Abstract
Background
It is well known that cerium oxide nanoparticles (CeNPs) have intense antioxidant activity. The antioxidant property of CeNPs are widely used in different areas of research, but little is known about the oxidative damage of Cu2+ associated with Type II diabetes mellitus (T2DM).
Material/Methods
In our research, the function of CeNPs was tested for its protection of β-cells from the damage of Cu2+ or H2O2. We detected hydroxyl radicals using terephthalic acid assay, hydrogen peroxide using Amplex Ultra Red assay, and cell viability using MTT reduction.
Results
We found that CeNPs can persistently inhibit Cu2+/H2O2 evoked hydroxyl radicals and hydrogen peroxide in oxidative stress of β-cells.
Conclusions
CeNPs will be useful in developing strategies for the prevention of T2DM.
MeSH Keywords: Diabetes Mellitus, Type 2; Hydrogen Peroxide; Islet Amyloid Polypeptide
Background
The number of people with Type II diabetes mellitus (T2DM) will reach 300 million by 2025, and T2DM is the most common form of diabetes, accounting for more than 90% of all diabetic cases [1]. T2DM is characterized by hyperglycemia caused by insufficiency insulin secretion or insensitivity to insulin action in secondary tissues [2], including skeletal muscle, adipose tissue, and liver tissues, and is associated with certain comorbidities including cardiovascular disease (CVD), obesity, and metabolic syndrome [3–5]. One of the major molecular mechanisms that has been proposed is hyperglycemia-induced oxidative stress which leads to deficits in β-cells and increased β-cells apoptosis [6,7]. In addition to the aforementioned hallmarks of T2DM, elevated serum Cu2+ levels are also commonly associated with T2DM [8]. Cu2+ is a cofactor for a number of enzymes such as cytochrome c oxidase and superoxide dismutase, which suggests an intermediary role for glutathione (GSH) [9]. Cu2+ levels are also commonly associated with several diseases, including Wilson disease and Alzheimer disease [10,11]. Under reducing cell-free conditions, particularly in the presence of the reducing agent GSH, Cu2+ tends to convert to the potent and reactive Cu+ ion that can produce H2O2 [12] rather than hA that has been found not only to produce but to also quench the H2O2 effect by several fold. Current research indicates that hA reduces the amount of H2O2 and decrease hydroxyl radical formation produced by Cu2+ and GSH [13]. This suggests that for T2DM it may be more important that Cu2+ is protected from reduced cellular agents such as GSH.
From these oxidative injuries, all stimulated intracellular reactive oxygen species (ROS) levels appear to cause nuclear and DNA damage resulting in apoptosis [14]. ROS and subsequent apoptosis correlate closely with the pathogenic mechanism and progression of T2DM. Antioxidative therapy has been the focus of clinical interest, however, antioxidants have had limited success, which has been attributed to their short half-lives, daily dosing requirements, side-effects, etc. [15–18]. Hence, much attention has been focused on searching for more powerful medicines and therapeutic strategies.
Cerium oxide nanoparticles (CeNPs), a rare earth oxide element from the lanthanide series of the periodic table, are widely used in oxygen sensing, anti-inflammatory action, and automotive catalytic converters [19,20]. The surface of the small size CeNPs exist in both Ce3+ and Ce4+ state attributing to a high surface area to volume ratio [21]. CeNPs reduce superoxide-produced hydrogen peroxide (H2O2) [22]. Then Ce4+ oxidizes H2O2 to O2 and regenerates Ce3+, and Ce3+ is also oxidized to Ce4+. It can form an auto-regenerative redox cycle on the surface of CeNPs between Ce3+ and Ce4+, and create oxygen defects to scavenge the free radicals [23]. Although CeNPs have nearly ideal antioxidant activity, little is known about their inhibition of oxidative damage to β-cells.
Hence, in our study, we aimed to discover whether the antioxidant activity of CeNPs protects β-cells from the damage of Cu2+ or H2O2 to the benefit of T2DM.
Material and Methods
Materials
CeNPs were purchased from Sciventions Inc., Canada as a 1.5 mg/mL aqueous suspension of sizes ranging from 1 nm to 10 nm, stabilized by polyacrylate sodium. Terephthalic acid (TPA) and MTT (thiazolyl blue tetrazolium bromide or (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) were obtained from Sigma-Aldrich (USA). Amplex Ultra Red reagent was purchased from Invitrogen (USA). Water with a resistivity of 18.2 MΩ cm was obtained through a Milli-Q system and was used in all the experiments.
