Abstract
Purpose
This study sought to examine trends in stereotactic radiosurgery (SRS) and in-hospital patient outcomes on a national level by utilizing national administrative data from the Nationwide Inpatient Sample (NIS) database.
Methods and materials
Using the NIS database, all discharges where patients underwent inpatient SRS were included in our study from 1998 – 2011 as designated by the ICD9-CM procedural codes. Trends in the utilization of primary and adjuvant SRS, in-hospital complications and mortality, and resource utilization were identified and analyzed.
Results
Our study included over 11,000 hospital discharges following admission for primary SRS or for adjuvant SRS following admission for surgery or other indication. The most popular indication for SRS continues to be treatment of intracranial metastatic disease (36.7%), but expansion to primary CNS lesions and other non-malignant pathology beyond trigeminal neuralgia has occurred over the past decade. Second, inpatient admissions for primary SRS have declined by 65.9% over this same period of time. Finally, as inpatient admissions for SRS become less frequent, the complexity and severity of illness seen in admitted patients has increased over time with an increase in the average comorbidity score from 1.25 in the year 2002 to 2.29 in 2011, and an increase in over-all in-hospital complication rate of 2.8 times over the entire study period.
Conclusions
As the practice of SRS continues to evolve, we have seen several trends in associated hospital admissions. Overall, the number of inpatient admissions for primary SRS has declined while adjuvant applications have remained stable. Over the same period, there has been associated increase in complication rate, length of stay, and mortality in inpatients. These associations may be explained by an increase in the comorbidity-load of admitted patients as more high-risk patients are selected for admission at inpatient centers while more stable patients are increasingly being referred to outpatient centers.
Keywords: stereotactic radiosurgery, Nationwide Inpatient Sample, usage trends, complications, mortality, radiosurgery outcomes
INTRODUCTION
Stereotactic radiosurgery (SRS) combines stereotactic targeting with focal, single or multisession, radiation techniques for the treatment of neurological disease. Originally conceived as an alternative treatment to stereotactic functional neurosurgical procedures, [1,2] the use of SRS has seen a dramatic rise in recent years due to its ability to effectively control oncologic and non-oncologic disorders while avoiding significant morbidities associated with traditional intracranial approaches. [3-5] Modern stereotactic radiosurgery is delivered via rigid frames or frameless stereotactic systems with high-resolution image-guided targeting of ionizing radiation that can be delivered in 1 to 5 sessions. [3] Since its integration into neurosurgical practice over three decades ago, the SRS landscape has seen profound changes in indications and effectiveness. [6] While initially directed mainly towards both benign and malignant intracranial tumors, [7] new applications have been reported for epilepsy, functional and even psychiatric indications. [8-10]
Despite the rise in popularity of SRS, no nationwide studies of overall utilization and outcomes for SRS in the US have been reported. Market research estimates reported that 32,335 patients underwent cranial LINAC SRS or Gamma Knife Surgery (GKS) in 2009 and the average increase inpatient volume at the time was 10% annually. Billing data from the Center for Medicare and Medicaid Services demonstrated that billing via CPT codes for SRS increased nearly 10 fold over the last two decades with over 15,000 codes billed annually in 2010 and 2011. [11] There remains, however, a lack of more comprehensive study of the practice of SRS on a national level within the literature. We believe that by studying the changes in in-patient practice patterns for SRS on a national level over the past decade, we can learn more about which patients are still receiving in-patient SRS, how the indications for SRS have expanded over time, and why there has been a shift in SRS from a primarily in-patient to an out-patient procedure. By utilizing the National Inpatient Sample (NIS), we describe the demographics, practice patterns, and costs associated with inpatient SRS in the US over the last decade.
METHODS AND MATERIALS
Data source
Data for this study were obtained from the Nationwide Inpatient Sample (NIS, Healthcare Cost and Utilization Project, Agency Healthcare Research and Quality) between 1998 and 2011. The NIS is a 20% stratified sample of nonfederal hospitals, accounting for approximately 8 million hospital stays from over 1,000 hospitals each year. Each data entry represents a single full hospitalization record with designations for hospitalization indications and procedures, as well as numerous patient and hospital demographic information. Detailed information on the design and validation of the NIS is available at http://www.hcup-us.ahrq.gov.
Patient selection
A retrospective analysis of patients admitted for stereotactic radiosurgery was conducted in the NIS database from 1998 to 2011. Appropriate individuals admitted for SRS procedures were identified using the International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM) procedure codes of 92.30 – 92.39. Indications for admission were determined based on procedure code ranking within the database. Patients with only stereotactic radiosurgery procedure coding or where stereotactic radiosurgery was the primary procedure code were classified as having been admitted primarily for radiosurgery. Patients identified as having received stereotactic radiosurgery as a secondary procedure code in conjunction with one or more other, more invasive, primary procedures (such as craniotomy) were classified as having received adjuvant radiosurgery.
