SUMMARY
In human motor learning, it is thought that the more information we have about our errors, the faster we learn. Here we show that additional error information can lead to improved motor performance without any concomitant improvement in learning. We studied split-belt treadmill walking that drives people to learn a new gait pattern using sensory prediction errors detected by proprioceptive feedback. When we also provided visual error feedback, participants acquired the new walking pattern far more rapidly and showed accelerated restoration of the normal walking pattern during washout. However, when the visual error feedback was removed during either learning or washout, errors reappeared with performance immediately returning to the level expected based on proprioceptive learning alone. These findings support a model with two mechanisms: a dual-rate adaptation process that learns invariantly from sensory prediction error detected by proprioception and a visual feedback dependent process that monitors learning and corrects residual errors, but shows no learning itself. We show that our voluntary correction model accurately predicted behavior in multiple situations where visual feedback was used to change acquisition of new walking patterns while the underlying learning was unaffected. The computational and behavioral framework proposed here suggests that parallel learning and error correction systems allow us to rapidly satisfy task demands without necessarily committing to learning, as the relative permanence of learning may be inappropriate or inefficient when facing environments that are liable to change.
Keywords: adaptation, split-belt treadmill, gait, motor learning, feedback, walking, locomotion
INTRODUCTION
When learning new movement patterns, we often seek feedback from motor “experts”: a tennis coach, a piano teacher, a dance instructor. We seek advice because we think that more feedback enables us to learn movement patterns faster. This idea expands beyond learning novel skills, as rehabilitation approaches rely heavily upon feedback to restore relatively automatic motor processes like walking. Consider a gait retraining session: a patient learning to walk with a knee brace might receive visual or auditory feedback from a therapist and proprioceptive feedback from his/her own body. How does the motor system use multiple forms of feedback simultaneously to learn new walking patterns?
Here we focus on understanding how visual error feedback affects walking adaptation. Adaptation is a learning process where a movement (e.g., walking) changes features (e.g., symmetry) in response to a perturbation while retaining its general identity [1]. When the perturbation is removed, baseline performance cannot be immediately recalled but is recovered through active deadaptation. Sensory prediction errors – discrepancies between expected and actual sensory consequences of movement – drive adaptation [2–4]. For example, during split-belt treadmill walking, people initially predict that they should walk as if both belts move at the same speed. Then, when one belt moves faster than the other, the prediction error is large and people begin to limp. Walking adaptation occurs as the prediction updates to minimize the error, resulting in a smooth, symmetric gait [5].
Prediction errors can be detected via proprioception alone [6–9]. Yet, walking adaptation appears to accelerate when people simultaneously see and feel their errors, though deadaptation was unaffected [10]. This is puzzling given that a prominent model of motor adaptation [11] predicts that changes in adaptation rate also change the deadaptation rate [12]. Here we consider that visual error feedback may not accelerate locomotor adaptation at all; instead, it may trigger a distinct voluntary mechanism to work alongside adaptation.
Recent findings support the idea that adaptation operates independently of voluntary changes in movement. Adaptation proceeds when people are told how to correct movement errors [3] or prohibited from correcting errors entirely [2]. Importantly, these studies enforced specific movement constraints (e.g., “aim your reach here” or “step here”). Here we did not constrain movement but instead provided an additional channel of sensory information about error via visual feedback. By allowing participants to see and feel their errors, we investigated whether walking adaptation is accelerated if multiple sensory modalities provide information about the same error.
We hypothesized that the locomotor system does not use visual error feedback to accelerate adaptation, but rather to voluntarily “clean up” errors not yet corrected by adaptation. To test this, we used a computational model to generate several counterintuitive predictions that bore out experimentally, clearly dissociating adaptation and voluntary correction during walking. Adaptation results in slow but lasting learning while voluntary correction is fast but fleeting. When combined, these mechanisms produce rapid, lasting changes to walking patterns.
RESULTS
Experiment 1: visual feedback of errors accelerates acquisition of a novel walking pattern
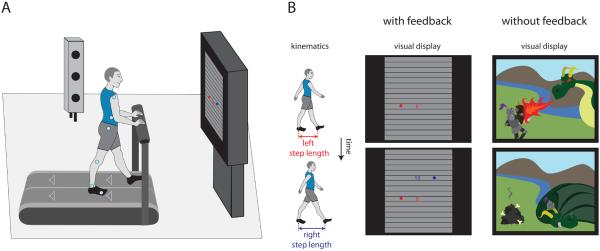
We first investigated whether participants could use a novel visual feedback system to restore symmetry more quickly during split-belt treadmill walking. The Feedback group received visual feedback of step lengths during split-belt adaptation when one belt moved twice as fast as the other (n=10). The No Feedback group adapted without visual feedback and instead watched a movie (n=10). We show the lab setup in Figure 1A and example kinematics with corresponding visual feedback in Figure 1B (further detail in supplemental text). We asked participants to walk with symmetric steps whenever the feedback was on and however felt most comfortable when the feedback was off.
Figure 1.
Lab setup for all experiments and visual display for Experiments 1-3. A) Participants walked on a split-belt treadmill while viewing visual feedback about their step lengths, or without visual feedback and instead a television show/movie. We recorded participant kinematics (pale blue markers) using three-dimensional motion capture. B) Example participant kinematics and corresponding visual display for Experiments 1-3 with visual feedback (left) and without visual feedback (right). Note that the visual feedback does not indicate real-time foot position, but rather step length (i.e., the distance between ankle markers) at heel-strike.