Cell culture
Rat insulinoma RINm5f cell line, which was derived from a rat islet cell tumor, was obtained from American Type Culture Collection (ATCC) (USA) and cultured in 5% CO2 in RPMI-1640 medium (ATCC, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were maintained at 37°C in a humidified incubator and passaged bi-weekly.
Detection of hydroxyl radical
Hydroxyl radical production was detected by TPA [24]. The treatments were co-incubated with fresh 5 mM TPA in 0.2 M phosphate buffered saline (PBS, pH 7) at room temperature for 60 minutes, 120 minutes, and 180 minutes. Total fluorescent intensity was measured at an excitation of 326 nm and emission of 432 nm on the SpectraMax M5 spectrofluorometer (Molecular Devices, LLC, USA). The fluorescence intensities of TPA are proportional to the amount of hydroxyl radicals produced in the system.
Detection of hydrogen peroxide
H2O2 production by CeNP, Cu2+, and GSH was detected using the Amplex Ultra Red hydrogen peroxide detection assay. The indicator solution was mingled with 100 μM Amplex Ultra Red reagent and 0.2 U/mL horseradish peroxidase (HRP) in 0.01 M PBS pH 7 or pH 5. Standard curve was made by concentrations ranging from 100 nM to 20 μM of H2O2. Following treatment, an equal volume of Amplex Ultra Red/HRP solution was reacted with each sample and incubated for 20 minutes at room temperature. Total fluorescent intensity was measured at an excitation of 530 nm and emission of 590 nm in the SpectraMax M5 spectrofluorometer (Molecular Devices, LLC, USA). The obtained fluorescence intensities were converted to peroxide concentrations using the standard curve.
MTT reduction assay
The reduction of MTT was used to assess cell viability. Following treatments, 100 uL of MTT solution in PBS (1 mg/mL) was added to each well and incubated for 3 hours at 37°C. Following which MTT was replaced by isopropanol with 1% HCl and kept on the shaker for 15 minutes. Colorimetric measurements of viable cell numbers were made at 570 nm against a background measurement of 690 nm with a Spectra Max M5 spectrofluorometer.
Results
The effect of cerium oxide nanoparticles on Cu2+-evoked hydroxyl radical production
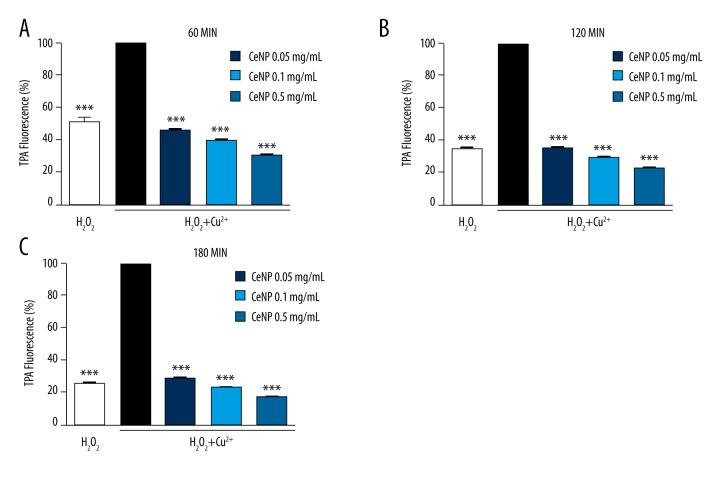
In this experiment, we tested the modulatory effect of CeNPs on Cu2+ catalyzed hydroxyl radical production. In the presence of H2O2, Cu2+ is known to catalyze hydroxyl radical production through the well-known Haber–Weiss chemistry [25,26]. In our study, CeNPs had a significant inhibitory effect on Cu2+/H2O2-induced hydroxyl radical formation (Figure 1). With the passage of time, the activity of CeNPs still maintained stabilization separately at 60 minutes (Figure 1A), 120 minutes (Figure 1B), and 180 minutes (Figure 1C). Taken together, our results indicated that CeNPs reduced Cu2+/H2O2 producing hydroxyl radicals significantly.
Figure 1.
