Abstract
Background
The purpose of our study was to determine the functional role of microRNA (miR)-16 in chronic inflammatory pain and to disclose its underlying molecular mechanism.
Material/Methods
Inflammatory pain was induced by injection of complete Freund’s adjuvant (CFA) to Wistar rats. The pWPXL-miR-16, PcDNA3.1- Ras-related protein (RAB23), and/or SB203580 were delivered intrathecally to the rats. Behavioral tests were detected at 0 h, 4 h, 1 d, 4 d, 7 d, and 14 d after CFA injection. After behavioral tests, L4–L6 dorsal spinal cord were obtained and the levels of miR-16, RAB23, and phosphorylation of p38 (p-p38) were evaluated by quantitative real-time PCR (qRT-PCR). In addition, luciferase reporter assay was performed to explore whether RAB23 was a target of miR-16, and qRT-PCR and Western blotting were used to confirm the regulation between RAB23 and miR-16.
Results
The level of miR-16 was significantly decreased in the CFA-induced inflammatory pain. Intrathecal injection of miR-16 alleviates pain response and raised pain threshold. The level of RAB23 was significantly increased in the pain model, and intrathecal injection of RAB23 aggravated pain response. Luciferase reporter assay confirmed that RAB23 was a direct target of miR-16, and RAB23 was negatively regulated by miR-16. In addition, we found that simultaneous administration of SB203580 and miR-16 further alleviates pain response compared to only administration of miR-16.
Conclusions
Our findings suggest that miR-16 relieves chronic inflammatory pain by targeting RAB23 and inhibiting p38 MAPK activation.
MeSH Keywords: Inflammation, MicroRNAs, p38 Mitogen-Activated Protein Kinases, Pain
Background
Inflammatory pain results from chronic inflammation that is initiated by tissue damage and inflammation secondary to a noxious stimulus. It is characterized by allodynia and hyperalgesia both at the site of damage and in adjacent tissue. Inflammatory pain impacts daily life of patients by altering general functions and activity, often leading to motor disabilities, and may also affect the prognosis of patients [1,2]. Currently available medication options for inflammatory pain are conventional analgesics, such as nonsteroidal anti-inflammatory drugs. However, the treatment is only partially effective and may be accompanied by serious side effects, making it become a major clinical problem [3]. Therefore, it is very important to identify novel targets and effective strategies for treatment of inflammatory pain.
MicroRNAs (miRNAs) represent a group of small noncoding RNAs with 18–23 nucleotide sequences. They negatively regulate multiple gene expressions at post-transcriptional levels and moderately promote mRNA degradation by annealing to complementary sequences in the 3′ untranslated regions (UTRs) of target mRNAs [4–7]. Many miRNAs have been found in the nervous system [8,9], and their functional roles in the development and pathophysiology of the nervous system have received much attention recently [10]. Down-regulation of microRNAs, such as miR-10a, -29a, -98, -99a, -124a, -134, and -183, has been shown in the trigeminal ganglion (TG) following inflammatory muscle pain induced by complete Freund’s adjuvant (CFA) in rats [11]. Additionally, decreased levels of miR-96, -182, and -183 were also described in the dorsal root ganglion (DRG) of rats in a model of chronic neuropathic pain [12]. Moreover, increased levels of miR-155 and miR-223 were detected in the mice after inflammatory pain induced by facial carrageenan injection [13]. However, little is known about the underlying specific molecular mechanisms of miRNAs regulation.
In the present study, we aimed to investigate the functional role of miR-16 in the inflammatory pain induced by CFA in rats, as well as the underlying molecular mechanisms of miR-16 regulation on target genes. Our study might provide new insight into the treatment of inflammatory pain.
Material and Methods
Animals and inflammatory pain model
Fifty 6-week old male Wistar rats (180–200 g) were obtained from Shanghai Laboratory Animal Center (Shanghai, China). The rats were housed individually in temperature-controlled micro isolator filter cages (25±2°C) under a 12-h light-dark cycle, with free access to food and water. The animals were acclimatized for at least 7 days before the experiment. The experiments were approved by Ethics Committee of our university and were performed in accordance with National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals. Inflammatory pain was induced by intra-plantar injection of 100 μl CFA (oil: saline=1: 1; Sigma-Aldrich, St. Louis, MO, USA) into the plantar surface of the right hind paw under general anesthesia. As a control, the same volume of saline was injected into the right hind paw. All the rats were sacrificed under deep anesthetized with 10% chloral hydrate (400 mg/kg) on day 14 after injection of CFA, and the L4–L6 dorsal spinal cords were obtained for further analysis.