We show the Experiment 1 protocols in Figure 2A. Both groups first walked for two minutes with the belts tied at 0.7 m/s without feedback (Baseline) and then with feedback (Baseline with Feedback). We measured step length asymmetry because it adapts robustly [5] and is thought to represent the movement error experienced during split-belt treadmill walking [13]:
All groups in Experiments 1-4 showed similar step length asymmetry during Baseline (F(6,62)=1.06, p=0.40) and Baseline with Feedback (F(6,62)=1.52, p=0.19).
Figure 2.
Experiment 1 protocol and results. A) Experiment 1 protocols are shown for the Feedback and No Feedback groups. The protocols are identical with the exception that the Feedback group received visual feedback of their individual step lengths during Adaptation while the No Feedback group watched a movie instead. B) Mean ± SE adaptation curves across participants within the Feedback (blue) and No Feedback (green) groups are shown. The curves are truncated in length to match the participant that took the fewest strides during Adaptation. Data points immediately following the adaptation curves show the step length asymmetry during plateau (mean ± SE of the last 30 strides) for each group. C) Mean deadaptation curves across participants within the Feedback (blue) and No Feedback (green) groups ± SE are shown.
Following the baselines, both groups walked for 10 minutes with the belt under the non-dominant leg at 1.4 m/s and the other at 0.7 m/s (Adaptation). During Adaptation, both groups showed similar initial step length asymmetry (t(18)=0.87, p=0.40) though the Feedback group restored symmetry faster (early: t(18)=2.15, p=0.045) and more completely (plateau: t(18)=2.20, p=0.040; Figure 2B). Following Adaptation, both groups walked with both belts at 0.7 m/s without feedback and watched a movie (Deadaptation). The groups were similar during Deadaptation (initial (t(18)=0.02, p=0.99), early (t(18)=1.27, p=0.22), plateau (t(18)=0.97, p=0.34); Figure 2C).
What are the potential mechanisms?
Since adaptation and deadaptation rates are thought to be related [12], how did the visual feedback accelerate Adaptation without affecting Deadaptation? We answered this question by comparing predictions from a pair of computational models – one that explains accelerated acquisition as accelerated adaptation, and another that explains accelerated acquisition as visual feedback-dependent voluntary correction. We began with the dual-rate adaptation model proposed by Smith and colleagues [11] because this model describes many behavioral phenomena across motor adaptation paradigms [11,14] (further detail in supplemental text).
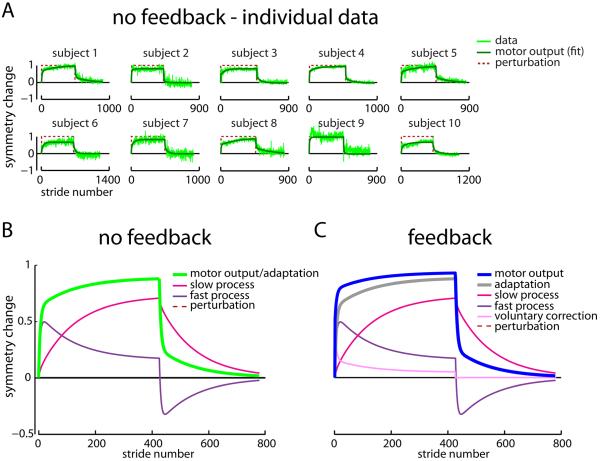
To compare how quickly participants restored step length symmetry, we transformed the step length asymmetry data to symmetry change (see supplemental text) and fit all models to symmetry change data. The dual-rate model with time-varying parameters can explain split-belt adaptation [15]; here we fit the model to data spanning Adaptation and Deadaptation with time-invariant parameters. We first fit the dual-rate model to No Feedback individual participant data (fits shown in Figure 3A, mean parameters and 95% confidence intervals for individual participant fits across all groups shown in Table S1). Because we aimed to make predictions about group behavior in future experiments, we also fit the model to the No Feedback group mean data shown in Figures 2B and 2C (fit shown in Figure 3B). The best-fit Af, As, Bf, and Bs parameters for the No Feedback group fit (Af=0.9335, As=0.9978, Bf=0.0918, Bs=0.0143; Figure S1A) were then used to predict behavior in all other groups in Experiments 1-4.
Figure 3.
Experiment 1 individual participant and group model fits. A) Dual-rate adaptation model fits are shown for Adaptation and Deadaptation symmetry change data from each participant in the No Feedback group. The data are shown in light green, the fit in dark green, and the perturbation (i.e., 1 for split belts, 0 for tied belts) in red. B) Dual-rate adaptation model fit to the group mean No Feedback data during Adaptation and Deadaptation. The motor output and adaptation (which overlap) are shown in green, slow process in magenta, fast process in purple, and perturbation in pink. C) Voluntary correction model fit to the Adaptation and Deadaptation group mean symmetry change data in the Feedback group. The motor output is shown in blue, adaptation in gray, slow process in magenta, fast process in purple, voluntary correction in pink, and perturbation in red. Note that the only difference between the fits on B and C is the presence of the voluntary correction process that also then changes the motor output. See also Figures S1 and S2.
