 (11.6) | .2673 | 16.6 (10.1) | 14.0 (8.5) | .0729 | −1.3 (.4096) |
| 2B | Sitagliptin (n=18) | Placebo (n=15) | Difference | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | (P-Value) | |
| MRS | 17.2 (11.4) | 15.0 (13.6) | .2602 | 18.5 (9.5) | 15.8 (8.6) | .1004 | −1.8 (.3245) |
| 2C | Sitagliptin (n=21) | Placebo (n=17) | Difference | ||||
|---|---|---|---|---|---|---|---|
| MRI-level | Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | (P-Value) |
| MRI-s | 19.7 (15.4) | 17.5 (12.1) | .1583 | 16.7 (10.4) | 14.0 (8.8) | .0853 | −0.5 (.8282) |
| MRI-m | 19.3 (14.6) | 17.3 (12.3) | .1194 | 16.5 (10.1) | 14.0 (8.7) | .0897 | −0.5 (.7938) |
| MRI-i | 18.2 (12.9) | 16.9 (12.2) | .1587 | 16.4 (10.0) | 14.2 (8.7) | .1420 | −0.9 (.5809) |
| MRI average | 19.1 (14.2) | 17.3 (12.2) | .1215 | 16.5 (10.1) | 14.1 (8.7) | .1013 | −0.6 (.7323) |
Data are expressed as means (sd) or mean difference with p-values in parentheses. Associated p-values are from t-test.
MRI-PDFF, Magnetic Resonance Imaging Proton Density Fat Fraction; MRS, Magnetic Resonance Spectroscopy; MRI-s, Magnetic Resonance Imaging superior; MRI-m, Magnetic Resonance Imaging middle; MRI-I, Magnetic Resonance Imaging inferior.
2A: MRI-PDFFs measured in all nine liver segments are used to calculate segmental and overall fat fraction averages at baseline and post-treatment between ezetimibe and placebo group.
2B: Longitudinal changes in MRS measurements.
2C: Internal cross-validation between MRI-PDFF and MRS-PDFF
Figure 1.
Percentage change in liver fat relative to baseline as assessed by MRI-PDFF and stratified by treatment group. The sitagliptin group is on the left in red and the placebo group is on the right in blue. There was no significant difference in the change in liver fat between the two groups (p=0.585).
Figure 2.
MRI-PDFF fat mapping throughout the whole liver of a representative study patient. The upper panels show MRI-PDFF measurements in the superior liver (regions 1, 2, 4a, 7, and 8) and the lower panels show MRI-PDFF in the inferior liver (regions 3, 4b, 5, and 6). The left column shows MRI-PDFF values at week 0 and the right column shows MRI-PDFF values at week 24. The patient's calculated liver fat fraction (averaged from the nine liver segments) decreased from 55.3% (Week 0) to 38.1% (Week 24).
MRS, used as a reference standard to quantify liver fat, was performed in co-localized ROIs with MRI-PDFF to demonstrate the internal validity of MRI-PDFF measurements. MRI-PDFF and MRS measurements correlated well with one another in both groups at baseline and post-treatment, and the correlation coefficients ranged from rs=0.96 to rs=0.99 (p<0.0001).
Effect of Sitagliptin on Hepatic Fibrosis as Assessed by MRE
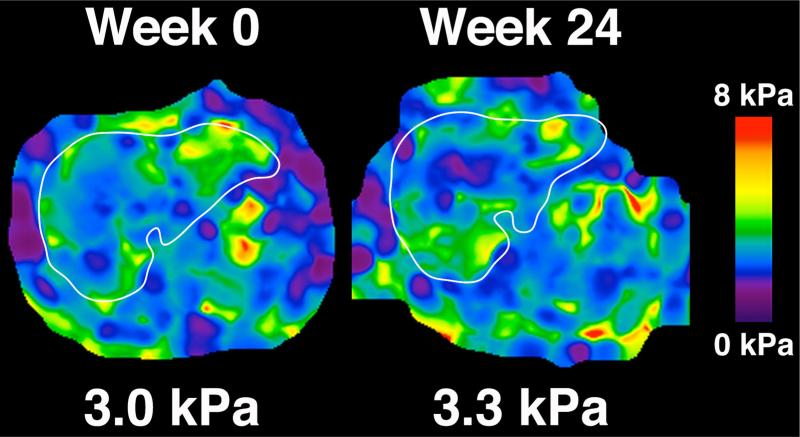
All 25 patients in the sitagliptin group and 22 patients in the placebo group had MRE at 60 Hz at baseline and end-of-treatment. The mean difference in MRE stiffness between the sitagliptin and placebo groups was −0.2, p=0.2631. Compared to baseline, there was no significant difference in end-of-treatment MRE in the sitagliptin group (2.6 to 2.7, p=0.3542) or the placebo group (2.8 to 2.7, p=0.5378) (Table 3). Figure 3 shows MRE elastograms depicting liver stiffness before and after treatment in one representative patient.