Effect of CeNPs on Cu2+/H2O2-induced hydroxyl radical formation after (A) 60 minutes (B) 120 minutes (C) 180 minutes. The final concentration 10 μM of Cu2+, 50 μM of H2O2 and 0.5 mg/mL, 0.1 mg/mL and 0.05 mg/mL of CeNPs were dissolved in ultrapure water with 96-well plate using TPA fluorescent assay. The hydroxyl radical production was decreased by CeNPs. (*** p<0.01)
The effect of cerium oxide nanoparticles on hydrogen peroxide scavenging
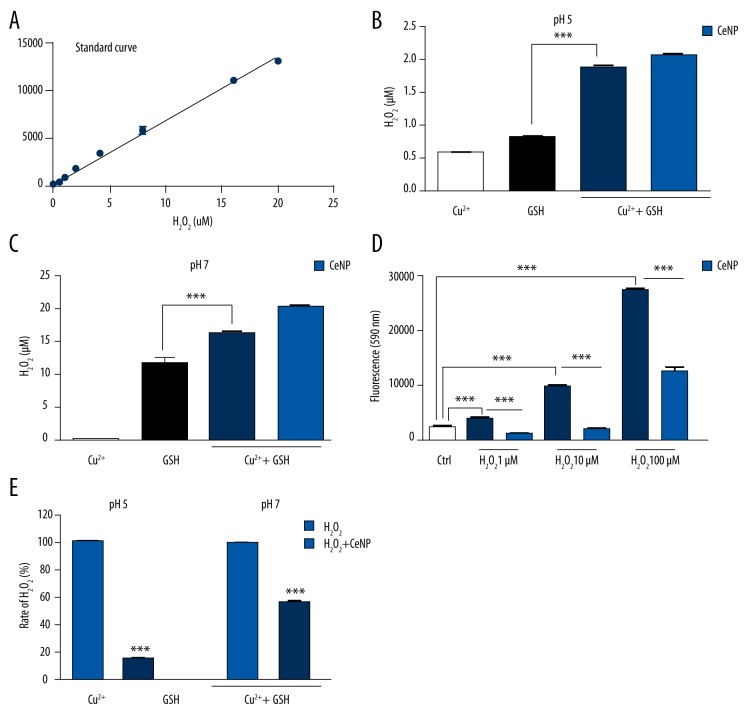
Interaction of Cu2+ with GSH produced an increase in H2O2 in the system, in contrast to the negligible stimulatory effect of Cu2+ on H2O2 production even at pH 5 (Figure 2B) and pH 7 (Figure 2C). The composition of the β-cells granule pH has been estimated to be 5–6 [27], close to the isoelectric point of insulin, and this is favorable for the solution of the hA. In conclusion, CeNPs had no significant effect on Cu2+/GSH evoked H2O2 formation. However, CeNPs at a concentration of 0.5 mg/mL caused a significant reduction in only H2O2 (1, 10, and 100 μM) accumulation (Figure 2D). CeNPs worked better in pH 5 than pH 7 (Figure 2E).
Figure 2.
Amplex Ultra Red hydrogen peroxide detection assay Amplex Ultra Red hydrogen peroxide detection assay standard curve (A). At pH 5 (B) and pH 7 (C), CeNPs did not inhibit H2O2 production. (D) While CeNPs inhibited accumulation of all the concentration of H2O2 (1, 10, and 100 μM). (E) CeNPs decreased the production of H2O2 (20 μM) more at pH 5 than pH 7. (** p<0.05; *** p<0.01)
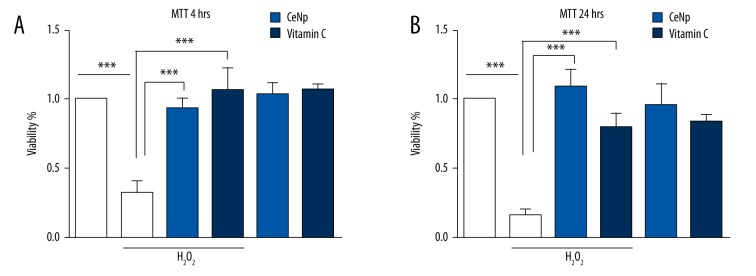
The effect of the CeNPs and vitamin C on toxicity of H2O2 in pancreatic insulinoma cells
We used the well-known MTT reduction assay to evaluate the effects of CeNPs (0.5 mg/mL) and vitamin C (40 μM) on H2O2 (50 μM) on RIN-m5f cell viability (Figure 3A). CeNPs and vitamin C protected cells from H2O2 induced toxicity. With the passage of time, CeNPs still maintained tremendous protection function, however, the effect of vitamin C was weakened by a quarter (Figure 3B). The mechanism of CeNPs protection of β-cells from apoptosis induced by ROS (such as hydroxyl radicals and hydrogen peroxide) are shown in Figure 4.
