Abstract
Background
Early-onset prostate cancer patients (aged ≤55 years) from Western countries have been well characterized in previous studies. However, the clinicopathological and prognostic characteristics of early-onset Chinese prostate cancer patients have not yet been assessed. This study aimed to examine the clinicopathological and prognostic factors of prostate cancer patients aged ≤55 years in a single Chinese center.
Material/Methods
One hundred six prostate cancer patients aged ≤55 years with complete clinicopathological data who were treated at our hospital between January 2000 and June 2014 were selected for this study. Survival rate was investigated by Kaplan-Meier analysis, and prognostic factors were examined by univariate and multivariate analysis.
Results
The median time from the onset of symptoms to diagnosis was 3.5 months (range, 2–55 months). The median time after endocrine therapy to development of androgen-independent prostate cancer was 10.5 months. A total of 54 patients died (50.9%), of whom 96.2% died from prostate cancer. The 1-, 3-, and 5-year overall survival rates were 88.7%, 66.2%, and 36.0%, respectively. Univariate and multivariate analysis showed that T staging, visceral metastasis, pathological pattern, and Gleason sum were independent prognostic factors in these patients.
Conclusions
Prostate cancer patients aged ≤55 years are often omitted or misdiagnosed in China. Furthermore, the pathology patterns in this age group were mostly complicated with a high degree of malignancy. Late staging, visceral metastasis, pathological pattern, and high Gleason score were independent prognostic factors in these patients. Comprehensive therapy combined with local therapy is an effective treatment strategy.
MeSH Keywords: China, Prognosis, Prostatic Neoplasms
Background
Prostate cancer (PCa) is generally described as a disease of aging and is rarely seen in young patients [1]. However, in the past few years, epidemiological studies have revealed that the onset age of PCa is 1–5 years earlier in Europe and America [2]. The morbidity of Pca patients aged <55 years accounts for this clearly increasing trend. Moreover, Pca mortality at the age of ≤55 years is extremely low, but rises rapidly in men aged >55 years. Based on the above-mentioned characteristics, we usually regard 50–55 years of age as the demarcation point between younger and older patients in the literature [3]. Nowadays in China, because of an aging population, changes in dietary intake, rural population urbanization, and the development of PSA examination techniques, PCa exposure factors have changed, resulting in an apparent escalating trend of morbidity. Among patients ≤55 years, an upward trend of incidence has become a cause of concern. We reviewed a series of foreign studies and found that the clinical manifestation, pathological features, treatment modalities, and prognosis significantly differ in PCa patients who are ≤55 years old. In this study, results from 106 patients aged ≤55 years from a single center in China are reported.
Material and Methods
The study protocol received approval from the Human Ethics Committee, Tianjin Medical University Cancer Institute and Hospital, China. Written informed consent was obtained from each subject prior to inclusion in the study. This study is registered with ClinicalTrials.gov (NCT02605226, NCT02615223).
Clinical materials
A total of 835 patients with PCa were admitted to Tianjin Medical University Cancer Institute and Hospital between January 2005 and June 2015, of which 106 patients aged ≤55 years were enrolled in this study. Histological diagnosis was classified according to the World Health Organization classification of tumors: “Pathology & genetics of tumours of the urinary system and male genital organs, 2004.” Clinical stages were defined by the American Joint Committee on Cancer system, 6th edition (2002). Age groups were stratified according to the Chinese Cancer Registry Annual Report [4]. Patients’ comorbidity profiles were scored using the Charlson Comorbidity Index (CCI) [5], and were stratified into two groups: 0 and ≥1.
Follow-up and efficacy evaluation
Follow-up was commenced on the day of diagnosis confirmation. Outpatient follow-up or telephone follow-up was up to June 2015. The primary endpoint of follow-up was death. Local therapy was defined as surgery, radioactive seed implantation, cryotherapy, or external beam radiotherapy. Castration-resistant prostate cancer (CRPC) was defined using two Prostate Cancer Working Group criteria: 25% PSA increase from the androgen deprivation therapy (ADT), PSA nadir, and a PSA increase to 2 ng/mL [6].