Table 3.
Sitagliptin versus Placebo: Longitudinal Changes in Liver Stiffness Values Using MRE at 60 Hz
| Sitagliptin (n=22) | Placebo (n=17) | Difference | ||||
|---|---|---|---|---|---|---|
| Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | (P-Value) |
| 2.6 (0.5) | 2.7 (1.0) | .3542 | 2.8 (0.9) | 2.7 (1.3) | .5378 | −0.2 (.2631) |
Data are expressed as means (sd). Associated p-values are from t-test.
Figure 3.
MRE (60 Hz) elastograms depicting of hepatic stiffness throughout the entire liver of a representative patient at week 0 (left panel) and week 24 (right panel).
Effect of Sitagliptin on AST, ALT, LDL, and HOMA-IR
There were no significant changes in AST, ALT, LDL, and HOMA-IR between the sitagliptin and placebo groups. Changes in biochemical and anthropometric variables between the sitagliptin and placebo groups are summarized in Table 4. Using natural logarithm of AST, ALT, and GGT in separate multiple linear regression models to compare the slopes of changes between the two groups, there were no significant differences in the slopes of sitagliptin versus placebo in any of the models (Supplementary Table 1). There was a significant decrease in glucose within the sitagliptin group (104.0 to 99.0, p=0.0352), although no significant glucose reduction was found between the sitagliptin and control groups (p=0.3852).
Table 4.
Changes in Anthropometric and Biochemical Variables between Sitagliptin and Placebo-Treated Patients
| Sitagliptin (n=25) | Placebo (n=22) | Difference | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | (P-Value) | |
| Weight (kg) | 92.8 ± 18.7 | 93.0 ± 18.9 | .7874 | 84.8 ± 20.5 | 84.6 ± 21.2 | .7949 | 0.0 (.7059) |
| BMI (kg/m2) | 31.9 ± 5.4 | 32.0 ± 5.5 | .7247 | 31.2 ± 4.6 | 31.1 ± 4.8 | .8249 | 0.0 (.6968) |
| ALT | 43.0 (26.0) | 34.0 (29.0) | .2905 | 40.0 (29.0) | 28.5 (30.0) | .2648 | −3.2 (.8569) |
| AST | 28.0 (15.0) | 27.0 (20.0) | .7458 | 28.5 (18.0) | 23.5 (11.0) | .5277 | −1.2 (.7583) |
| AST/ALT | 0.7 (0.3) | 0.8 (0.3) | .1046 | 0.8 (0.4) | 0.8 (0.3) | .4205 | 0.0 (.5325) |
| Glucose | 104.0 (27.0) | 99.0 (19.0) | .0352 | 105.0 (36.0) | 95.5 (27.5) | .5723 | −5.9 (.3852) |
| Insulin | 21.0 (14.0) | 24.0 (28.0) | .1731 | 22.0 (9.0) | 21.5 (16.0) | .9354 | 4.9 (.4213) |
| Hgb A1C | 6.1 (0.5) | 6.1 (0.4) | .4101 | 6.1 (0.8) | 6.2 (0.8) | .6744 | 0.8 (.8779) |
| Triglycerides | 185.0 (52.0) | 156.0 (109.0) | .6191 | 149.0 (84.0) | 151.5 (93.0) | .5672 | −12.7(.8866) |
| Total Cholesterol | 191.0 (48.0) | 186.0 (58.0) | .9688 | 193.0 (49.0) | 188.0 (52.0) | .2394 | −4.2 (.3208) |
| LDL | 100.0 (42.0) | 98.0 (42.5) | .9765 | 99.0 (62.0) | 101.0 (55.0) | .9763 | 0.0 (.7984) |