Figure 3.
The effect of CeNPs (0.1 mg/mL) and vitamin C (40 μM) on toxicity of H2O2 in RIN-m5f cells. CeNPs and vitamin C protect RIN-m5f cells from the oxidative stress of H2O2 after 4 hours (A) and 24 hours (B) respectively. (** p<0.05; *** p<0.01)
Figure 4.
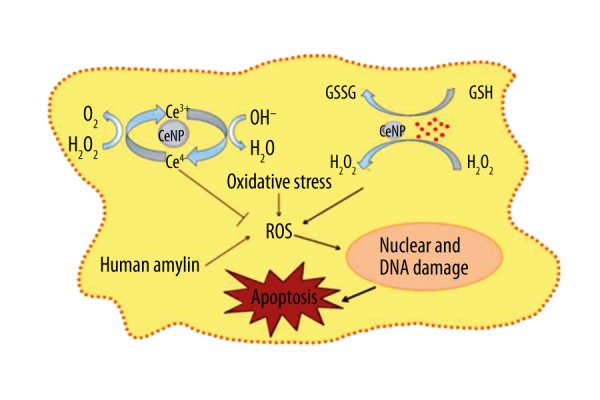
The mechanism of CeNPs protecting β-cells from apoptosis induced by reactive oxygen species (such as hydroxyl radicals, hydrogen peroxide, Cu2+).
Discussion
In previous studies, CeNPs had been reported to have the ability to counteract H2O2 challenge and apoptosis in breast fibrosarcoma cells, exerting antioxidant and anti-apoptotic effects on cardiomyocytes, neuronal cells, macrophages, and mice with autoimmune encephalomyelitis [23,28,29]. Although the antioxidant properties of CeNPs in biological systems has been reported [29–31], nothing is known about Cu2+/H2O2, or Cu2+/glutathione in β-cells, although it has been reported that in T2DM, Cu2+/H2O2, Cu2+/glutathione catalyzed formation of reactive oxygen species (ROS) and induced cytotoxicity.
CeNP has been shown to be a superoxide dismutase mimetic enzyme [32]. Investigators established important parameters for CeNP, including that different synthesis methods and surface chemical properties of CeNP show different catalytic activity. Studies have analyzed the surface of CeNP and found that under trivalent oxidation environments, abundant cerium atoms on the surface of CeNP could cause excess H2O2 to restore to a lower reduction level of its chemical state. It was also proposed that CeNP had a simulative catalase nature [33]. In mammals, there are many kinds of enzymes involved in H2O2 level modulation, including glutathione peroxidase, superoxide reductase, and catalase. Initially there was no correlation between catalase with high catalytic activity and CeNP with low activity. However research now suggests that peroxide is likely to be the most stable and abundant active oxygen in the body, and thus a catalyst such that reduced peroxide levels may be a key to preventing inflammation and metal catalytic oxidation reaction. In addition, the catalase simulative activity of CeNP, which is reversible, is related to the activity of SOD simulation on the surface of cerium. In other words, when the surface of CeNP cerium atoms are in the higher concentration of trivalent oxidation state, catalase activity is weak. Accidentally, an initial study discovered that the phosphate ion can affect the activity of CeNP. It showed that phosphoric acid can make CeNP from SOD to catalase mimetic enzyme conversed to each other in vitro closely tied with cerium reduction status.
The reaction of the chemicals in the test tube was considered to mimic the chemical reaction of CeNP with phosphate in the body, despite only milligram molecular levels of phosphate in most biological systems. So in our experiment, the phosphate buffer acted as a solvent to observe antioxidant activity of CeNP. The experimental results showed that the CeNP removal of hydrogen peroxide in the phosphate buffer was significantly higher than in PBS (Figure 2). Visible phosphate made CeNP reflect the nature of the catalase mimetic enzyme.