Vector construction and transduction
The miR-16 lentivirus expression vector pWPXL-miR-16 was constructed and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) based on the manufacturer’s instructions. The pri-miR-16 sequence was amplified from normal genomic DNA and inserted into the pWPXL vector. Full-length RAB23 cDNA was amplified from the total RNA of the tissues by reverse transcriptase PCR, and cloned into the eukaryotic expression vector pcDNA3.1 (Invitrogen). A blank plasmid was used as a control. The vector was identified by the double endonuclease restriction of BamH I and Xho I. Lentivirus expression plasmids (pWPXL-miR-16 or pcDNA3.1-RAB23) were co-transfected into HEK293T cells using Lipofectamine 2000 (Invitrogen).
Intrathecal cannulation and drugs administration
The rats underwent intrathecal cannulation as described before [14]. Briefly, the animals were anesthetized with pentobarbital sodium (40 mg/kg intraperitoneally). After a 3-cm dorsal midline incision was made at the level of the T3–T4 vertebrae, a polyethylene catheter (PE-10, Clay-Adams, Parsippany, NJ, USA) was implanted into the subarachnoid space of the lumbar and sacral enlargement. The polyethylene catheter was filled with normal saline. MiR-16 (20 μL), RAB23 (20 μL), or SB203580 (30 nmol/10 μL, Sigma, St. Louis, MO, USA) was delivered via the intrathecal cannula with the help of an automatic microinjection device for 4 continuous days. After administration of these drugs, the muscles and skin were sutured and the rats were housed in individual cages to recover.
Behavioral tests
The animals received pretesting for at least 3 consecutive days before the operation and at 0 h, 4 h, 1 d, 4 d, 7 d, and 14 d after the injection. For the mechanical hyperalgesia testing, von Frey filaments (Stoelting, Kiel, WI, USA) were used. A series of gradually increasing pressures was applied to the hind paws of the animals. The pressure was applied for 5–6 s for 10 times each filament. The minimal force that initiated paw withdrawal was recorded as the mechanical withdrawal thresholds (MWTs). For the thermal preference testing, a radiant heat (BME-410A, Beijing, China) was placed under the plantar surface of the hind paw. Each hind paw was tested 3 times with an interval of 3 min. The thermal withdrawal latencies (TWLs) were recorded when the hind paw was withdrawn from the heat source with an interval of 40 s to avoid tissue damage. For the cold allodynia testing, a drop of acetone was gently applied to each hind paw with a syringe connected to a thin polyethylene tube. A brisk paw withdrawal response was considered as a sign of cold hyperalgesia. The test was repeated 3 times with a 5–10 min interval between each test.
Cell cultures
The mouse neuroblastoma and rat neuron hybrid ND8/34 cell line obtained from Sigma-Aldrich (St. Louis, MO, USA) was used in our experiment. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO Laboratory, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Life Technologies), 100 U/mL penicillin (GIBCO), and 100 μg/ml streptomycin (GIBCO) at 37°C in an atmosphere of 5% CO2.
Target prediction
MiR-16 target sites on the 3′UTR of potential target genes were predicted by bioinformatics analysis using TargetScan 6.2 (http://www.targetscan.org) and/or microRNA.org (http://www.microrna.org).
Luciferase reporter assay
The pMIR-report-3′UTR plasmid for the RAB23 gene was constructed. The wild-type (WT) or mutated (Mut) RAB23 3′-UTR sequence was isolated, amplified, and subcloned into the region directly downstream of a cytomegalovirus promoter-driven firefly luciferase cassette in a pMIR-report vector (Cambridge, MA) at the SpeI and HindIII sites. For the reporter assay, ND8/34 cells were transfected with 0.5 μg pMIR-RAB23 3′UTR-WT or pMIR- RAB23 3′UTR-Mut and 50 nM antisense oligonucleotide (ASO)-miR-16, ASO-negative control (NC), miR-16 mimics, and corresponding NC (Sangon Biotech Co., Ltd., Shanghai, China) using the Lipofectamine 2000 reagent (Invitrogen) at 70–80% cell confluence. At 48 h post-transfection, luciferase activity was assessed by using the dual-luciferase activity assay system (Promega, Madison, USA) according to the manufacturer’s instructions. Firefly luciferase activity was normalized to Renilla luciferase activity. Tests were performed in triplicate.