Statistical methods
The statistical analysis was carried out using SPSS software version 17.0 (IBM Corporation, Chicago, Illinois, USA). Population statistics and basic features were summarized through descriptive data, measurement data used the independent-sample t-test, enumeration data comparison adopted the chi-square test, and the overall survival curve was calculated using the Kaplan-Meier method. Nine prognostic factors (PSA value, CCI score, T staging, lymph node metastasis, bone metastasis, visceral metastasis, pathological type, Gleason sum, and local therapy) were evaluated by univariate analysis. Significant variables of prognostic factors were used in the subsequent multivariate analysis using Cox’s proportional hazards model. All P values <0.05 were considered to indicate statistical significance.
Results
The clinical and pathological characteristics of patients are shown in Table 1.
Table 1.
Patient clinicopathological features.
| Patient characteristics | |
|---|---|
| Age, yr, median (range) | 53 (16–55) |
| Follow-up, mo, median (range) | 53 (2–109) |
| PSA level, ng/dl, median (range) | 82.4 (0.14–5385.00) |
| From symptom to Dx, mo, median (range) | 4 (1–20) |
| CCI score no. (%) | |
| 0 | 90 (84.9%) |
| ≥1 | 16 (15.1%) |
| Pathological pattern, no. (%) | |
| Acinous carcinoma | 94 (88.7%) |
| Other | 12 (11.3%) |
| Status of lymph nodes metastases, no. (%) | |
| Metastases | 40 (37.7%) |
| No metastases | 66 (62.3%) |
| Status of bone metastases, no. (%) | |
| Metastases | 56 (52.8%) |
| No metastases | 50 (47.2%) |
| Status of Visceral metastases, no. (%) | |
| Metastases | 20 (20.8%) |
| Liver Metastases | 12 (11.3%) |
| Lung Metastases | 10 (9.4%) |
| No metastases | 86 (79.2%) |
| Clinic T stage, no. (%) | |
| T2 | 28 (26.4%) |
| T3 | 45 (42.4%) |
| T4 | 29 (27.4%) |
| Unstaged | 4 (3.8%) |
| Gleason sum, no. (%) | |
| ≤6 | 10 (9.4%) |
| 7 | 22 (20.8%) |
| 8–10 | 69 (65.1%) |
| Unscored | 5 (4.7%) |
| Primary treatment | |
| Local therapy | 17 (16.0%) |
| Local therapy + ADT | 24 (22.6%) |
| Local therapy + chemotherapy | 3 (2.8%) |
| ADT | 61 (57.5%) |
| Chemotherapy | 1 (0.9%) |
PSA – prostate specific antigen; Dx – diagnosis; CCI – Charleston comorbidity index, CRPC – castration resistant prostate cancer; ADT – androgen deprivation therapy; mo, month.
Age of onset
The median age of onset was 53 years (range, 16–55 years). Patients aged 55 years, 45–49 years, 40–44 years, 35–39 years, 30–34 years, and below 30 years accounted for 78.3% (83/106), 11.3% (12/106), 2.8% (3/106), 2.8% (3/106), 0.9% (1/106), and 3.8% (4/106) of cases, respectively. The median age of onset for patients with acinus carcinoma and non-acinus carcinoma was 53 and 46 years, respectively. The statistical difference was obvious (t=61.801, P<0.001).
Main clinical symptoms and manifestations
A total of 73.6% (78/106) of patients were hospitalized because of nonspecific lower urinary tract symptoms, which are commonly accompanied by dysuria; patients hospitalized on account of metastatic bone pain, elevated PSA discovered by health examination, hematuria, deep venous thrombosis, and discovery of metastatic cervical lymph nodes, lower limb movement disorder, and difficult defecation accounted for 8.5% (9/106), 6.6% (7/106), 3.8% (4/106), 2.8% (3/106), 1.9% (2/106), 1.9% (2/106), and 0.9% (1/106) of cases, respectively. The median time from symptom manifestation to diagnosis clarification was 3.5 months (range, 2–55 months).