In the present study, we showed that CeNP had a significant inhibitory effect on Cu2+/H2O2-induced hydroxyl radical formation and protected the β-cells from damage form H2O2. We further tested whether Cu2+ can promote H2O2 production during interactions with GSH, which are pernicious to β-cells. Studies have shown that CeNP can only inhibit the apoptosis of oxidative damage. In the mechanism of action, it was found that the expending of glutathione was an important indicator that CeNP inhibited apoptosis caused by oxidative stress. This suggested that CeNP may have an impact on GSH metabolism, in particular where it is likely to have an impact is on transport proteins that transport glutathione to the extracellular fluid and on the control of REDOX in cell membranes. However, anti-oxidation of CeNP was invalid in coexisting Cu2+ with GSH at either neutral pH or acidic pH. This was likely because Cu2+ acted as a catalytic agent to promote GSH enhancing the concentration of H2O2 and causing an irreversible reaction in the system. In our study, we also found that CeNP and vitamin C, both of which act as harmless materials in mammals, can protection against oxidative stress of β-cells. Furthermore, with the passing of time, the function of vitamin C became weaker than that of CeNPs, because the former is a relative unstable substance in β-cells.
In future studies, we intend to research the interaction of CeNP and GSH in vitro and anti-diabetic effect and mechanism of CeNP in vivo.
Conclusions
We found that CeNPs can persistently inhibit Cu2+/H2O2 and evoked hydroxyl radicals and oxidative stress of β-cells, which will be tested further. These results can be applied to the development of strategies for the prevention of T2DM.
Footnotes
Statement
All authors declared that there were no conflicts in this work.
Source of support: This research was supported by a grant from the National Natural Science Foundation of China (No. 81271697, 31571017, 81271697, 81171431), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20120061110021), and the Project of Science and Technology Department of Jilin Province, China (No. 20130206069GX)
References
- 1.O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med. 1988;318:1225–30. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 2.Kuo YH, Lin CH, Shih CC. Caffeamide 36-13 regulates the antidiabetic and hypolipidemic signs of high-fat-fed mice on glucose transporter 4, AMPK phosphorylation, and regulated hepatic glucose production. J Agric Food Chem. 2015;63:2479–89. doi: 10.1155/2014/821569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gjesing AP, Pedersen O. ‘Omics’-driven discoveries in prevention and treatment of type 2 diabetes. Eur J Clin Invest. 2012;42:579–88. doi: 10.1111/j.1365-2362.2012.02678.x. [DOI] [PubMed] [Google Scholar]
- 4.Seshasai SRK, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolberg JA, Jorgensen T, Gerwien RW. Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort. Diabetes Care. 2009;32:1207–12. doi: 10.2337/dc08-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan YW, Lam FC, Leung PC, et al. Antihyperglycemic and antioxidative effects of a herbal formulation of Radix Astragali, Radix Codonopsis and Cortex Lycii in a mouse model of type 2 diabetes mellitus. Phytother Res. 2009;23:658–65. doi: 10.1002/ptr.2694. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura S, Okabayashi S, Ageyama N, et al. Transthyretin amyloidosis and two other aging-related amyloidoses in an aged vervet monkey. Vet Pathol. 2008;45:67–72. doi: 10.1354/vp.45-1-67. [DOI] [PubMed] [Google Scholar]
- 8.Guardadomendoza R, Davalli AM, Chavez AO, et al. Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Nati Sci USA. 2009;106:13992–97. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maryon EB, Molloy SA, Kaplan JH. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am J Physiol Cell Physiol. 2013;304:768–79. doi: 10.1152/ajpcell.00417.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi H, Yano M, Fujita Y, Wakusawa S. Compound overload of copper and iron in patients with Wilson’s disease. Med Mol Morphol. 2006;39:121–26. doi: 10.1007/s00795-006-0326-7. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES. Serum copper concentration and coronary heart disease among US adults. Am J Epidemiol. 2000;151:1182–88. doi: 10.1093/oxfordjournals.aje.a010168. [DOI] [PubMed] [Google Scholar]