Quantitative real-time (qRT-PCR)
Total RNA, including miRNA, was extracted from the tissues and ND8/34 by using a mirVana miRNA Isolation Kit (Ambion, Inc., Austin, TX, USA) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 1 mg of total RNA by using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Darmstadt, Germany) with reverse primer. QRT-PCR was performed to validate the miRNA expression of miR-16 and mRNA expression of RAB23 and p-p38 using the SYBR Green reaction kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. U6 snRNA and GAPDH were used as an internal control for the expression of miRNA and mRNA, respectively. The relative expression levels of the genes were analyzed using 2−ΔΔCT method. All experiments were run in triplicate.
Western blotting
Tissues were sonicated in the lysis buffer and centrifuged. The protein concentration was determined using the BCA Protein Assay Kit (Thermo Scientific, Pierce Protein Research Products, Rockford, USA). The protein samples were resolved by 10–12% sodium dodecyl sulfonate (SDS) - polyacrylamide gel electrophoresis (PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes. Thereafter, the membranes were blocked with 5% nonfat milk and incubated overnight at 4°C with anti-phosphorylation-p38 (p-p38) antibody (1:500; Cell Signaling), followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling). Anti-GAPDH antibody (Cell Signaling) was recorded as a reference. The membranes were then assessed using enhanced chemiluminescence and densitometric analysis. The data were analyzed by NIH Image J software (NIH Image, Bethesda, MD, USA).
Statistical analysis
All data are expressed as mean ± standard deviation (SD). The results were analyzed by the non-paired or paired Student’s t-test. All statistical analyses were performed using Statistic Package for Social Science (SPSS, version 18.0, SPSS Inc., Chicago, Illinois, USA) statistical software. P-value of <0.05 was considered to be statistically significant.
Results
MiR-16 alleviated inflammatory pain induced by CFA
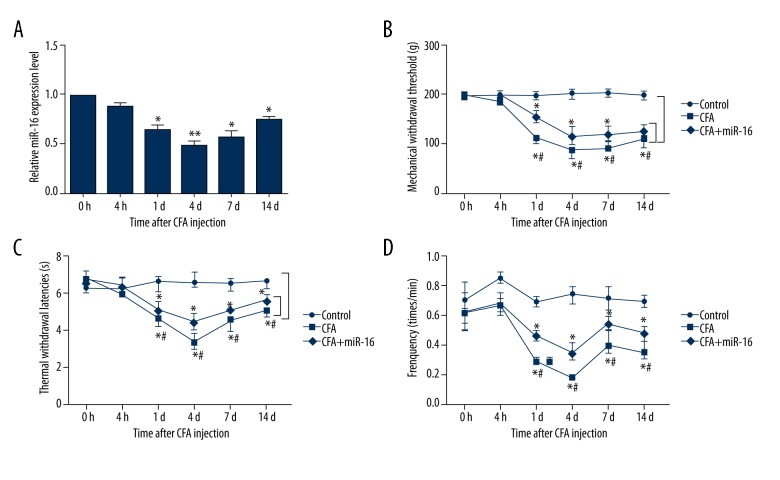
To investigate the functional role of miR-16 in the inflammatory pain, we first analyzed the expression levels of miR-16 in the tissues by using qRT-PCR. As indicated in Figure 1A, the results showed that the expression levels of miR-16 were significantly reduced at 1 d (P<0.05), 4 d (P<0.01), 7 d (P<0.05), and 14 d (P<0.05) compared to 0 h after injection of CFA, with the lowest level at 4 d. However, no significant differences were found at 4 h after CFA injection. The results indicated that miR-16 might play a significant role in inflammatory pain. To better understand the biological functions of miR-16 in the inflammatory pain, we performed intrathecal injection of miR-16 via the intrathecal cannula. The effects of miR-16 on behavioral tests including hind paw withdrawal responses to mechanical, thermal, and cold stimulation were determined. We found that the MWTs, TWLs, and frequency responses to cold stimulation were statistically reduced by CFA; however, application of miR-16 significantly increased the MWTs, TWLs, and frequency responses to cold stimulation compared to the CFA group at 1 d, 4 d, 7 d, and 14 d after injection (all P<0.05) (Figure 1B–1D). These results suggested that miR-16 could alleviate CFA-induced inflammatory pain.