Patient and health-care system–related characteristics
Patient age, gender, race, comorbidities, admission source, hospital size, teaching status, payer information, and treatment modality were obtained from the NIS database. Patients were also categorized according to race as “white,” “black,” “Hispanic,” “others,” and “not stated.” Comorbidities were assessed using the ‘NIS Severity’ data files, a supplemental dataset beginning in 2002 which describes patient comorbidities based on AHRQ review of historical patient information prior to de-identification and entry into the core dataset. Utilization of this information provides improved capture of comorbidities. Total comorbidity score was determined for each case by adding one point per comorbidity ascertained in this fashion. Hospital size (bed number) was categorized into “small,” “medium,” and “large”. Payer information was categorized into “Medicare,” “Medicaid,” “private,” and “others.” The admission source for each discharge was also determined as either from emergency room, hospital transfer, or elective admissions. Clinical indications for radiosurgery were determined by way of billed primary admission diagnoses. All patient refined DRG mortality risk and severity predictors, risk stratifying variables assigned by 3M Health Information Systems beginning in 2002, were also obtained from the NIS database and used as a means to better describe patients’ illness burden. Illness severity is graded on a categorical scale from 1-4 for minor to extreme loss of function, and mortality risk is graded on a categorical scale from 1-4 for minor to extreme likelihood of mortality during admission.
Outcomes
Outcome variables were selected to evaluate efficiency and efficacy of care. Mortality, length of hospital stay, total hospital charges, post-operative complications, and discharge disposition were obtained via the NIS database. In-hospital complications were obtained using the following ICD-9-CM codes: neurologic complications (997.00–997.09); respiratory complications (518.4, 518.5, 518.81–518.84, and 997.3); cardiac complications (410 and 997.1); gastrointestinal complications (535.0, 570, 575.0, 577.0, and 997.4); urinary and renal complications (584 and 997.5); pulmonary embolism (415.1); and wound-related complications including infection, dehiscence, seroma, and hematoma (998.1, 998.3, 998.5, 998.83, and 999.3). Disposition of patients was categorized into “routine,” “transfer to short-term hospital,” “other transfers,” “home health care,” and “died in hospital.” Other transfers include to skilled nursing facility, intermediate care, and other types of non-hospital rehabilitation facilities.
Data analysis
All charges were adjusted for inflation using the consumer price index, adjusting to 2011 US dollars. When specified, the aggregate sum of estimated discharges using the discharge weight (NIS variable DISCWT) is used to provide estimates of annual U.S. volume. A linear regression model was applied to analyze the time trends of length of stay and total incurred charges. All statistical analyses were performed using SAS Version 4.3 (Cary, NC). Baseline categorical characteristics were compared using the chi-squared test, ANOVA, and student’s T-test where appropriate. A P-value < 0.05 was considered statistically significant.
RESULTS
Patient and hospital demographics
Table 1 contains detailed information regarding patient and hospital demographics for all stereotactic radiosurgery cases captured from 1998 to 2011 in the NIS database. A total 11,662 cases were observed during this time period. Mean age was 56.4 years with 6,190 females (53.1%) and 5,435 males (46.6%). Of those treated, 8,382 (71.9%) admissions were for primary stereotactic radiosurgery where SRS was the primary procedure code and purpose of the admission. The remainder represent adjuvant SRS admissions where SRS was a secondary procedure code and not the main purpose of the admission. Adjuvant SRS in these instances occurred following admission for surgery or other indication. Most cases were performed at large hospitals (58.3%; 6,796) with the majority within teaching institutions (66.8%; 7,787). 70.1% of cases were routinely scheduled with the most commonly utilized modality being multi-source photon emitters such as Gamma Knife (52.3%).
Observed surgical caseload
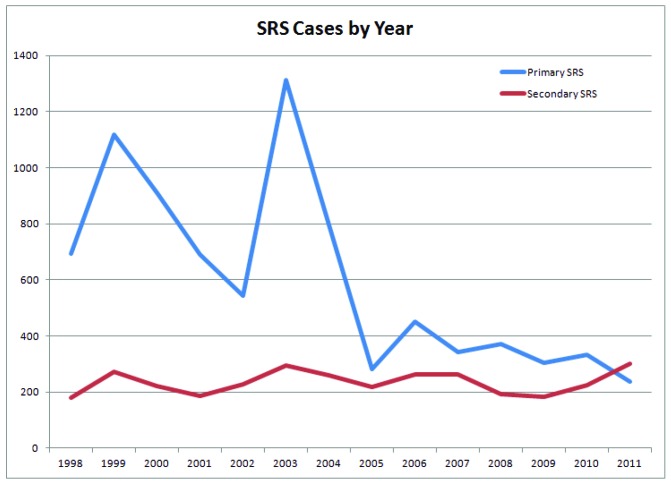
The number of admitted inpatients for primary stereotactic radiosurgery has declined over time, from 695 observed NIS cases in 1998 to 237 cases in 2011. Two notable drops in caseload are noted to begin in 1999 and 2003. In contrast, adjuvant SRS cases have remained stable over this period of time (Fig. 1). The five most common indications for radiosurgery through this time period were for secondary brain and spinal cord metastases (36.7%; 3,079), trigeminal neuralgia (10.7%; 899), meningiomas (9.6%; 808), cranial nerve schwannomas (6.7%; 565), and cerebrovascular anomalies (5.6%; 465). Radiosurgery was most commonly used as an adjuvant treatment in resections of secondary brain and spinal cord metastases (19.5%; 638), primary neoplasms of frontal lobe (8.3%, 272), meningiomas (7.5%; 246), primary neoplasms of temporal lobe involving hippocampus/uncus (6.4%; 210), and benign pituitary neoplasms (5.1%; 167). (Table 2)
Figure 1.
Stereotactic radiosurgery cases per year. The number of admitted inpatients for primary stereotactic radiosurgery has declined over time, from 695 observed NIS cases in 1998 to 237 cases in 2011. Two notable drops in caseload are noted to begin in 1999 and 2003.