- 12.Eskici G, Axelsen PH. Copper and oxidative stress in the pathogenesis of Alzheimer’s disease. Biochemistry. 2012;51:6289–311. doi: 10.1021/bi3006169. [DOI] [PubMed] [Google Scholar]
- 13.Lee EC, Ha E, Singh S, et al. Copper(II)-human amylin complex protects pancreatic cells from amylin toxicity. Phys Chem Chem Phys. 2013;15:12558–71. doi: 10.1039/c3cp44542a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tikkanen L, Matsushima T, Natori S, Yoshihira K. Mutagenicity of natural naphthoquinones and benzoquinones in the Salmonella/microsome test. Mutat Res. 1983;124:25–34. doi: 10.1016/0165-1218(83)90182-9. [DOI] [PubMed] [Google Scholar]
- 15.Inbaraj JJ, Chignell CF. Cytotoxic action of juglone and plumbagin: A mechanistic study using HaCaT keratinocytes. Chem Res Toxicol. 2004;17:55–62. doi: 10.1021/tx034132s. [DOI] [PubMed] [Google Scholar]
- 16.Rippmann JF, Hobbie S, Daiber C, et al. Phosphorylation-dependent proline isomerization catalyzed by Pin1 is essential for tumor cell survival and entry into mitosis. Cell Growth Differ. 2000;11:409–16. [PubMed] [Google Scholar]
- 17.Varga Z, Bene L, Pieri C, et al. The effect of juglone on the membrane potential and whole-cell K + currents of human lymphocytes. Biochem Biophys Res Commun. 1996;218:828–32. doi: 10.1006/bbrc.1996.0147. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Zhao Y, Guo Y, et al. Significant longevity-extending effects of a tetrapeptide from maize on Caenorhabditis elegans under stress. Food Chem. 2012;130:254–60. [Google Scholar]
- 19.Masui T, Hirai H, Imanaka N, et al. Synthesis of cerium oxide nanoparticles by hydrothermal crystallization with citric acid. Sci Lett. 2002;21:489–91. [Google Scholar]
- 20.Hirst SM, Karakoti AS, Tyler RD, et al. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5:2848–56. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 21.Naganuma T, Traversa E. Stability of the Ce3+ valence state in cerium oxide nanoparticle layers. Nanoscale. 2012;4:4950–53. doi: 10.1039/c2nr30406f. [DOI] [PubMed] [Google Scholar]
- 22.Karakoti AS, Singh S, Kumar A, et al. PEGylated nanoceria as radical scavenger with tunable redox chemistry. J Am Chem Soc. 2009;131:14144–45. doi: 10.1021/ja9051087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu J, Wang K, Kolattukudy PE. Cerium oxide nanoparticles inhibit oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J Pharmacol Exp Ther. 2011;338:53–61. doi: 10.1124/jpet.111.179978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan EB, Unthank JK, Castillo-Melendez M, et al. Novel method for in vivo hydroxyl radical measurement by microdialysis in fetal sheep brain in utero. J Appl Physiol. 2005;98:2304–10. doi: 10.1152/japplphysiol.00617.2004. [DOI] [PubMed] [Google Scholar]
- 25.Manevich Y, Held KD, Biaglow JE. Coumarin-3-carboxylic acid as a detector for hydroxyl radicals generated chemically and by gamma radiation. Radi Res. 1997;148:580–91. [PubMed] [Google Scholar]
- 26.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radicals Biol Med. 1995;18:321–36. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 27.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 28.Clark A, Zhu A, Kai S, Petty HR. Cerium oxide and platinum nanoparticles protect cells from oxidant-mediated apoptosis. J Nanopart Res. 2011;13:5547–55. doi: 10.1007/s11051-011-0544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estevez AY, Erlichman JS. The potential of cerium oxide nanoparticles (nanoceria) for neurodegenerative disease therapy. Nanomedicine. 2014;9:1437–40. doi: 10.2217/nnm.14.87. [DOI] [PubMed] [Google Scholar]
- 30.Park EJ, Choi J, Park YK, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245:90–100. doi: 10.1016/j.tox.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Hou Y, Cheng G, et al. Cerium oxide nanoparticles protect endothelial cells from apoptosis induced by oxidative stress. Biol Trace Elem Res. 2013;154:156–66. doi: 10.1007/s12011-013-9678-8. [DOI] [PubMed] [Google Scholar]
- 32.Korsvik C, Patil S, Seal S, Self WT. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun (Camb) 2007;10:1056–58. doi: 10.1039/b615134e. [DOI] [PubMed] [Google Scholar]
- 33.Pirmohamed T, Dowding JM, Singh S, et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun (Camb) 2010;46(16):2736–38. doi: 10.1039/b922024k. [DOI] [PMC free article] [PubMed] [Google Scholar]