| FFA | 0.5 (0.4) | 0.5 (0.3) | .5772 | 0.5 (0.3) | 0.4 (0.3) | .3824 | 0.3 (.6832) |
| Alk Phos | 73.0 (29.0) | 74.0 (36.0) | .5821 | 79.5 (18.0) | 79.0 (24.0) | .1253 | −0.6 (.1819) |
| GGT | 34.0 (38.0) | 32.0 (30.0) | .2628 | 29.0 (37.0) | 23.0 (31.0) | .2707 | −4.4 (.8919) |
| Total Bilibrubin | 0.4 (0.1) | 0.4 (0.2) | .9063 | 0.5 (0.3) | 0.4 (0.2) | .5706 | 0.0 (.6558) |
| Direct Bilirubin | 0.1 (0.0) | 0.1 (0.0) | .1753 | 0.1 (0.0) | 0.1 (0.0) | .0054 | 0.0 (.3680) |
| Albumin | 4.6 (0.4) | 4.5 (0.3) | .1642 | 4.5 (0.2) | 4.5 (0.2) | .6617 | 0.0 (.2611) |
| Protime | 10.4 (1.2) | 10.3 (0.9) | .7183 | 10.3 (0.7) | 10.3 (0.7) | .6683 | 1.9 (.7936) |
| HOMA-IR | 5.9 (4.8) | 6.8 (5.3) | .5770 | 5.4 (2.5) | 4.9 (6.0) | .5791 | 0.8 (.5560) |
Data are expressed as mean ± standard deviation or median (IQR) with p-values from paired t-test or Wilcoxon signed rank sum test. P-value difference was determined using Wilcoxon-Mann-Whitney test or independent samples t-test. BMI, Body Mass Index; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; Hgb A1c, hemoglobin A1c; LDL, Low-Density Lipoprotein; FFA, Free Fatty Acids; Alk Phos, Alkaline Phosphatase; GGT, Gamma-Glutamyl Transferase; HOMA-IR, homeostatic model assessment of insulin resistance
Effect of Sitagliptin on Hepatic Fibrosis as Assessed by FIBROSpect® II
There was no significant difference between fibrosis changes in the sitagliptin group versus changes in fibrosis in the placebo group, as assessed by FIBROSpect® II (p=0.3057, Table 5). In the sitagliptin group, there were no significant changes in FIBROSpect® II, alpha-2 macroglobulin, hyaluronic acid, and TIMP1. In the control group, there was a significant increase in FIBROSpect® II (p=0.0306) and hyaluronic acid (p=0.0125) from baseline to end-of-treatment.
Table 5.
Sitagliptin versus Placebo: Longitudinal changes in FIBROSpect II in the sitagliptin and placebo groups
| Sitagliptin (n=25) | Placebo (n=22) | Difference | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | (P-Value) | |
| FIBROSpect II Index | 21.0 (16.8) | 21.7 (15.7) | .7460 | 18.6 (19.0) | 22.4 (19.4) | .0306 | 3.0 (.3057) |
| Alpha-2 macroglobulm | 169.7 (56.1) | 167.9 (60.7) | .7680 | 157.7 (38.1) | 157.1 (39.6) | .8319 | 1.2 (.8636) |
| Hyaluronic Acid | 57.7 (52.2) | 61.6 (45.0) | .6223 | 70.3 (125.3) | 94.7 (153.9) | .0125 | 20.5 (.0911) |
| Tissue Inhibitor Metaloproteinase Type 1 | 877.7 (228.9) | 875.9 (266.1) | .9710 | 812.0 (149.8) | 848.9 (180.3) | .1489 | 38.3 (.4221) |
Data are expressed as means (sd) or mean difference with p-values in parentheses. Associated p-values are from t- test.