Table 2.
Top diagnosis for SRS. A) The five most common associated indications for primary radiosurgery were for secondary brain and spinal cord metastases (36.7%; 3,079), trigeminal neuralgia (10.7%; 899), meningiomas (9.6%; 808), cranial nerve schwannomas (6.7%; 565), and cerebrovascular anomalies (5.6%; 465). B) Radiosurgery was most commonly used as an adjuvant treatment in resections of secondary brain and spinal cord metastases (19.5%; 638), primary neoplasms of frontal lobe (8.3%, 272), meningiomas (7.5%; 246), primary neoplasms of temporal lobe involving hippocampus/uncus (6.4%; 210), and benign pituitary neoplasms (5.1%; 167).
A)
| Top Ten Associated Diagnosis for Primary SRS | n | % | |
| 1 | Metastasis (198.3) | 3079 | 36.74 |
| 2 | Trigeminal neuralgia (350.1) | 899 | 10.73 |
| 3 | Meningioma (225.2) | 808 | 9.64 |
| 4 | Schwannoma (225.1) | 565 | 6.74 |
| 5 | AVM (747.81) | 465 | 5.55 |
| 6 | Primary malignant CNS tumor, frontal lobe (191.1) | 261 | 3.11 |
| 7 | Primary malignant CNS tumor, corpus callosum (191.8) | 228 | 2.72 |
| 8 | Benign pituitary lesions (227.3) | 177 | 2.11 |
| 9 | Primary malignant CNS tumor, temporal lobe (191.2) | 169 | 2.02 |
| 10 | Primary malignant CNS tumor, parietal lobe (191.3) | 157 | 1.87 |
| B) | |||
| Top Ten Primary Diagnosis for Secondary SRS | n | % | |
| 1 | Metastasis (198.3) | 638 | 19.45 |
| 2 | Primary malignant CNS tumor, frontal lobe (191.1) | 272 | 8.29 |
| 3 | Meningioma (225.2) | 246 | 7.5 |
| 4 | Primary malignant CNS tumor, temporal lobe (191.2) | 210 | 6.4 |
| 5 | Benign pituitary lesions (227.3) | 167 | 5.09 |
| 6 | Primary malignant CNS tumor, parietal lobe (191.3) | 153 | 4.66 |
| 7 | Parkinson’s disease (332.0) | 81 | 2.47 |
| 8 | Primary malignant CNS tumor (NOS) (191.9) | 69 | 2.1 |
| 9 | Primary malignant CNS tumor, corpus callosum (191.8) | 56 | 1.71 |
| 10 | Benign primary CNS neoplasm (225.0) | 53 | 1.62 |
SRS – stereotactic radiosurgery
AVM – arteriovenous malformation
CNS – central nervous system
NOS – not otherwise specified
Comorbidities and post-procedural complications
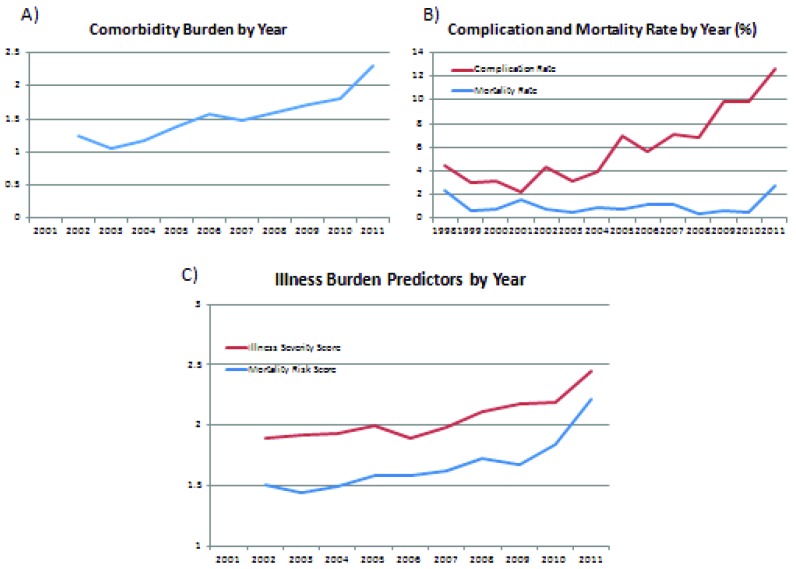
Since the NIS began tabulating patient comorbidities in 2002, the mean comorbidity score has increased over time from an average 1.25 per patient in 2002 to 2.29 in 2011 (p < .05, Fig. 2a). Furthermore, the overall in-hospital complication rate has also increased over time from 4.5% in 1998 to 12.7% in 2011 (p < .05, Fig. 2b). Of note, these complications are aggregated from the entire inpatient admission and are not necessarily directly related to SRS. Illness burden predictors of disease severity and pre-operative mortality risk predictors, which were also initiated in the NIS database beginning in 2002, have also increased from 1.9 and 1.51 respectively in 2002 to 2.45 and 2.21 in 2011 (Fig. 2c)
Figure 2.