Baseline PSA value
A total of 13 patients had a PSA that was less than 4.0 ng/mL, accounting for 12.3% of the group. There were four patients with acinus carcinoma and nine patients with non-acinus carcinoma. The statistical difference was obvious (P<0.001) (Table 2).
Table 2.
Comparison of metastasis characteristics in patients with adenocarcinoma and non-acinus carcinoma and PSA levels.
| Variable | Adenocarcinoma (n=94) (%) | Non-acinus carcinoma (n=12) (%) | χ2 | P |
|---|---|---|---|---|
| Lymphatic metastasis | 1.475 | 0.225 | ||
| Absent | 62 (66.0) | 10 (83.3) | ||
| Present | 32 (34.0) | 2 (16.7) | ||
| Osseous metastasis | 6.362 | 0.012 | ||
| Absent | 42 (44.7) | 10 (83.3) | ||
| Present | 52 (55.3) | 2 (16.7) | ||
| Visceral metastases | 0.043 | 0.836 | ||
| Absent | 76 (80.9) | 10 (83.3) | ||
| Present | 18 (19.1) | 2 (16.7) | ||
| PSA | 53.313 | <0.001 | ||
| <4 (ng/ml) | 5 (5.3) | 10 (83.3) | ||
| ≥4 (ng/ml) | 89 (94.7) | 2 (16.7) |
Main complications
Patients with a CCI score of 0 and patients with a score of ≥1 accounted for 84.9% and 15.1% of cases, respectively. Six patients experienced two complications, and 10 patients had one complication. Type 2 diabetes was the most common complication (8.5%, 9/106), followed by hypertension (7.5%, 8/106) and coronary heart disease (2.8%, 3/106).
Pathological characteristics
Patients with acinus carcinoma and non-acinus carcinoma accounted for 88.8% and 11.3% of cases, respectively, including 16.7% neuroendocrine neoplasm, 41% sarcomatoid carcinoma, 16.7% rhabdomyosarcoma, and 8.3% leiomyosarcoma, stromal sarcoma, and squamous-cell carcinoma. Gleason grades (sum) demonstrated that 8.5%, 20.8%, and 61.0% of patients had a Gleason score of 2–6, 7, and 8–10, respectively. Four patients with sarcoma and one patient with squamous carcinoma were not suitable for Gleason scoring.
Clinical staging
Patients in T2 staging accounted for 26.4% of cases, including 67.9% of T2N0M0 cases. Nine other patients were diagnosed with lymphatic metastasis and/or distant metastasis, and nine patients with sarcoma were not suitable for tumor-node-metastasis (TNM) staging (3.8%). The lymphatic metastasis rate was 32.1% (34/106). Among them, 32 patients with acinus carcinoma and two patients with non-acinus carcinoma had lymphatic metastasis; however, this was not statistically significant (P=0.225). The occurrence rate of osseous metastasis was 50.9%. Fifty-two patients with acinus carcinoma and two patients with non-acinus carcinoma had osseous metastasis. The lymphatic metastasis occurrence was statistically significant (P=0.012). The occurrence rate of visceral metastases was 18.9%. Among them, 18 patients with acinus carcinoma and two patients with non-acinus carcinoma had visceral metastases; however, this was not statistically significant (P=0.836) (Table 2).
Treatment methods
Primary treatment was as follows: 17 patients received local therapy, 24 patients received local therapy combined with ADT, 3 patients received local therapy combined with chemotherapy, 61 patients received ADT, and 1 patient was treated with chemotherapy only. To summarize, 44 patients underwent local therapy and accounted for 41.5% of cases; 85 patients (80.2%) were administered endocrinotherapy.
A total of 91.8% (78/85) patients developed castration-resistant disease, and the median duration of PCa sensitivity to ADT (defined as the time from ADT initiation to the onset of castration resistance) was 10.5 months (range, 6–23 months). Treatment of castration-resistant patients included chemotherapy with docetaxel (79.5%, 62/78), second-line hormonal therapy (11.5%, 9/78), and novel agents (9.0%, 7/78).