Figure 1.
MiR-16 alleviates inflammatory pain induced by CFA. (A) Relative miR-16 expression level after infection of CFA; (B) Mechanical withdrawal threshold after injection; (C) Thermal withdrawal latencies after injection; (D) Frequency responses to cold stimulation after injection. MiR – microRNA; CFA – complete Freund’s adjuvant; * P<0.05 compared to 0 h after injection or control group; ** P<0.01 compared to 0 h after injection; # P<0.05 compared to CFA group.
RAB23 aggravated inflammatory pain induced by CFA
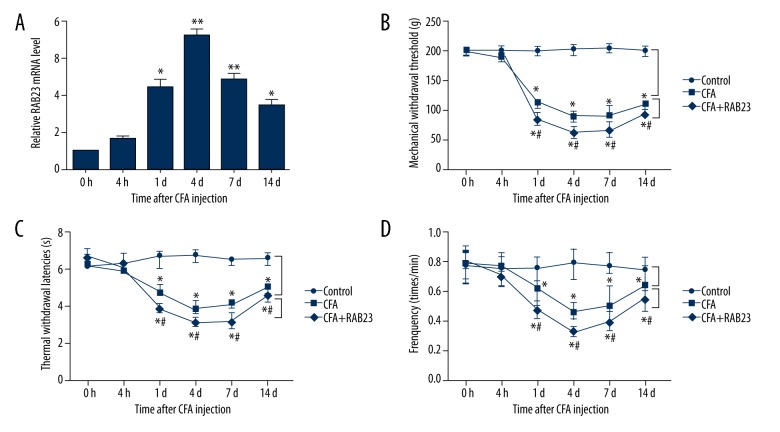
RAB23 has been reported to be a critical negative regulator of the Sonic hedgehog (SHH) signaling pathway [15] that modulates nociceptive sensitization [16]. A previous study has suggested that blocking SHH signaling prevents the development of morphine analgesic tolerance in a model of inflammatory pain [17]. Therefore, we explored the functional role of RAB23 in the inflammatory pain induced by CFA. The mRNA expression levels of RAB23 in the tissues were assessed by qRT-RCP. As shown in Figure 2A, the mRNA expression levels of RAB23 were significantly increased at 1 d (P<0.05), 4 d (P<0.01), 7 d (P<0.01), and 14 d (P<0.05) after injection, with the highest level at 4 d after injection, but there were no significant differences at 4 h after injection. Also, the effects of RAB23 on behavioral tests were studied after intrathecal injection of RAB23. The data demonstrated that the MWTs, TWLs, and frequency responses to cold stimulation were all significantly lower with intrathecal injection of RAB23 than those in the CFA group at 1 d, 4 d, 7 d, and 14 d after injection (all P<0.05) (Figure 2B–2D), suggesting that RAB23 aggravated inflammatory pain induced by CFA.
Figure 2.
RAB23 aggravates inflammatory pain induced by CFA. (A) Relative RAB23 expression level after infection of CFA; (B) Mechanical withdrawal threshold after injection; (C) Thermal withdrawal latencies after injection; (D) Frequency responses to cold stimulation after injection. MiR – microRNA; CFA – complete Freund’s adjuvant; RAB – Ras-related protein; * P<0.05 compared to 0 h after injection or control group; ** P<0.01 compared to 0 h after injection; # P<0.05 compared to CFA group.