Comorbidity and illness burden correlate with increasing complication rates. A) The mean comorbidity score has increased over time from an average 1.25 per patient in 2002 to 2.29 in 2011. B) The overall in-hospital complication rate has also increased over time from 4.5% in 1998 to 12.7% in 2011. (red) Mortality rate over this same period has remained stable from 2.29% in 1998 to 2.79% in 2011. (blue) C) Illness burden predictors of disease severity (red) and pre-operative mortality risk predictors (blue), which were also initiated in the NIS database beginning in 2002, have also increased from 1.9 and 1.51 respectively in 2002 to 2.45 and 2.21 in 2011.
Table 1.
Baseline characteristics. Includes detailed information regarding patient and hospital demographics for all stereotactic radiosurgery cases captured from 1998 to 2011 in the NIS database.
| Baseline Characteristics | |||
| Age | Mean | 56.4 | |
| SD | 17.7 | ||
| n | % | ||
| Sex | Female | 6,190 | 53.08 |
| Male | 5,435 | 46.60 | |
| No information | 37 | 0.32 | |
| Race | White | 7,484 | 64.17 |
| Black | 583 | 5.00 | |
| Hispanic | 689 | 5.91 | |
| Asian | 164 | 1.41 | |
| Native American | 37 | 0.32 | |
| Other | 267 | 2.29 | |
| No information | 2,438 | 20.91 | |
| Healthcare Coverage | Medicare | 4,521 | 38.77 |
| Medicaid | 936 | 8.03 | |
| Private | 5,653 | 48.47 | |
| self | 238 | 2.04 | |
| No charge | 31 | 0.27 | |
| Other | 268 | 2.30 | |
| No information | 15 | 0.13 | |
| Admission Source | ED | 1,097 | 9.41 |
| Hospital/facility transfer | 457 | 3.92 | |
| Scheduled | 8,180 | 70.14 | |
| No information | 1,928 | 16.53 | |
| Treatment Method | Unspecified | 3,073 | 26.35 |
| Single Source Photon (LINAC) | 658 | 5.64 | |
| Multi-Source Photon (GK) | 6,099 | 52.30 | |
| Particulate | 56 | 0.48 | |
| Other | 1,776 | 15.23 | |
| Hospital Type | Non-teaching | 1,612 | 13.82 |
| Teaching | 7,787 | 66.77 | |
| No information | 2,263 | 19.40 | |
| Hospital Size | Small | 854 | 7.32 |
| Medium | 1,749 | 15.00 | |
| Large | 6,796 | 58.27 | |
| No information | 2,263 | 19.40 | |
Overall in-hospital complication rates between 1998 and 2011 were 1.0% for neurologic, 1.9% for respiratory, 0.5% for cardiac, 0.3% for gastrointestinal, 0.8% for urinary and renal, 0.6% for pulmonary embolism, 0.8% for wound-related, and 5.9% for overall complications. (Table 3a) Mortality rate over this same period has remained stable from 2.29% in 1998 to 2.79% in 2011. When stratified according to number of complications, in 1998, 95.5% had zero, 3.7% had one, 0.6% had two, and 0.2% had three. In contrast, in 2011, 87.3% of patients had zero, 9.9% had one, 2.2% had two, and 0.5% had three (Table 3b).
Table 3.
Overall outcomes and complications. A) Overall outcomes in terms of length of stay (LOS, 4.28 days), total charges ($50,200.15), as well as mortality risk and illness severity scores are reported. Overall in-hospital complication rates between 1998 and 2011 were 1.0% for neurologic, 1.9% for respiratory, 0.5% for cardiac, 0.3% for gastrointestinal, 0.8% for urinary and renal, 0.6% for pulmonary embolism, 0.8% for wound-related, and 5.9% for overall complications. B) When discharges were stratified according to number of complications, in 1998, 95.5% had zero, 3.7% had one, 0.6% had two, and 0.2% had three. In contrast, in 2011, 87.3% of patients had zero, 9.9% had one, 2.2% had two, and 0.5% had three.
A)
| Outcomes | Mean | SD | |||
| Mean LOS | 4.28 | 7.18 | |||
| Mean Total Charges | 50,200.15 | 56,087.65 | |||
| Predicted Mortality Risk Score | 1.64 | 0.82 | |||
| Predicted Illness Severity Score | 2.03 | 0.71 | |||
| Comorbidity Burden | 1.47 | 1.43 | |||
| Complications | n | % | Overall Complication Rate (per 100) | ||
| Neurologic | 121 | 17.61 | 1.0 | ||
| Respiratory | 221 | 32.17 | 1.9 | ||
| Cardiac | 58 | 8.44 | 0.5 | ||
| Gastrointestinal | 31 | 4.51 | 0.3 | ||
| Urinary and Renal | 93 | 13.54 | 0.8 | ||
| Pulmonary Embolism | 69 | 10.04 | 0.6 | ||
| Wound-related complication | 94 | 13.68 | 0.8 | ||
| Total Complications | 687 | 100.00 | 5.9 | ||
| B) | |||||
| No. complications | No. cases (%) | Mean Age | Mean LOS | Mean total Charge | Weighted Average Charges |
| 1998 | |||||
| 0 | 835 (95.54) | 53.9 | 3.49 | 31,958.55 | 30,532.48 |
| 1 | 32 (3.66) | 56.3 | 19.72 | 102,380.79 | 3,748.50 |
| 2 | 5 (0.57) | 57 | 37.2 | 368,264.61 | 2,106.78 |
| 3 | 2 (0.23) | 65 | 16 | 80,699.28 | 184.67 |
| 2011 | |||||
| 0 | 469 (87.34) | 58 | 6.51 | 98,308.17 | 85,859.46 |
| 1 | 53 (9.87) | 57.7 | 13.62 | 162,165.25 | 16,005.14 |
| 2 | 12 (2.23) | 66.8 | 27 | 299,960.08 | 6,703.02 |
| 3 | 3 (0.56) | 48.7 | 26.33 | 325,183.33 | 1,816.67 |
Hospital total charges and discharge disposition
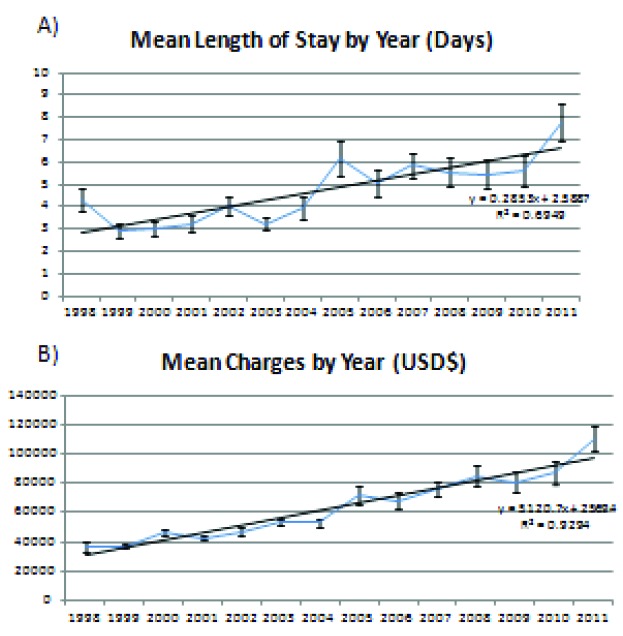