Treatment effects and influencing factors
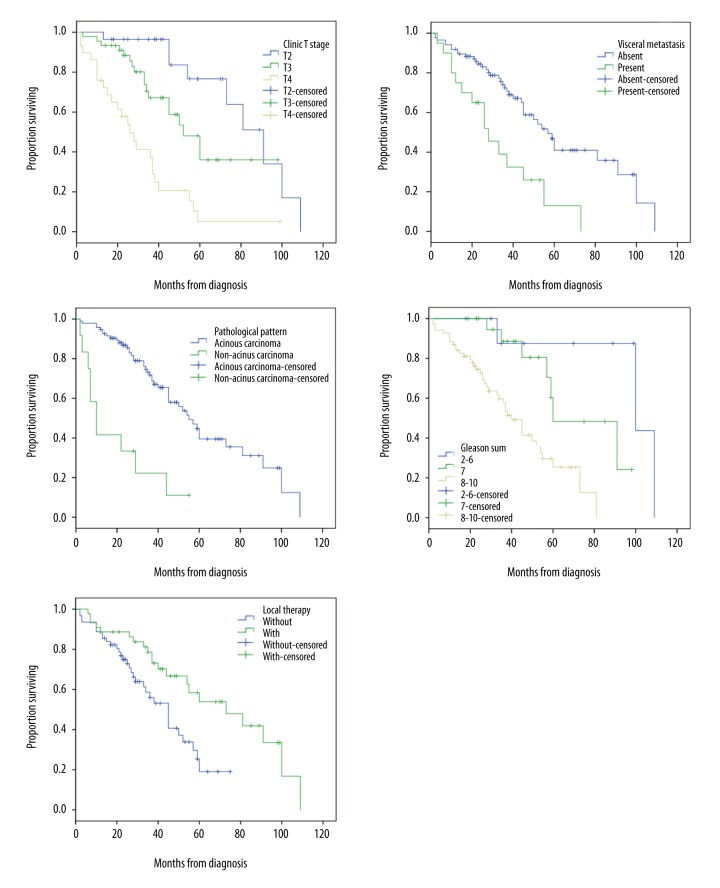
The median follow-up was 53 months (range, 12–98 months). During this period, 50.9% (54/106) of patients died. Patients who died of tumor-related and non-tumor-related diseases accounted for 96.3% and 3.7% of cases, respectively. The median overall survival (OS) was 52 months (range, 2–109 months). The 1-, 3- and 5-year OS rates were 88.7%, 66.2%, and 36.0%, respectively. Univariate (Table 3) and multivariate (Table 4) analysis showed that T staging (P<0.001), visceral metastasis (P=0.027), pathological pattern (P=0.023), Gleason sum (P<0.001), and local therapy (P=0.013) were independent prognostic factors in these patients (Figure 1).
Table 3.
Univariate analysis of clinicopathological factors and survival time of prostate cancer patients of ≤55 years.
| Predictor | Median survival (95%CI) (mo) | χ2 | P value |
|---|---|---|---|
| PSA | 0.016 | 0.899 | |
| <20 ng/ml(n=31) | 59 (27.5–90.5) | ||
| ≥20 ng/ml(n=75) | 52 (41.0–63.0) | ||
| CCI score | 0.045 | 0.832 | |
| 0 (n=90) | 54 (40.9–67.1) | ||
| ≥1 (n=16) | 44 (35.4–52.6) | ||
| T staging | 31.789 | <0.001 | |
| T2 (n=28) | 91 (70.1–111.9) | ||
| T3 (n=45) | 52 (39.1–64.9) | ||
| T4 (n=29) | 26 (18.0–34.0) | ||
| Lymphatic metastasis | 3.141 | 0.076 | |
| Absent (n=66) | 57 (40.1–73.9) | ||
| Present (n=40) | 37 (33.7–40.3) | ||
| Osseous metastasis | 0.051 | 0.822 | |
| Absent (n=50) | 54 (43.6–64.4) | ||
| Present (n=56) | 45 (37.1–52.9) | ||
| Visceral metastasis | 11.641 | 0.001 | |
| Absent (n=86) | 57 (48.3–65.7) | ||
| Present (n=20) | 28 (16.2–39.8) | ||
| Pathological pattern | 23.159 | <0.001 | |
| Acinous carcinoma (n=94) | 55 (44.6–65.4) | ||
| Non-acinus carcinoma(n=12) | 10 (5.0–15.0) | ||
| Gleason sum | 18.364 | <0.001 | |
| 2–6 (n=10) | 100 (5.5–194.5) | ||
| 7 (n=22) | 60 (32.5–87.5) | ||
| 8–10 (n=69) | 40 (32.6–47.4) | ||
| Local therapy | |||
| Without(n=62) | 45 (35.9–54.1) | 7.274 | 0.007 |
| With(n=44) | 73 (42.4–103.6) |
Table 4.