The dual-rate model requires changing its parameters to account for the faster acquisition in the Feedback group. This would suggest that visual feedback accelerates adaptation by changing learning and/or retention. We show a dual-rate model fit with adjusted parameters (i.e., accelerated adaptation) to the Feedback group mean data in Figure S2.
Alternatively, accelerated acquisition could be explained by leaving the dual-rate adaptation parameters unchanged and instead adding a distinct, visual feedback-dependent voluntary correction process. This process corrects residual error unaccounted for by adaptation and shows no retention:
where 0≤Bv≤1. All other parameters are defined and constrained similarly to the dual-rate model. Note that, despite the voluntary correction, the error term e remains the difference between the perturbation and adaptation (x, as in the dual-rate model) and not the difference between the perturbation and motor output (xnew) because the motor output and adaptation are no longer equivalent. Importantly, in this model, voluntary correction then depends on adaptation while adaptation is independent of voluntary correction. The nervous system detects the prediction error e between the perturbation and adaptation then applies voluntary correction to further reduce this error and improve performance. It is possible that adaptation alone fails to correct this error more completely because adaptation is conservative and depends on prior knowledge of environmental consistency [16].
We show a voluntary correction model fit to the Feedback group mean data (using Af, As, Bf, and Bs parameters from the No Feedback group mean fit and fitting the new Bv parameter) in Figure 3C (parameters: Af=0.9335, As=0.9978, Bf=0.0918, Bs=0.0143, Bv=0.4148). The same fit is overlaid on data in Figure S1B and individual participant fits using the voluntary correction model are shown in Figure S1C. In sum, the dual-rate model explains accelerated acquisition as accelerated adaptation (Figure S2) and the voluntary correction model explains accelerated acquisition as unchanged adaptation with a distinct voluntary correction process (Figure 3C)
Experiment 2: visual feedback drives more complete acquisition during Adaptation with no effect on Deadaptation
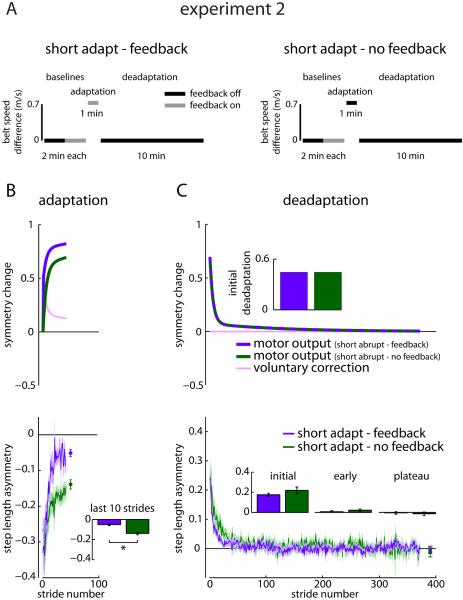
The models explain accelerated acquisition through different mechanisms, so which is utilized by the nervous system? We next tested several competing predictions of the two models. In Experiment 2, we tested two groups similar to Experiment 1 but shortened Adaptation. We did this to investigate whether a change in the amount of symmetry restored (i.e., plateau) during Adaptation affected initial Deadaptation. We should note that we observed a significant difference in the plateaus between the groups in Experiment 1. However, the difference was small (Feedback vs. No Feedback: −0.011 ± 0.027 vs. −0.051 ± 0.056) and it was difficult to interpret effects on Deadaptation. Here we tested a Short Adapt-Feedback group (n=10) that received visual step length feedback during a one-minute Adaptation and a Short Adapt-No Feedback group (n=10) that adapted for one minute without visual feedback (Figure 4A). Both groups then underwent a ten-minute Deadaptation without feedback.
Figure 4.
Experiment 2 protocol and results. A) Experiment 2 protocols are shown for the Short Adapt-Feedback and Short Adapt-No Feedback groups. These protocols are identical to those tested in Experiment 1 except Adaptation is truncated to one minute. B) Adaptation predictions from the voluntary correction model based on parameters set in Experiment 1 (top) and mean ± SE adaptation curves across participants within the Short Adapt-Feedback (purple) and Short Adapt-No Feedback (dark green) groups (bottom) are shown. Data points immediately following the adaptation curves show the step length asymmetry (mean ± SE) of the last 10 strides of Adaptation for each group. * indicates a significant difference with p < 0.05. C) Deadaptation predictions from the voluntary correction model based on parameters set in Experiment 1 (top) and mean deadaptation curves across participants within the Short Adapt - Feedback (purple) and No Feedback (dark green) groups ± SE (bottom) are shown. See also Figures S3 and S4.
The dual-rate model predicts that a large between-group difference in Adaptation plateau causes a large difference in initial Deadaptation (Figure S3). In other words, more adaptation causes a stronger aftereffect. However, the voluntary correction model allows Adaptation plateaus to differ without influencing Deadaptation, provided that visual feedback is removed after Adaptation (Figures 4B and 4C, top). In Experiment 2, the difference in Adaptation plateau more than doubled when we truncated Adaptation (mean of last ten strides, Short Adapt-Feedback vs. Short Adapt-No Feedback: −0.051 ± 0.032 vs. −0.139 ± 0.041; t(18)=5.37, p<0.001) compared to Experiment 1. However, Deadaptation remained similar between the Short Adapt groups (Figures 4B and 4C, bottom; initial, early, and plateau all p>0.20). We show simulations generated by the Af, As, Bf, and Bs parameters from the No Feedback group mean fit and Bv parameter from the Feedback group mean fit overlaid on Short Adapt-No Feedback and Short Adapt-Feedback group data in Figures S4A and S4B and individual participant fits using the voluntary correction model in Figures S4C and S4D. These findings provided initial compelling evidence that visual feedback drives voluntary changes in walking while underlying adaptation is unchanged.