The mean length of stay has shown considerable increase on a year-to-year basis from 4.3 days in 1998 to 7.8 days in 2011 (p < .05, Fig. 3a). Mean hospital charges were $50,200 between 1998 and 2011. However the inflation-adjusted mean hospital charges have increased over time from $36,583 in 1998 to $110,497 in 2011 (p < .05, Fig. 3b). Table 3b expands upon the relationship of complications on hospital length of stay and charges. The adjusted mean hospital charges were $31,958 for patients without complications and $102,380 for those with one complication in 1998 compared to $98,308 for patients without complications and $162,165 for those with one complication in 2011 (p < .05).
Routine discharges accounted for 82.3% of discharges throughout the entire time period but has decreased over time from 84.8% in 1998 to 58.9% in 2011 (p < .05, Supple Fig. 1a). Consequently, the proportion of patients discharged with home health care or transferred to other subacute facilities have both increased from 2.8% and 9.6%, respectively in 1998 to 15.6% and 21.4% in 2011 (p < .05, Supple Fig. 1b).
DISCUSSION
We present a nationwide study of inpatient trends following stereotactic radiosurgery. Our survey of over 11,000 hospital discharges with primary SRS or for adjuvant SRS following admission for surgery or other indication uncovered several trends worth noting. First, while the most popular indication for SRS continues to be treatment of intracranial metastatic disease, expansion to primary CNS lesions and other non-malignant pathology beyond trigeminal neuralgia has occurred over the past decade. Second, inpatient admissions for primary SRS have declined over this same period of time. The progressive decrease in inpatient admissions primarily for SRS correspond well to two different nationwide reimbursement policy changes occurring in 1997 and 2005 that ultimately decreased hospital reimbursements for the procedure. Finally, as inpatient admissions primarily for SRS become less frequent, presumably due to increased transition of procedures to ambulatory centers, the complexity and severity of illness seen in patients admitted for SRS has increased over time.
SRS was originally developed by Lars Leksell for the treatment of trigeminal neuralgia [1]. Several subsequent randomized control trials of radiation therapy for intracranial metastasis demonstrated improved outcomes when compared to whole brain radiation therapy (WBRT) [12-15]. As a result, SRS became more widely utilized for the treatment of metastases, ultimately becoming the leading indication for SRS use in the US. This is consistent with results from our study of inpatient admissions where metastasis to the brain remains the most common indication for both primary stand-alone and adjuvant utilization of SRS. (Table 2) However, as neurosurgeons and radiation oncologists continue to develop and advance the boundaries of radiosurgery, indications for SRS have broadened more recently to include treatment of AVMs [16,17], schwannomas [18-20], meningiomas [21,22], and gliomas [23,24]. Our study supports the popularity of these other newer indications over the past decade. Technological advancements within the field including fractionation and real-time imaging have allowed for the wider applicability of radiosurgery for these newer indications with increasing efficacy and safety.
Two precipitous drops in admissions primarily for SRS are observed in 1998-99 and 2004-05. Surrounding these two time points were major changes in the Centers for Medicare & Medicaid Services (CMS) inpatient reimbursement schema for SRS. First, in 1997 there was a change in SRS procedure codes from diagnosis-related group (DRG) 1 to DRG 7 and 8 [25]. Procedure codes in DRG 1, including craniotomies, are reimbursed at higher rates than those in DRG 7 and 8, and thus provided more financial support to hold SRS procedures in an inpatient setting where more ancillary support was present. As inpatient reimbursements for each SRS procedure declined due to DRG coding changes coupled with demonstrated safety improvements via technological advancements, financial incentives likely shifted SRS procedures to ambulatory centers where fewer ancillary staff was needed. (Fig. 1) Then, in 2005, CMS removed temporary bundled codes for SRS. This move forced hospitals to use existing traditional radiation therapy coding for SRS, which billed at significantly lower rates [25,26]. These changes in CMS policy and ultimate decline in SRS reimbursements coupled with improved safety and outcomes from continued innovation, have likely stimulated movement of SRS out of inpatient facilities and into the outpatient setting, where the majority of procedures are performed today.