Adverse events
There were no significant adverse events documented as part of this study. No patient in the sitagliptin arm dropped out of the study, and three patients in the placebo arm dropped out of the study. The dropouts from the placebo arm were not associated with any study adverse events.
DISCUSSION
Main findings
This randomized, double-blind, placebo-controlled clinical trial showed sitagliptin was not significantly better than placebo for reducing liver fat as measured by MRI-PDFF. Sitagliptin was also not significantly better than placebo for improving the secondary endpoints of AST, ALT, LDL, and HOMA-IR. Sitagliptin did not significantly decrease fibrosis as measured by MRE, although patients in the placebo group showed an increase in fibrosis as estimated by FIBROSpect® II and hyaluronic acid.
This trial provides new data as proof of concept to conduct clinical trials in higher risk patients such as pre-diabetics and diabetics without the need to do a liver biopsy in order to efficiently screen agents that have a role in the treatment of NASH in early phase trials. Our previous trials included a liver biopsy, but this trial protocol was approved by the United States Federal Drug Administration without the need for baseline liver biopsy as the patient population was considered likely to have NASH on biopsy. It provides independent validation of the methodology used in the MOZART Trial37 to give further evidence that measurements of hepatic fat fraction using MRI-PDFF is accurate and may be used to longitudinally measure hepatic fat changes over time. All MRI-PDFF measurements were confirmed by co-localized MRS measurements. This trial also provides validation of the methodology used for co-localization of MRE before and after to examine changes in liver stiffness in a clinical trial involving patients with NAFLD. This trial shows that FIBROSpect® II may have utility in monitoring longitudinal changes in fibrosis in future NASH clinical trials. Finally, this trial provides convincing evidence that sitagliptin-treated patients can be included in future NASH trials as it is not likely to modify NASH treatment.
Rationale for using MRI-PDFF for assessing liver fat
MRI-PDFF was used to assess the primary outcome of liver fat change because it is non-invasive and does not subject patients to ionizing radiation like computed tomography (CT) scans. Additionally, it allows for objective, quantitative fat fraction measurements throughout different segments of the liver with minimal sampling variability.43 In previous NAFLD clinical trials, MRI-PDFF was shown to be more sensitive than histology for assessing quantitative changes in liver fat.34, 39 Compared to ultrasound, MRI-PDFF is not operator-dependent and provides more objective measures. MRI-PDFF is also more accurate than CT without subjecting patients to ionizing radiation. MRI-PDFF is also much more practical to perform than MRS, which can only be performed at specialized centers, is difficult to perform and analyze from a technical standpoint, and can only be used to estimate liver fat content within a 2×2×2 cm3 cube (voxel) in the liver.34
Strengths and limitations
The strength of this study lies in its use of a randomized, double-blind, allocation-concealed, placebo-controlled clinical trial study design to evaluate the effectiveness of sitagliptin versus placebo for treating NAFLD. MRI-PDFF, a novel, precise, and accurate noninvasive imaging biomarker, was used to evaluate the primary outcome of changes in liver fat. The study was conducted by experienced investigators from multidisciplinary backgrounds and with special expertise in the noninvasive assessment of NAFLD. Our study protocol for measuring quantitative changes in liver fat within ROIs in each of the nine liver segments can be utilized in future NASH clinical trials. We demonstrate the utility of advanced MR methods, including MRE, for measuring longitudinal changes in liver stiffness in clinical trials. Finally, we demonstrate the utility of the non-invasive, biomarker-based test FIBROSpect® II in monitoring longitudinal changes in hepatic fibrosis in NASH clinical trials.