Experiment 3: removal of visual feedback during Adaptation or Deadaptation causes a re-emergence of error
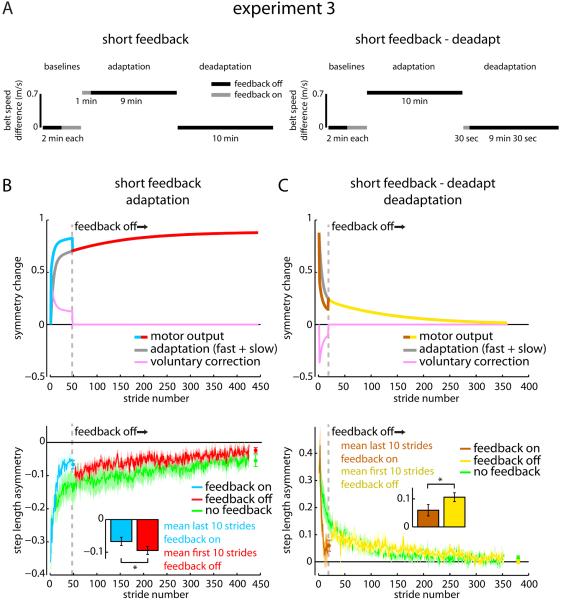
In Experiment 3, we considered an interesting prediction of the voluntary correction model that cannot be explained by changes in dual-rate parameters: previously corrected asymmetry should reappear if visual feedback is removed during Adaptation. Because voluntary correction is visual feedback-dependent and exhibits no retention, the model predicts that walking regresses to the pattern expected from adaptation alone whenever feedback is withheld, even if the belt speeds are unchanged. This prediction is rather counterintuitive because it predicts walking to regress from a desirable, energetically efficient [17], symmetric pattern to an undesirable, potentially energetically costly, asymmetric pattern without changing the treadmill speeds. Will the locomotor system lose its desired walking pattern simply because the means used to acquire it are removed?
We studied a Short Feedback group (n=10) that underwent a ten-minute Adaptation but received visual step length feedback during only the first minute (Figure 5A). As expected, we observed accelerated acquisition while the feedback was on. However, as would be predicted by the voluntary correction model (Figure 5B, top), asymmetry reappeared immediately once we removed the feedback (mean of last ten strides of Adaptation with feedback vs. mean of first ten strides of Adaptation without feedback: t(9)=2.63, p=0.027). More specifically, the walking pattern regressed to that observed in the No Feedback group from Experiment 1 (Figure 5B, bottom).
Figure 5.
Experiment 3 protocol and results. A) Experiment 3 protocols are shown for the Short Feedback and Short Feedback-Deadapt groups. B) Adaptation predictions from the voluntary correction model based on parameters set in Experiment 1 (top) and mean ± SE adaptation curves across participants within the Short Feedback group when the feedback was on (cyan) and off (red) as well as the No Feedback (green) group from Experiment 1 (bottom) are shown. Data points immediately following the adaptation curves show the step length asymmetry (mean ± SE) of the last 10 strides for each group. * indicates a significant difference with p < 0.05. C) Deadaptation predictions from the voluntary correction model based on parameters set in Experiment 1 (top) and mean deadaptation curves across participants within the Short Feedback-Deadapt group when the feedback was on (brown) and off (yellow) as well as the No Feedback (green) group from Experiment 1 (bottom) are shown. See also Figure S5.
The voluntary correction model also predicts a similar phenomenon when the visual feedback is removed during Deadaptation (Figure 5C, top). We find this particularly interesting because it means that participants could use feedback to restore a symmetric, everyday walking pattern in a normal environment (i.e., tied belts) and yet revert to an abnormal asymmetric pattern once the feedback is removed. We tested this in a Short Feedback–Deadapt group (n=10) that underwent a ten-minute Adaptation without feedback and then a ten-minute Deadaptation with feedback during only the first 30 seconds. We observed exactly what the voluntary correction model predicted: accelerated acquisition while the feedback was on and a rebound in asymmetry once it was removed (mean of last ten strides of Deadaptation with feedback vs. mean of first ten strides of Deadaptation without feedback: t(9)=2.31, p=0.046). We show simulations generated by the Af, As, Bf, and Bs parameters from the No Feedback group mean fit and Bv parameter from the Feedback group mean fit overlaid on Short Feedback and Short Feedback-Deadapt group mean data in Figures S5A and S5B and individual participant fits using the voluntary correction model in Figures S5C and S5D. Thus, even when the visual feedback helps restore a normal walking pattern in a normal environment, voluntary correction does not influence locomotor adaptation.