Multivariate analysis of prostate cancer patients of ≤55 years.
| Predictor | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| T staging | 2.644 | 1.678–4.166 | <0.001 |
| Visceral metastases | 2.232 | 1.097–4.542 | 0.027 |
| Pathological pattern | 2.996 | 1.166–7.699 | 0.023 |
| Gleason sum | 4.454 | 2.029–9.774 | <0.001 |
| Local therapy | 0.408 | 0.202–0.825 | 0.013 |
Figure 1.
Overall survival stratified by T staging, visceral metastases, pathological pattern, Gleason sum, and local therapy.
Discussion
Despite the relatively lower incidence of PCa in Asian countries [7], an upward trend has become apparent in recent years. According to data from the 2012 Chinese Cancer Registration Annual Report, between 1998 and 2008, the increases in incidence in patients aged ≤55 years are similar to the data on the same age group in the USA, up to 50% or more. In China, the population aged ≤55 years is an important work force with a longer life expectancy, and understanding the clinical characteristics and influencing factors of PCa, in parallel with early prevention and intervention, is critical not only in this population but particularly in developing countries.
PCa onset in patients aged ≤55 years is not easy to detect. In this group, only 26.4% of patients were diagnosed at the limited-disease stage (T2 stage), which differs significantly from the percentage in developed countries [8]. The causes were outlined as follows: patients have no typical clinical symptoms, and when a progressed tumor is involved in the urethra and other adjacent organs, it is easily mixed up with prostatic hyperplasia. Moreover, China has not yet established a perfect screening mechanism, so it is hard to discover PCa via active monitoring. Additionally, some doctors lack the necessary understanding or don’t think they need to give attention to young PCa patients, which is the main reason for missed diagnoses or misdiagnoses. This, then, results in a longer time from seeing a doctor to obtaining a correct diagnosis, at which point most patients are at intermediate and advanced stages.
PCa clinical development is relatively slow. Therefore, the impacts of PCa screening are more obvious than for other tumors. Epidemiological studies show that tumor de-differentiation is the main mechanism of PCa progression. Different conclusions have been made regarding whether PSA screening enables diagnosis at an earlier stage [9–11]. However, these sub-clinical (preclinical stage) tumors [12] can be identified up to an average of 10 years in advance, which may allow early-stage diagnosis [9,10]. Based on PSA screening, while the incidence in developed countries is higher than that in developing Asian countries, the mortality is lower [13] and shows a downward trend [14]. Compared with PSA values of older patients, PSA values of young patients suffer from less interference [15], so these patients are most likely to benefit [16,17] from PSA screening. Therefore, it is critical to set up a screening system suitable for clinical practice in China that pays particular attention to high-risk populations aged ≤55 years.
Patients in Asian countries present with high-grade PCa, in contrast to Europe and America [18]. Previous research also revealed that young PCa patients at a high-grade local progressive stage were 3–5-fold [19] more common than elderly patients who died because of PCa. Our data showed that, among PCa patients ≤55 years of age, poorly differentiated adenocarcinoma accounted for 68.1% (64/94) of cases, a percentage that is much higher than the SEER data [20]. Additionally, studies suggest that the lower the PSA level, the poorer the differentiation [21]. Neuroendocrine tumors and sarcomatoid carcinoma may lose their ability during differentiation, and non-acini carcinomas do not even secrete PSA, showing a normal PSA level. In this group, non-acini carcinoma accounts for 11.3% (12/106) of cases, which is significantly higher than the percentage observed in the USA (1.5%) [20]. These are important explanations for the poor prognosis of young Chinese PCa patients.