However, we acknowledge the following limitations. The study was performed at a center which is highly specialized for advanced imaging in NASH. While MRI-PDFF has been shown to be effective for measuring changes in hepatic steatosis in our research center, additional multi-center trials are needed to assess the utility of MRI-PDFF for measuring hepatic steatosis changes in more diverse patient populations. MRI-PDFF changes only provide information regarding changes in liver fat, and does not provide any information regarding improvement in liver inflammation, cellular injury, and fibrosis. Therefore, additional non-invasive biomarkers of inflammation, cellular injury, and fibrosis are needed to ascertain a more comprehensive assessment of treatment response. Although we found little change in hepatic stiffness from the beginning to end-of-treatment, longer studies may be needed to assess the utility of MRE for measuring longitudinal changes in hepatic stiffness. Finally, hepatic inflammation plays an important role in morbidity and mortality in NAFLD patients, and further studies are needed to develop novel biomarkers to assess changes in necroinflammation activity, ballooning degeneration, and fibrogenesis, since MRI-PDFF, MRE and FIBROSpect® II cannot detect these changes.
Implications for future research
This study shows that sitagliptin at 100 mg/day for six months has little effect in treating NAFLD compared to placebo. This study suggests that MRI-PDFF, MRE, and FIBROSpect® II may have utility as non-invasive biomarkers to assess treatment response in NASH clinical trials, although further studies are needed to validate the use of MRI-PDFF and MRE. Further studies are also needed to assess the utility of MRI-PDFF, MRE, and FIBROSpect® II for measuring hepatic fat and fibrosis in multicenter clinical trials and also in clinical trials of longer duration.
Supplementary Material
Lay Summary.
In a randomised, double-blind, placebo-controlled study, the anti-diabetic drug sitagliptin was no more effective than placebo for improving liver fat and liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). This study demonstrates that noninvasive magnetic resonance imaging (MRI) imaging techniques, including MRI-proton density fat fraction (MRI-PDFF) and magnetic resonance elastography (MRE), can be used to assess treatment response in NAFLD clinical trials.
Acknowledgments
Funding Support: This work was supported by an investigator initiated study grant to Dr. Rohit Loomba (RL) by Merck Inc. The study was conducted at the Clinical and Translational Research Institute, University of California at San Diego. RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. JC is supported by NIH T32 training grant 5TL1TR000098. Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303.
Role of Funding Agencies: Funding agencies did not have any role in the design and conduct of the study, collection, management, analysis or interpretation of the data; preparation, review, or approval of the manuscript.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- DPP-4
dipeptidyl peptidase 4
- GLP-1
glucagon-like peptide-1
- GIP
glucose-dependent insulinotropic polypeptide
- HbA1c
hemoglobin A1c
- SREBP-1c
sterol regulatory element bind protein-1c
- T2DM
type 2 diabetes mellitus
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GGT
gamma glutamyl transpeptidase
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- MRI-PDFF
MRI-proton density fat fraction
- MRE
MR elastography
- ROI
regions of interest
- MRS
MR spectroscopy
- TIMP1
tissue inhibitor metalloproteinase I
- CT
computed tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: The authors report no conflict of interests.
Author contributions:
Jeffrey Cui – data collection, interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission
Len Philo – critical revision of the manuscript, approved final submission
Phirum Nguyen – data collection, critical revision of the manuscript, approved final submission
Heather Hofflich – patient referral, data collection, critical revision of the manuscript, approved final submission
Carolyn Hernandez – data collection, critical revision of the manuscript, approved final submission
Ricki Bettencourt – Analysis of data, critical revision of the manuscript, approved final submission
Lisa Richards – data collection, critical revision of the manuscript, approved final submission
Joanie Salotti – data collection, critical revision of the manuscript, approved final submission
Archana Bhatt – data collection, critical revision of the manuscript, approved final submission
Jonathan Hooker – data collection, drafting of the manuscript, critical revision of the manuscript, approved final submission
William Haufe – data collection, critical revision of the manuscript, approved final submission
Catherine Hooker – data collection, critical revision of the manuscript, approved final submission
David A Brenner – critical revision of the manuscript, approved final submission
Claude B Sirlin – Study concept and design, analysis and interpretation of data, drafting of the manuscript, MRI analysis, critical revision of the manuscript, obtained funding, study supervision, approved final submission.