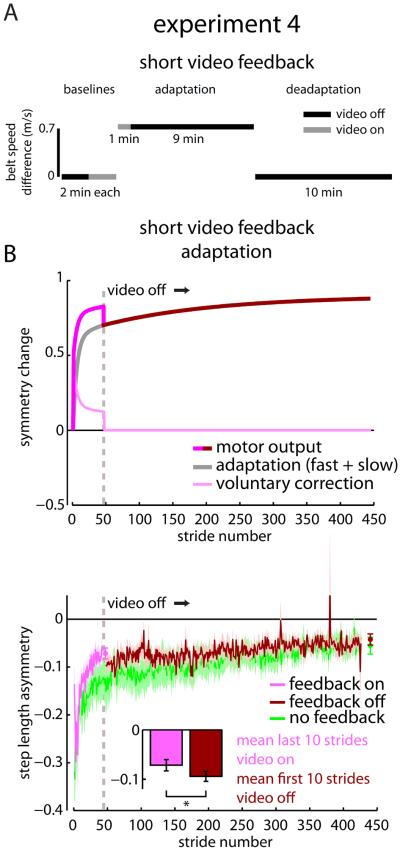
Experiment 4: real-time continuous visual feedback of walking has no effect on adaptation
In Experiment 4, we tested whether more salient visual feedback affected adaptation. Specifically, we wondered whether continuous, real-time information about gait kinematics (beyond simply step length) might influence adaptation. We tested a Video Feedback group (n=9) that was similar to the Short Feedback group from Experiment 3, only here participants viewed real-time digital video feedback of a sagittal view of their feet and lower legs (as in [10]) instead of our virtual feedback screen. We asked the participants to walk with their step lengths as symmetric as possible (an investigator demonstrated what was meant by step length) whenever the video was displayed. Following the baselines, participants underwent a ten-minute Adaptation followed by a ten-minute Deadaptation. Similar to the Short Feedback group, we removed the video feedback after one minute during Adaptation (Figure 6A). Again, the voluntary correction model predicts that asymmetry will reappear once the video is removed with adaptation unaffected (Figure 6B, top).
Figure 6.
Experiment 4 protocol and results. A) Experiment 4 protocol is shown for the Short Video Feedback group. B) Adaptation predictions from the voluntary correction model based on parameters set in Experiment 1 (top) and mean ± SE adaptation curves across participants within the Short Video Feedback group when the feedback was on (magenta) and off (dark red) as well as the No Feedback (green) group from Experiment 1 (bottom) are shown. Data points immediately following the adaptation curves show the step length asymmetry (mean ± SE) of the last 10 strides for each group. * indicates a significant difference with p < 0.05. See also Figures S6 and S7 and Table S1.
As observed in the Short Feedback group, participants used the video feedback to restore symmetry faster but regressed once the video was removed (Figure 6B, bottom; mean of last ten strides of Adaptation with feedback vs. mean of first ten strides of Adaptation without feedback: t(8)=2.70, p=0.027). We show a simulation generated by the Af, As, Bf, and Bs parameters from the No Feedback group mean fit and Bv parameter from the Feedback group mean fit overlaid on Short Video Feedback group mean data in Figure S6A, an example of the video feedback seen by the participants in Figure S6B, and individual participant fits using the voluntary correction model in Figure S6C. These findings indicate that multiple types of visual feedback are irrelevant for adaptation, as even real-time video of the participants' own walking patterns drove voluntary correction without influencing adaptation.
Why does a voluntarily corrected walking pattern revert to a presumably less desirable pattern after feedback is removed? After all, it appears that voluntary correction drove faster acquisition of the same symmetric walking pattern that would later be learned through dual-rate adaptation—why doesn't the nervous system recognize this? Perhaps the walking pattern acquired using the feedback was different (and potentially more energetically costly) than the pattern learned through adaptation. For example, the participants could have taken “choppier” steps with increased cadence and shortened step lengths to achieve symmetry on the feedback display at the expense of minimizing energetic cost. We would then not expect participants to retain this pattern once feedback was removed because the prediction error remains and there is now no reason to incur additional energy cost.
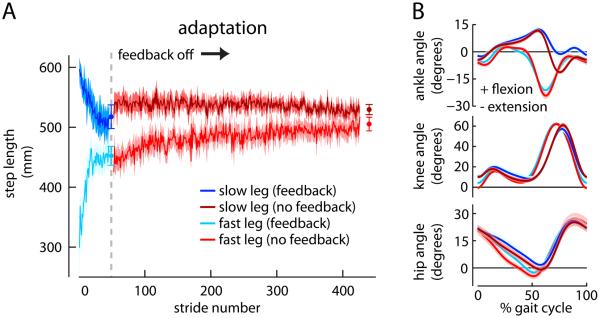
Figure 7A shows the step lengths adopted by the Short Feedback group during Adaptation. Step lengths changed similarly to previous split-belt walking experiments [5]. The sagittal joint angle kinematics acquired while the feedback was on were also mostly consistent with prior findings (Figure 7B); however, the fast limb ankle plantarflexion was diminished during the swing phase (though not significantly: t(18)=1.60, p=0.127). This leaves an open question whether this subtle change in ankle kinematics was sufficient for the nervous system to render the pattern inefficient, discard it, and pursue another through adaptation. This may be possible as the ankle exhibits large kinematic [5] and kinetic [18] changes during split-belt walking. However, we think the most likely explanation is that voluntary changes in movement do not influence one's prediction about the belt speeds, and thus the prediction error driving adaptation is unchanged.
Figure 7.