A relatively high percentage of PCa patients have metastasis, which mostly occurs to the bone. Many occurrences are concomitant with liver and/or lung metastasis, while viscus metastasis is an independent risk factor (HR: 2.232, 95% CI: 1.097–4.542) that affects prognosis. Past reports showed that the survival and prognosis of patients with bone metastasis and internal organ metastasis were significantly inferior to the survival and prognosis of patients with simple bone metastasis [22]. mCRPC patients who underwent docetaxel therapy had the worst prognosis because of liver metastasis, followed by lung metastasis, while the prognosis of patients with bone metastasis is better [23]. The mechanism with regards to the poor prognosis of patients with viscus metastasis is not clear. Current studies suggest that differences exist in the clones of various metastatic positions, as well as gene expression, microenvironment, and treatment response. This is thought to cause differences in prognosis [24].
Five-year OS (36.0%) in PCa patients aged ≤55 years in this group is similar to that of patients in Japan (36.3%), but significantly lower than that of European and American populations (82%) [25,26]. Endocrine therapy is a first-line treatment for PCa but not satisfactory in this age group. The median time after endocrine therapy before PCa patients aged ≤55 years developed CRPC was only 10.5 months, similar to that reported by Ribeirio et al. [27] (11 months). Hussain et al. [28] suggested that the shorter the effective time of hormonal therapy, the worse the prognosis. Humphreys et al. [29] discovered that the median time before development of CRPC and the OS of PCa patients aged <55 years were significantly inferior to those of patients in the older age group. The specific mechanism underlying this difference remains unclear. Recently, some researchers proposed that genetic factors may significantly influence young PCa patients. Indeed, Forrest et al. [30] suggested that 43% of PCa patients <55 years had genetic susceptibility. Moreover, low-age PCa was associated with genetic susceptibility to gene rearrangement [31], showing that androgen mediates related ETS gene fusion, directly mediates AR activities, and affects endocrine treatment outcomes.
Young patients have a good tolerance to drugs and aggressive treatment because of fewer concomitant diseases. In the USA, approximately 84.9% of young patients undergo local therapy [19] to achieve a cure; however, only 41.5% (44/106) of patients in this group in our study were given local therapy, which represents a significant difference. This is likely to be because the disease was in the advanced stage in these patients. At present, with regard to metastatic PCa, greater emphasis is placed on whether local therapy will achieve a benefit. The SEER data [32] between 2004 and 2010 showed that local radical treatment or radiotherapy may benefit survival of patients with metastatic PCa. The study results also suggest that, compared with simple endocrine therapies, combined local therapy contributes to increased OS (P=0.013). This indicates that local therapy has an important role in the treatment of low-age PCa.
As a retrospective study, the present work has some limitations. First, PET-CT scans were not routinely performed in the evaluation of patients; thus, lymph node and visceral metastases may be underestimated. Second, some potential risk factors were not included and analyzed, such as obesity [33] and the consumption of dietary fat [34]. Finally, as hereditary PCa is common among men with early-onset PCa [3], the impact of genetic factors on these patients should also be considered.
Conclusions
PCa patients aged ≤55 years are easily omitted or misdiagnosed, and are more likely to be diagnosed at an advanced stage. Therefore, it is critical to set up a screening system suitable to this population. The pathology patterns at this age are complicated and characterized by a high degree of malignancy and a relatively high percentage of metastasis. Late staging, visceral metastasis, pathological pattern, and high Gleason score were independent prognostic factors in these patients. Endocrine therapy alone is not sufficient to counteract the poor prognosis of this population. Comprehensive therapy combined with local therapy has contributed to improved prognosis and is worthy of further exploration.