Our reported mortality rate and complication rates are higher than commonly reported outpatient rates in the literature. This likely reflects a selection effect where only the sickest and medically complex patients that are unable to safely undergo outpatient SRS are admitted to the inpatient setting for SRS and included in the NIS. The increase in disease burden and medical comorbidities we have observed over this time frame are likely due to an exacerbation of this selection effect given the continued improvements in precision and SRS technology allowing for more patients to safely undergo outpatient procedures despite medical comorbidities. It must be acknowledged that as primary SRS case volume dropped precipitously in 2005, adjuvant SRS secondary to other primary procedures has increased as a proportion of all inpatient SRS, and increases in morbidity and complications may be due to these primary procedures (ie, craniotomy). However, the ratio of primary to adjuvant SRS remained relatively constant from 2005 onwards (Fig. 1) while all other indicators of disease burden continued to increase over time. Thus, changes in the inpatient primary SRS population must account for a significant portion of these changes. Given the improvements in methodology and technical precision, as well as the expansion of SRS to more benign disease processes, it is unlikely that this increase in disease burden reflects a trend of the general baseline SRS population.
Inpatient hospital stay duration for SRS has demonstrated a constant increase over our study period. (Fig. 3a) Given the increases in Charleson comorbidity scores and illness severity predictors seen over this same period, this trend likely reflects the increasingly challenging post-procedural recovery due to the increasing medical complexity of admitted patients. (Fig. 2) This concept is supported by the steady decrease in routine discharges home with a corresponding increase in discharges to intermediate care facilities or home with home health care. (Supple Fig. 1) Total inpatient charges associated with SRS admissions have also demonstrated progressive increases over our study period, likely secondary to accrued hospital costs from increased length of stay. (Fig. 3b)
Figure 3.
Mean length of stay and mean charges. A) The mean length of stay has shown considerable increase on a year-to-year basis from 4.3 days in 1998 to 7.8 days in 2011 (p < .05). B) Mean hospital charges were $50,200 between 1998 and 2011, however the inflation-adjusted mean hospital charges have increased over time from $36,583 in 1998 to $110,497 in 2011 (p < .05).
Supplemental Figure 1.
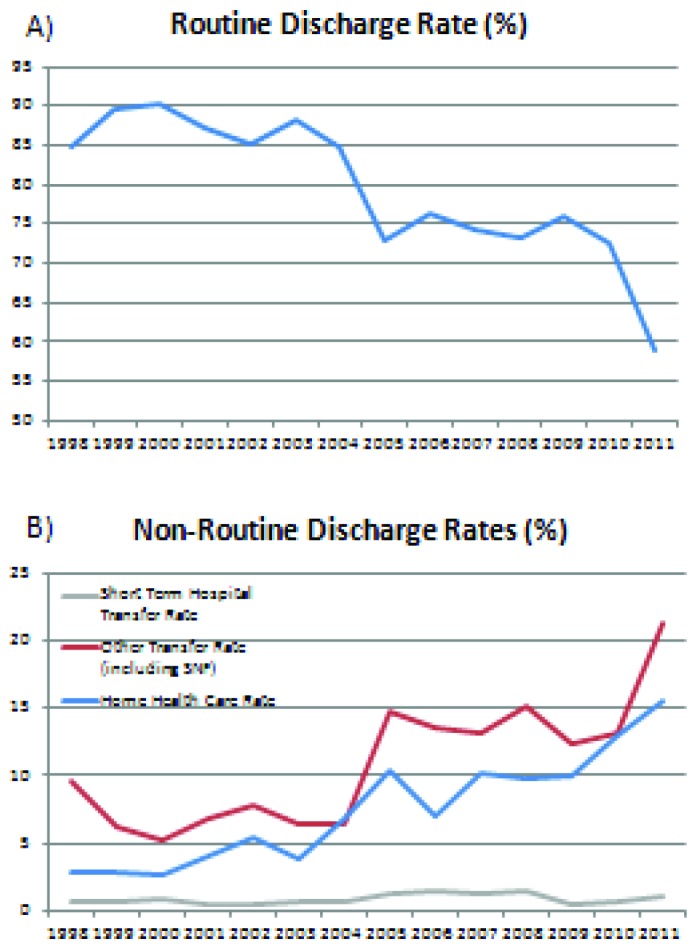
Routine and non-routine discharge rates. A) Routine discharges accounted for 82.3% of discharges throughout the entire time period but has decreased over time from 84.8% in 1998 to 58.9% in 2011 (p < .05). B) The proportion of patients discharged with home health care (blue) or transferred to other subacute facilities (red) have both increased from 2.8% and 9.6%, respectively in 1998 to 15.6% and 21.4% in 2011 (p < .05).