Experiment 3 individual step lengths and joint kinematics. A) Mean ± SE slow and fast step lengths during Adaptation in the Short Feedback group when the feedback was on (slow in dark blue, fast in cyan) and off (slow in dark red, fast in light red). Data points immediately following the adaptation curves show the step length asymmetry (mean ± SE) of the last 10 strides for each feedback condition. B) Mean ± SE ensemble ankle (top), knee (middle), and hip (bottom) angles for the Short Feedback group averaged over the last five strides when the feedback was on and off. Positive values indicate flexion angles and negative indicate extension.
DISCUSSION
In this study, we used two different visual feedback methods to demonstrate that voluntary movement correction and locomotor adaptation work differently to change walking patterns. This result held regardless of whether the feedback was represented by an abstract virtual environment or a direct video feed of the feet and lower legs. Locomotor adaptation can be explained by the familiar dual-rate adaptation model characterized by fast and slow learning processes [11] while voluntary correction corrects residual errors and shows no retention.
We think that two representations of error drove these separate mechanisms simultaneously. Visual error feedback drove voluntary correction of walking patterns and sensory prediction error detected by proprioceptive feedback drove locomotor adaptation. We suspect that other feedback modalities, such as auditory instruction, could also drive voluntary correction; however, walking adaptation appears to be driven purely by prediction error (i.e., the treadmill initially moves at unexpected speeds).
The findings presented here show the remarkably invariant nature of locomotor adaptation. They are consistent with other work showing that adaptation proceeds unaltered when people are either told how to correct movement errors [3] or prohibited from correcting errors entirely [2]. Indeed, it is difficult to accelerate the adaptation rate through behavioral means, as the tools available are limited to electrical stimulation of the cerebellum [19] and prior experience (i.e., savings; [20,21]). The aftereffects following adaptation are similarly robust, as they persist even when voluntary correction provides a “shortcut” to the familiar walking pattern.
Voluntary correction, on the other hand, adds flexibility to locomotor control without affecting adaptation. This is not the case in other motor skill learning paradigms (e.g. object manipulation, reaching), where continuous feedback appears to accelerate learning but people actually learn less [22]. The ability to change walking voluntarily does not result in lasting learning but does facilitate coordination of a steady, controlled walking pattern, even while subconscious learning processes (e.g., adaptation) recalibrate locomotion to new environments. It is interesting that the voluntary correction studied here was relatively slow and incomplete (with Bv much smaller than 1). We suspect that Bv < 1 because participants prioritize stability over voluntary correction upon immediate exposure to the split-belt perturbation, and thus do not immediately use the visual feedback to generate symmetric steps. Further, the feedback provided in Experiment 3 was step length feedback (not real-time foot position feedback) and likely requires a learning curve before the participants fully understand how their step lengths map to the screen. In Experiment 4, the video feedback is real-time but it is difficult to tell exactly how symmetric step lengths are using the less quantitative video feedback. This probably also influenced the efficacy of the voluntary correction.
We must consider that these mechanisms represent only a subset of those used to change walking patterns. Voluntary correction could benefit mechanisms other than adaptation. Take for example, energy optimization – this process can robustly drive changes in walking [23]. Visual feedback might be useful for helping people find an energetically optimal solution faster by adding extra feedback to fine-tune exploration or maintain a new pattern, rather than default to costly preprogrammed gait patterns [24]. We also believe that energy optimization is distinct from the adaptation tested here (though adaptation can result in a more energy efficient pattern [17]). Unlike adaptation, energy optimization has not been shown to be cerebellum-dependent [25] or driven by prediction error, but instead results in a remapping between a movement pattern and its expected energetic consequences. It is unclear whether this remapping changes perception (as has been observed in split-belt walking [26,27]) or generalizes to other contexts.
It is important to note that our key finding is that voluntary correction and adaptation are fundamentally distinct mechanisms that can be dissociated during walking, and that we are not suggesting a prescribed role for each type of sensory feedback (vision versus proprioception) in all learning situations. Indeed, other work has shown that visual feedback can drive locomotor learning mechanisms that do not require adaptation or prediction error, such as sequence learning [28]. In our study, visual feedback drove voluntary correction and not adaptation because the split-belt perturbation is mechanical (i.e., the belts move at different speeds) and the prediction error is detected through proprioception. The visual feedback simply provided an additional channel of performance feedback that was used for voluntary correction. However, our prior work showed that visual feedback can drive gait adaptation when a visual prediction error is present, such as showing one leg flexing less than it actually does [29]. Participants adapted to that visual feedback by increasing flexion in the perturbed leg and subsequently showed aftereffects when the visual perturbation was removed.
It is also beneficial to contrast our work with prior work on reaching. If people are told where to aim their reach to counteract a visuomotor rotation, they initially hit the target but begin to miss as they adapt [3]. When the aim is flexible, aiming balances with adaptation to reduce movement errors [30]. This has led to the interpretation that voluntary aiming represents the fast state of the dual-rate adaptation model [31]. While this may be the case in reaching, it is unlikely to be in walking. The fast and slow states of dual-rate adaptation learn from the same error signal and sum to update the same motor output [11]. Therefore, errors should not immediately reappear after they are corrected without a change in the perturbation, which we observed here. Further, adaptation in the No Feedback group was better explained by a dual-rate rather than single-rate adaptation model (see supplemental text), suggesting that the fast and slow processes contribute to locomotor adaptation even in the absence of visual feedback.