Acknowledgements
The authors thank the Chinese Cancer Registry and the Prostate Cancer Registry of Tianjin Medical University and Hospital. We would like to thank those men with prostate cancer who have donated their time during this research.
Footnotes
Conflict of interest
None declared.
Source of support: This study is registered with ClinicalTrials.gov (NCT02605226, NCT02615223). This work was supported by the National Natural Science Foundation of China (No. 81471761, No.81501568) and the National Key Clinical Specialist Construction Programs of China (No. 2013-544)
References
- 1.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 2.Neppl-Huber C, Zappa M, Coebergh JW, et al. EUNICE Survival Working Group. Changes in incidence, survival and mortality of prostate cancer in Europe and the United States in the PSA era: Additional diagnoses and avoided deaths. Ann Oncol. 2012;23:1325–34. doi: 10.1093/annonc/mdr414. [DOI] [PubMed] [Google Scholar]
- 3.Hussein S, Satturwar S, Van der Kwast T. Young-age prostate cancer. J Clin Pathol. 2015;68:511–15. doi: 10.1136/jclinpath-2015-202993. [DOI] [PubMed] [Google Scholar]
- 4.Hao J, Chen WQ. Chinese Cancer Registry Annual Report (2012) Beijing: Military Medical Science Press; 2012. National Office for Cancer Prevention and Control, National Center for Cancer Registry, Disease Prevnetion and Control Bureau, MOH. [Google Scholar]
- 5.Zelefsky MJ, Marion C, Fuks Z, Leibel SA. Improved biochemical disease-free survival of men younger than 60 years with prostate cancer treated with high dose conformal external beam radiotherapy. J Urol. 2003;170:1828–32. doi: 10.1097/01.ju.0000093720.46502.24. [DOI] [PubMed] [Google Scholar]
- 6.Scher HI, Morris MJ, Basch E, Heller G. End points and outcomes in castration-resistant prostate cancer: From clinical trials to clinical practice. J Clin Oncol. 2011;29:3695–704. doi: 10.1200/JCO.2011.35.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 8.Becker A, Tennstedt P, Hansen J, et al. Functional and oncological outcomes of patients aged <50 years treated with radical prostatectomy for localised prostate cancer in a European population. BJU Int. 2014;114:38–45. doi: 10.1111/bju.12407. [DOI] [PubMed] [Google Scholar]
- 9.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62:3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Penney KL, Stampfer MJ, Jahn JL, et al. Gleason grade progression is uncommon. Cancer Res. 2013;73:5163–68. doi: 10.1158/0008-5472.CAN-13-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pashayan N, Pharoah P, Neal DE, et al. PSA-detected prostate cancer and the potential for dedifferentiation – estimating the proportion capable of progression. Int J Cancer. 2011;128:1462–70. doi: 10.1002/ijc.25471. [DOI] [PubMed] [Google Scholar]
- 12.Draisma G, Postma R, Schroder FH, et al. Gleason score, age and screening: Modeling dedifferentiation in prostate cancer. Int J Cancer. 2006;119:2366–71. doi: 10.1002/ijc.22158. [DOI] [PubMed] [Google Scholar]
- 13.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittemore AS, Cirillo PM, Feldman D, Cohn BA. Prostate specific antigen levels in young adulthood predict prostate cancer risk: Results from a cohort of Black and White Americans. J Urol. 2005;174:872–76. doi: 10.1097/01.ju.0000169262.18000.8a. discussion 876. [DOI] [PubMed] [Google Scholar]
- 16.Strope SA, Andriole GL. Prostate-specific antigen-based risk assessment in younger men. Eur Urol. 2012;61:8–9. doi: 10.1016/j.eururo.2011.08.052. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- 17.Heijnsdijk EA, de Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: A simulation study based on ERSPC data. J Natl Cancer Inst. 2015;107:366. doi: 10.1093/jnci/dju366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi H, Epstein JI, Wakui S, et al. Differences in prostate cancer grade, stage, and location in radical prostatectomy specimens from United States and Japan. Prostate. 2014;74:321–25. doi: 10.1002/pros.22754. [DOI] [PubMed] [Google Scholar]