Historically, given multiple changes in CMS reimbursement policies, SRS has largely migrated to an outpatient practice. Patients who are admitted to the hospital setting to undergo treatment likely reflect a unique population of patients who are medically complex. This cohort of patients, captured in our study, demonstrated higher comorbidity indices as well as higher length of stays over time than traditionally reported in the literature. These factors should be taken into account when reviewing inpatient SRS series as they likely do not represent a homogenous patient population with outpatient series. Inpatient SRS case series may reflect poorer outcomes when compared to outpatient series due to an innate selection effect for sicker patients. Amid growing concerns about the impact of policies and CMS reimbursement schemes on the provision and appropriate reimbursement of care [26], it will be important to consider the recent trends in inpatient SRS utilization reported in this study as an example of the large impact such changes can have on the field of radiosurgery.
Limitations
As with all retrospective analyses utilizing large administrative databases, inherent limitations are present. Because the NIS database is an inpatient database, we are unable to elucidate outpatient procedural characteristics. From the discrepant mortality rates found within our cohort, it is likely that the inpatient population reflects a sicker patient population due to selection effects and cannot be directly generalized to the average outpatient. Thus, the results of this study may not be applied to the vast majority of SRS procedures that are occurring in the outpatient setting. Certainly there are many SRS cases not captured within the NIS database, but nevertheless the data do provide an interesting and reasonable estimate of trends in utilization within this growing field, which are well correlated with historical events over the last decade.
Data entry is subject to the accuracy or bias of administrators entering and curating data. However, these biases should be uniform throughout the database. NIS provides only inpatient events with hospital discharge marking the end to follow-up. As such, it is difficult to assess recurrence or long term complications, which may be presently underestimated in the literature. Additionally, minimal functional outcome data is present in the database, limiting conclusions we may draw on functional status post-procedure.
CONCLUSION
As the practice of stereotactic radiosurgery continues to evolve, there have been several trends in associated hospital admissions. Overall, the number of inpatient admissions for SRS has declined while adjuvant applications have remained stable. Given the total overall increased use of SRS per national reports, this shift likely reflects a move of patients from inpatient to outpatient centers. Over the same period, an associated increase in complication rate, length of stay, and mortality in inpatients have also been observed. Consequentially, hospital admission costs have similarly shown expected increases as well. These associations may be explained by an observed increase in comorbidity-load of admitted patients as sicker, more high-risk patients are selected for at inpatient centers while more stable patients are increasingly being referred to outpatient centers, in part due to changes in the reimbursement landscape surrounding SRS.
Ackowledgments
We gratefully acknowledge support for this study from Craig and Kimberly Darian and Carol Bade to Dr. Steven Chang.
Footnotes
Authors’ disclosure of potential conflicts of interest
Dr. Allen Ho and Alexander Li are members of and received grants from the Stanford Society of Physician Scholars which were utilized during the conduct of the study. This research was supported by the Office of the Dean, Stanford School of Medicine.
This work was supported in part by the Stanford Clinical and Translational Science Award (CTSA) to Spectrum (UL1 TR001085). The CTSA program is led by the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author contributions
Conception and design: Allen L. Ho, Steven D. Chang
Data collection: Allen L. Ho, Alexander Y. Li, Eric S. Sussman, Arjun V. Pendharkar
Data analysis and interpretation: Allen L. Ho, Alexander Y. Li, Eric S. Sussman, Arjun V. Pendharkar, Aditya Iyer, Steven D. Chang
Manuscript writing: Allen L. Ho, Alexander Y. Li, Eric S. Sussman, Arjun V. Pendharkar, Aditya Iyer, Patricia A. Thompson, Armine T. Tayag, Steven D. Chang
Final approval of manuscript: Allen L. Ho, Alexander Y. Li, Eric S. Sussman, Arjun V. Pendharkar, Aditya Iyer, Patricia A. Thompson, Armine T. Tayag, Steven D. Chang
REFERENCES
- 1. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta chirurgica Scandinavica 1951;102:316-319. [PubMed] [Google Scholar]
- 2. Lunsford LD, Maitz A, Lindner G. First united states 201 source cobalt-60 gamma unit for radiosurgery. Applied neurophysiology 1987;50:253-256. [DOI] [PubMed] [Google Scholar]
- 3. Barnett GH, Linskey ME, Adler JR, Cozzens JW, Friedman WA, Heilbrun MP, Lunsford LD, Schulder M, Sloan AE, American Association of Neurological S. Congress of Neurological Surgeons Washington Committee Stereotactic Radiosurgery Task F Stereotactic radiosurgery--an organized neurosurgery-sanctioned definition. Journal of neurosurgery 2007;106:1-5. [DOI] [PubMed] [Google Scholar]
- 4. Pannullo SC, Fraser JF, Moliterno J, Cobb W, Stieg PE. Stereotactic radiosurgery: A meta-analysis of current therapeutic applications in neuro-oncologic disease. J Neurooncol 2011;103:1-17. [DOI] [PubMed] [Google Scholar]