The findings reported here also explain how watching oneself walk drove faster acquisition in our previous study [10]. It now appears that participants likely did not learn faster in that study, but rather brought online a distinct voluntary correction process. However, one notable inconsistency between that study and this one is that visual feedback was removed during adaptation in both, but asymmetry reappeared only in the present study. This is likely because in our previous study [10], participants were asked to maintain their gait pattern when the feedback was removed, and thus voluntary correction remained engaged.
CONCLUSION
Understanding how locomotor control and learning mechanisms operate to make quickly-acquired and long-lasting changes in walking patterns remains a key area of interest in gait rehabilitation research. Here, we present a novel computational and behavioral framework to illustrate how voluntary correction and adaptation mechanisms influence gait patterns. Voluntary correction is feedback-dependent, corrects errors not yet corrected by adaptation, and shows no retention while adaptation is characterized as dual-rate learning. These mechanisms can be leveraged simultaneously to facilitate fast, lasting changes in walking patterns and represent a promising step forward in understanding the mechanisms available to change walking patterns.
EXPERIMENTAL PROCEDURES
Participants
Sixty-nine healthy young people participated (demographic detail in supplemental text). All participants were naïve to split-belt treadmill walking, free of neurological, musculoskeletal, or cardiovascular conditions, and participated in only one of the three experiments. We recorded leg dominance as the leg used to kick a soccer ball. They also provided written informed consent in accordance with the Johns Hopkins Medicine Institutional Review Board prior to participating and were compensated for participation.
Data collection
Participants walked on a custom-built split-belt treadmill (Woodway USA, Waukesha, WI) and we collected kinematic data similarly to prior studies [2,21] (detail in supplemental text). We controlled the belts using custom Vizard software (WorldViz, Santa Barbara, CA).
Behavioral data analysis
Our primary outcome measure is step length (i.e., anterior-posterior distance between the ankle markers at heel-strike) asymmetry. In Experiment 1, we analyzed Adaptation and Deadaptation at three distinct time periods: initial (mean of the first five strides), early (mean of strides 6–200), and plateau (mean of the last 30 strides) [32]. We compared these periods between the Feedback and No Feedback groups using independent samples t-tests. In Experiment 2, we compared the mean of the last ten strides of Adaptation as well as initial, early, and plateau Deadaptation between the Short Adapt–Feedback and Short Adapt–No Feedback groups using independent samples t-tests. In Experiments 3 and 4, we compared the mean of the last ten strides of Adaptation with feedback to the mean of the first ten strides of Adaptation without feedback in the Short Feedback group using paired samples t-tests. Similarly, we compared the mean of the last ten strides of Deadaptation with feedback to the mean of the first ten strides of Deadaptation without feedback in the Short Feedback–Deadapt group using a paired samples t-test. We performed all statistical analyses using MATLAB (The Mathworks, Natick, MA; α=0.05).
Computational modeling
After calculating symmetry change for each No Feedback participant (see supplemental text), we fit the dual-rate model to individual data spanning Adaptation and Deadaptation without changing the parameters between conditions (fitting details in supplemental text). We also fit the dual-rate model to the No Feedback group mean data. This fit yielded the following parameters: Af=0.9335, As=0.9978, Bf=0.0918, and Bs=0.0143 (residuals between simulations and group mean data shown in Figure S7). We used these parameters to make predictions about group data in the Feedback group and all groups in Experiments 2, 3, and 4. We then fit the voluntary correction model to the Feedback group mean data using these four parameters and allowed a new free parameter Bv. This fit yielded Bv=0.4148. We used these parameters to make predictions about all group data in Experiments 2–4. We also fit the voluntary correction model with Af, As, Bf, Bs, and Bv as free parameters to all individual participants across all experiments. As mentioned by Smith and colleagues [11], the qualitative predictions made by the models do not depend on specific parameter values but hold as long as Af<As, Bf>Bs, and all parameters are ≤1 and ≥0.
We also considered that visual feedback might accelerate adaptation. To test this hypothesis, we fit the dual-rate model to the Feedback group mean data. We began with the same initial conditions and this fit yielded: Af=0.9689, As=1.0, Bf=0.0791, and Bs=0.0169. Note that this model accounts for accelerated symmetry restoration by increasing the magnitudes of the retention parameters (Af and As) and maintaining the learning parameters (Bf and Bs) relatively constant when compared to the No Feedback group fit above. The model fit for the group mean Feedback data is shown in Figure S2 and the model's predictions for the Short Adapt–Feedback and Short Adapt–No Feedback groups in Experiment 2 are shown in Figure S3.
Supplementary Material
Highlights.
People can use visual error feedback to acquire a new walking pattern faster.
Visual error feedback triggers voluntary correction of walking errors.
Voluntary correction and motor adaptation work in parallel to change walking.
Voluntary correction monitors adaptation; adaptation learns from prediction error.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants F32 NS090751 to RTR, F31 NS092241 to AWL, and R01 HD048741/R37 NS090610 to AJB. We thank Tziporah Thompson for illustrations on Figure 1, Anthony Gonzalez for assistance with data collections, and Kristan Leech for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS Conceptualization, RTR, AWL, and AJB; Methodology, RTR, AWL, and AJB; Investigation, RTR; Formal Analysis, RTR; Writing – Original Draft, RTR; Writing – Review & Editing, RTR, AWL, and AJB; Funding Acquisition, RTR, AWL, and AJB; Resources, AJB; Supervision, AJB.