- 19.Lin DW, Porter M, Montgomery B. Treatment and survival outcomes in young men diagnosed with prostate cancer: A Population-based Cohort Study. Cancer. 2009;115:2863–71. doi: 10.1002/cncr.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scosyrev E, Messing EM, Mohile S, et al. Prostate cancer in the elderly: Frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118(12):3062–70. doi: 10.1002/cncr.26392. [DOI] [PubMed] [Google Scholar]
- 21.Izumi K, Ikeda H, Maolake A, et al. The relationship between prostate-specific antigen and TNM classification or Gleason score in prostate cancer patients with low prostate-specific antigen levels. Prostate. 2015;75:1034–42. doi: 10.1002/pros.22985. [DOI] [PubMed] [Google Scholar]
- 22.Drake CG. Visceral metastases and prostate cancer treatment: ‘die hard,’ ‘tough neighborhoods,’ or ‘evil humors’. Oncology (Williston Park) 2014;28:974–80. [PMC free article] [PubMed] [Google Scholar]
- 23.Halabi s, Kelly WK, Zhou H, et al. The site of visceral metastases (mets) to predict overall survival (OS) in castration-resistant prostate cancer (CRPC) patients (pts): A meta-analysis of five phase III trials. J Clin Oncol. 2014;32(Suppl) abstr 5002. [Google Scholar]
- 24.Drake CG, Kwon ED, Fizazi K, et al. Results of subset analyses on overall survival (OS) from study CA184-043: Ipilimumab (Ipi) versus placebo (Pbo) in post-docetaxel metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2014;32(Suppl 4) abstr 2. [Google Scholar]
- 25.Innos K, Lang K, Parna K, Aareleid T. Age-specific cancer survival in Estonia: recent trends and data quality. Clin Epidemiol. 2015;7:355–62. doi: 10.2147/CLEP.S87699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Onozawa M, Miyazaki J, et al. Prognostic impact of young age on stage IV prostate cancer treated with primary androgen deprivation therapy. Int J Urol. 2014;21:578–83. doi: 10.1111/iju.12389. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro M, Ruff P, Falkson G. Low serum testosterone and a younger age predict for a poor outcome in metastatic prostate cancer. Am J Clin Oncol. 1997;20(6):605–8. doi: 10.1097/00000421-199712000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Hussain MH, Goldman B, Tangen CM, et al. Use of prostate-specific antigen progression (PSA-P) to predict overall survival (OS) in patients (pts) with metastatic prostate cancer (PC): Data from S9346 and S9916. J Clin Oncol. 2008;26(Suppl 15):5015. [Google Scholar]
- 29.Humphreys MR, Fernandes KA, Sridhar SS. Impact of age at diagnosis on outcomes in men with castrate-resistant prostate cancer (CRPC) J Cancer. 2013;4:304–14. doi: 10.7150/jca.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forrest MS, Edwards SM, Houlston R, et al. CR-UK/BPG UK prostate cancer study collaborators. Association between hormonal genetic polymorphisms and early-onset prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:95–102. doi: 10.1038/sj.pcan.4500785. [DOI] [PubMed] [Google Scholar]
- 31.Weischenfeldt J, Simon R, Feuerbach L, et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell. 2013;23:159–70. doi: 10.1016/j.ccr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65:1058–66. doi: 10.1016/j.eururo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Rohrmann S, Roberts WW, Walsh PC, Platz EA. Family history of prostate cancer and obesity in relation to high-grade disease and extraprostatic extension in young men with prostate cancer. Prostate. 2003;55(2):140–46. doi: 10.1002/pros.10211. [DOI] [PubMed] [Google Scholar]
- 34.Lophatananon A, Archer J, Easton D, et al. Dietary fat and early-onset prostate cancer risk. Br J Nutr. 2010;103:1375–80. doi: 10.1017/S0007114509993291. [DOI] [PubMed] [Google Scholar]