- 5. Kooy HM, Nedzi LA, Loeffler JS, Alexander E, 3rd, Cheng CW, Mannarino EG, Holupka EJ, Siddon RL. Treatment planning for stereotactic radiosurgery of intra-cranial lesions. International journal of radiation oncology, biology, physics 1991;21:683-693. [DOI] [PubMed] [Google Scholar]
- 6. Sheehan JP, Yen CP, Lee CC, Loeffler JS. Cranial stereotactic radiosurgery: Current status of the initial paradigm shifter. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32:2836-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kondziolka D, Lunsford LD, Loeffler JS, Friedman WA. Radiosurgery and radiotherapy: Observations and clarifications. Journal of neurosurgery 2004;101:585-589. [DOI] [PubMed] [Google Scholar]
- 8. Barbaro NM, Quigg M, Broshek DK, Ward MM, Lamborn KR, Laxer KD, Larson DA, Dillon W, Verhey L, Garcia P, Steiner L, Heck C, Kondziolka D, Beach R, Olivero W, Witt TC, Salanova V, Goodman R. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: Seizure response, adverse events, and verbal memory. Annals of neurology 2009;65:167-175. [DOI] [PubMed] [Google Scholar]
- 9. Sheehan JP, Patterson G, Schlesinger D, Xu Z. Gamma knife surgery anterior capsulotomy for severe and refractory obsessive-compulsive disorder. Journal of neurosurgery 2013;119:1112-1118. [DOI] [PubMed] [Google Scholar]
- 10. Young RF, Li F, Vermeulen S, Meier R. Gamma knife thalamotomy for treatment of essential tremor: Long-term results. Journal of neurosurgery 2010;112:1311-1317. [DOI] [PubMed] [Google Scholar]
- 11. Lunsford LD, Chiang V, Adler JR, Sheehan J, Friedman W, Kondziolka D. A recommendation for training in stereotactic radiosurgery for us neurosurgery residents. Journal of neurosurgery 2012;117 Suppl:2-4. [DOI] [PubMed] [Google Scholar]
- 12. Fuller BG, Kaplan ID, Adler J, Cox RS, Bagshaw MA. Stereotaxic radiosurgery for brain metastases: The importance of adjuvant whole brain irradiation. International journal of radiation oncology, biology, physics 1992;23:413-418. [DOI] [PubMed] [Google Scholar]
- 13. Sanghavi SN, Miranpuri SS, Chappell R, Buatti JM, Sneed PK, Suh JH, Regine WF, Weltman E, King VJ, Goetsch SJ, Breneman JC, Sperduto PW, Scott C, Mabanta S, Mehta MP. Radiosurgery for patients with brain metastases: A multi-institutional analysis, stratified by the rtog recursive partitioning analysis method. International journal of radiation oncology, biology, physics 2001;51:426-434. [DOI] [PubMed] [Google Scholar]
- 14. Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, Hudgins WR, Weiner R, Harsh GRt, Sneed PK, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. International journal of radiation oncology, biology, physics 1994;28:797-802. [DOI] [PubMed] [Google Scholar]
- 15. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. Jama 2006;295:2483-2491. [DOI] [PubMed] [Google Scholar]
- 16. Starke RM, Yen CP, Ding D, Sheehan JP. A practical grading scale for predicting outcome after radiosurgery for arteriovenous malformations: Analysis of 1012 treated patients. Journal of neurosurgery 2013;119:981-987. [DOI] [PubMed] [Google Scholar]
- 17. Lunsford LD, Niranjan A, Kondziolka D, Sirin S, Flickinger JC. Arteriovenous malformation radiosurgery: A twenty year perspective. Clinical neurosurgery 2008;55:108-119. [PubMed] [Google Scholar]
- 18. Hansasuta A, Choi CY, Gibbs IC, Soltys SG, Tse VC, Lieberson RE, Hayden MG, Sakamoto GT, Harsh GRt, Adler JR, Jr., Chang SD. Multisession stereotactic radiosurgery for vestibular schwannomas: Single-institution experience with 383 cases. Neurosurgery 2011;69:1200-1209. [DOI] [PubMed] [Google Scholar]
- 19. Hasegawa T, Fujitani S, Katsumata S, Kida Y, Yoshimoto M, Koike J. Stereotactic radiosurgery for vestibular schwannomas: Analysis of 317 patients followed more than 5 years. Neurosurgery 2005;57:257-265; discussion 257-265. [DOI] [PubMed] [Google Scholar]
- 20. Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: Summary of experience in 829 cases. Journal of neurosurgery 2005;102 Suppl:195-199. [PubMed] [Google Scholar]
- 21. Chang SD, Adler JR, Jr., Martin DP. Linac radiosurgery for cavernous sinus meningiomas. Stereotactic and functional neurosurgery 1998;71:43-50. [DOI] [PubMed] [Google Scholar]
- 22. Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, Niranjan A, Flickinger JC. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery 2008;62:53-58; discussion 58-60. [DOI] [PubMed] [Google Scholar]
- 23. Shrieve DC, Alexander E, 3rd, Wen PY, Fine HA, Kooy HM, Black PM, Loeffler JS. Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery 1995;36:275-282; discussion 282-274. [DOI] [PubMed] [Google Scholar]
- 24. Souhami L, Seiferheld W, Brachman D, Podgorsak EB, Werner-Wasik M, Lustig R, Schultz CJ, Sause W, Okunieff P, Buckner J, Zamorano L, Mehta MP, Curran WJ., Jr Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of radiation therapy oncology group 93-05 protocol. International journal of radiation oncology, biology, physics 2004;60:853-860. [DOI] [PubMed] [Google Scholar]
- 25. Emerick R. Regulatory and reimbursement aspects of radiosurgery. In: Regine C. editor Principles and practice of stereotactic radiosurgery. New York, NY: Springer Science+Business Media; 2008. pp. 673-680. [Google Scholar]
- 26. Heilbrun MP, Adler JR. The 2009 devaluation of radiosurgery and its impact on the neurosurgery-radiation oncology partnership. Journal of neurosurgery 2010;113:10-15. [DOI] [PubMed] [Google Scholar]