REFERENCES
- 1.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119:1199–211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- 2.Long AW, Roemmich RT, Bastian AJ. Blocking trial-by-trial error correction does not interfere with motor learning in human walking. J. Neurophysiol. 2016;115:2341–8. doi: 10.1152/jn.00941.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J. Neurosci. 2006;26:3642–5. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J. Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- 5.Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J. Neurophysiol. 2005;94:2403–15. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- 6.Torres-Oviedo G, Bastian AJ. Seeing is believing: effects of visual contextual cues on learning and transfer of locomotor adaptation. J. Neurosci. 2010;30:17015–22. doi: 10.1523/JNEUROSCI.4205-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiZio P, Lackner JR. Congenitally blind individuals rapidly adapt to coriolis force perturbations of their reaching movements. J. Neurophysiol. 2000;84:2175–80. doi: 10.1152/jn.2000.84.4.2175. [DOI] [PubMed] [Google Scholar]
- 8.Tong C, Wolpert DM, Flanagan JR. Kinematics and dynamics are not represented independently in motor working memory: evidence from an interference study. J. Neurosci. 2002;22:1108–13. doi: 10.1523/JNEUROSCI.22-03-01108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheidt RA, Conditt MA, Secco EL, Mussa-Ivaldi FA. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J. Neurophysiol. 2005;93:3200–13. doi: 10.1152/jn.00947.2004. [DOI] [PubMed] [Google Scholar]
- 10.Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J. Neurophysiol. 2010;103:1954–62. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang VS, Shadmehr R. Persistence of motor memories reflects statistics of the learning event. J. Neurophysiol. 2009;102:931–40. doi: 10.1152/jn.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JT, Vining EP, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain. 2009;132:722–33. doi: 10.1093/brain/awn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huberdeau DM, Krakauer JW, Haith AM. Dual-process decomposition in human sensorimotor adaptation. Curr. Opin. Neurobiol. 2015;33:71–7. doi: 10.1016/j.conb.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Mawase F, Shmuelof L, Bar-Haim S, Karniel A. Savings in locomotor adaptation explained by changes in learning parameters following initial adaptation. J. Neurophysiol. 2014;111:1444–54. doi: 10.1152/jn.00734.2013. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez Castro LN, Hadjiosif AM, Hemphill MA, Smith MA. Environmental consistency determines the rate of motor adaptation. Curr. Biol. 2014;24:1050–61. doi: 10.1016/j.cub.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: the energy cost of walking tracks motor adaptation. J. Physiol. 2013;591:1081–95. doi: 10.1113/jphysiol.2012.245506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roemmich RT, Stegemöller EL, Hass CJ. Lower extremity sagittal joint moment production during split-belt treadmill walking. J. Biomech. 2012;45:2817–21. doi: 10.1016/j.jbiomech.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J. Neurophysiol. 2012;107:2950–7. doi: 10.1152/jn.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malone LA, Vasudevan EV, Bastian AJ. Motor adaptation training for faster relearning. J. Neurosci. 2011;31:15136–43. doi: 10.1523/JNEUROSCI.1367-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roemmich RT, Bastian AJ. Two ways to save a newly learned motor pattern. J. Neurophysiol. 2015;113:3519–30. doi: 10.1152/jn.00965.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winstein CJ. Knowledge of results and motor learning--implications for physical therapy. Phys. Ther. 1991;71:140–9. doi: 10.1093/ptj/71.2.140. [DOI] [PubMed] [Google Scholar]
- 23.Selinger JC, O'Connor SM, Wong JD, Donelan JM. Humans can continuously optimize energetic cost during walking. Curr. Biol. 2015;25:2452–6. doi: 10.1016/j.cub.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Snaterse M, Ton R, Kuo AD, Donelan JM. Distinct fast and slow processes contribute to the selection of preferred step frequency during human walking. J. Appl. Physiol. 2011;110:1682–90. doi: 10.1152/japplphysiol.00536.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J. Neurosci. 2006;26:9107–16. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vazquez A, Statton MA, Busgang SA, Bastian AJ. Split-belt walking adaptation recalibrates sensorimotor estimates of leg speed but not position or force. J. Neurophysiol. 2015;114:3255–67. doi: 10.1152/jn.00302.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen L, Prokop T, Dietz V. Adaptational effects during human split-belt walking: influence of afferent input. Exp. Brain. Res. 1998;118:126–30. doi: 10.1007/s002210050262. [DOI] [PubMed] [Google Scholar]
- 28.Choi JT, Jensen P, Nielsen JB. Locomotor sequence learning in visually guided walking. J. Neurophysiol. 2016;115:2014–20. doi: 10.1152/jn.00938.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Statton MA, Toliver A, Bastian AJ. A dual-learning paradigm can simultaneously train multiple characteristics of walking. J. Neurophysiol. 2016;115:2692–700. doi: 10.1152/jn.00090.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor JA, Krakauer JW, Ivry RB. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J. Neurosci. 2014;34:3023–32. doi: 10.1523/JNEUROSCI.3619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDougle SD, Bond KM, Taylor JA. Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. J. Neurosci. 2015;35:9568–79. doi: 10.1523/JNEUROSCI.5061-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long AW, Finley JM, Bastian AJ. A marching-walking hybrid induces step length adaptation and transfers to natural walking. J. Neurophysiol. 2015;113:3905–14. doi: 10.1152/jn.00779.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.