Rohit Loomba – Study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 5.Wong VW, Chu WC, Wong GL, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–15. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 6.Arulanandan A, Ang B, Bettencourt R, et al. Association Between Quantity of Liver Fat and Cardiovascular Risk in Patients With Nonalcoholic Fatty Liver Disease Independent of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2015;13:1513–20. e1. doi: 10.1016/j.cgh.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Noureddin M, Rinella ME. Nonalcoholic Fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis. 2015;19:361–79. doi: 10.1016/j.cld.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–51. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazick J, Donithan M, Neuschwander-Tetri BA, et al. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38:1347–55. doi: 10.2337/dc14-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idilman R, Mizrak D, Corapcioglu D, et al. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;28:200–8. doi: 10.1111/j.1365-2036.2008.03723.x. [DOI] [PubMed] [Google Scholar]
- 11.Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–82. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doycheva I, Loomba R. Effect of metformin on ballooning degeneration in nonalcoholic steatohepatitis (NASH): when to use metformin in nonalcoholic fatty liver disease (NAFLD). Adv Ther. 2014;31:30–43. doi: 10.1007/s12325-013-0084-6. [DOI] [PubMed] [Google Scholar]
- 13.Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–10. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 14.Ratziu V, Charlotte F, Bernhardt C, et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–53. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 15.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 16.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–84. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 19.Herman GA, Bergman A, Liu F, et al. Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol. 2006;46:876–86. doi: 10.1177/0091270006289850. [DOI] [PubMed] [Google Scholar]
- 20.Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12(Suppl 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 21.Tremblay AJ, Lamarche B, Deacon CF, et al. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:366–73. doi: 10.1111/j.1463-1326.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 22.Monami M, Lamanna C, Desideri CM, et al. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther. 2012;29:14–25. doi: 10.1007/s12325-011-0088-z. [DOI] [PubMed] [Google Scholar]
- 23.Giampietro O, Giampietro C, Bartola LD, et al. Sitagliptin as add-on therapy in insulin deficiency: biomarkers of therapeutic efficacy respond differently in type 1 and type 2 diabetes. Drug Des Devel Ther. 2013;7:99–104. doi: 10.2147/DDDT.S38346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki T, Tomeno W, Yoneda M, et al. Non-alcoholic fatty liver disease adversely affects the glycemic control afforded by sitagliptin. Hepatogastroenterology. 2012;59:1522–5. doi: 10.5754/hge11689. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz Y, Yonal O, Deyneli O, et al. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg. 2012;75:240–4. [PubMed] [Google Scholar]
- 26.Kim D, Kim WR, Talwalkar JA, et al. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411–9. doi: 10.1148/radiol.13121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920–8. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loomba R, Schork N, Chen CH, et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology. 2015;149:1784–93. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loomba R, Cui J, Wolfson T, et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am J Gastroenterol. 2016 doi: 10.1038/ajg.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453–61. doi: 10.1002/hep.28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imajo K, Kessoku T, Honda Y, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626–637. e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 32.Patel K, Gordon SC, Jacobson I, et al. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935–42. doi: 10.1016/j.jhep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Zaman A, Rosen HR, Ingram K, et al. Assessment of FIBROSpect II to detect hepatic fibrosis in chronic hepatitis C patients. Am J Med. 2007;120:280, e9–14. doi: 10.1016/j.amjmed.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–32. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SC, Heba E, Wolfson T, et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol. 2015;13:1337–1345. e6. doi: 10.1016/j.cgh.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology. 2015;61:1239–50. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarrinpar A, Gupta S, Maurya MR, et al. Serum microRNAs explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: a prospective study. Gut. 2015 doi: 10.1136/gutjnl-2015-309456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–40. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel NS, Peterson MR, Brenner DA, et al. Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:630–9. doi: 10.1111/apt.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reeder SB, Cruite I, Hamilton G, et al. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J Magn Reson Imaging. 2011;34 doi: 10.1002/jmri.22580. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–9. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reeder SB. Emerging quantitative magnetic resonance imaging biomarkers of hepatic steatosis. Hepatology. 2013;58:1877–80. doi: 10.1002/hep.26543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41:1271–80. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2016;43:83–95. doi: 10.1111/apt.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Talwalkar JA, Yin M, et al. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749–56. doi: 10.1148/radiol.11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.