RAB23 was a direct target of miR-16
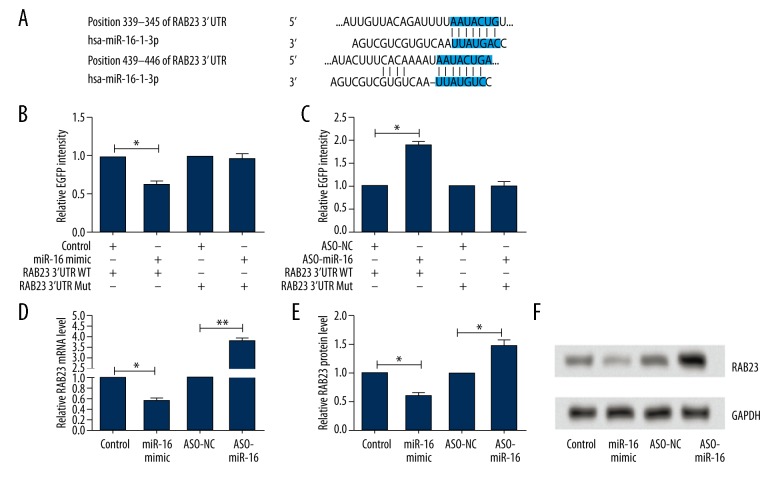
Since both miR-16 and RAB23 were involved in inflammatory pain, we therefore speculated that RAB23 might be a target gene of miR-16. To predict whether the RAB23 gene mRNA 3′-UTR might contain a miR-16 binding site, TargetScan 6.2 and microRNA.org were used in our study to confirm the prediction. Our results indicate that the 3′-UTR of RAB23 mRNA comprises several predicted miR-16-binding sites (Figure 3A). To validate whether RAB23 was indeed regulated by miR-16, ND8/34 cells were used and the reporter plasmids (RAB23 3′UTR WT or Mut) were generated. The luciferase reporter assay showed that the relative enhanced green fluorescent protein (EGFP) intensity was significantly decreased by co-transfection of miR-16 mimic with RAB23 3′UTR WT in ND8/34 cells, but was significantly increased by co-transfection of ASO-miR-16 with RAB23 3′UTR WT compared with their corresponding control groups (both P<0.05). No significant differences were found by co-transfection of miR-16 mimic or ASO-miR-16 with Mut (Figure 3B, 3C). We further investigated the regulative relationship between RAB23 and miR-16 by using qRT-PCR and Western blotting. The results demonstrated that both the mRNA and protein levels of RAB23 in ND8/34 cells were significantly downregulated by transfection of miR-16 mimic but were significantly upregulated by transfection of ASO-miR-16 (P<0.05 or P<0.01) (Figure 3D–3F). These results suggested that miR-16 inhibits the expression of RAB23.
Figure 3.
RAB23 is a direct target of miR-16. (A) Bioinformatics analysis confirms that RAB23 gene 3′-UTR carries a putative miR-16 binding site; (B, C) Relative EGFP intensity after overexpression or silencing the expression of miR-16; (D, E) Relative RAB23 mRNA level and protein level after overexpression or silencing the expression of miR-16; (F) Representative pictures of Western blot; MiR – microRNA; RAB – Ras-related protein; EGFP – enhanced green fluorescent protein; UTR – untranslated regions; WT – wide-type; Mut – mutant; ASO – antisense oligonucleotide; NC – negative control; * P<0.05 compared to corresponding control; ** P<0.01 compared to corresponding control.
MiR-16 alleviated inflammatory pain by inhibition of p38 MAPK
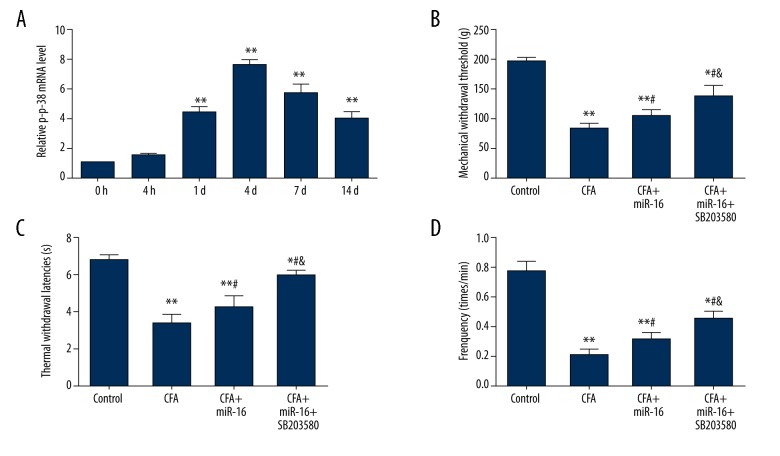
We explored whether the effects of miR-16 on inflammatory pain were involved in signaling pathways. Previous studies have suggested that the p38 MAPK signaling pathway is activated in inflammatory pain [18,19]. Therefore, we hypothesized that the effects of miR-16 on inflammatory pain may be related to the p38 MAPK pathway. To confirm this hypothesis, we assessed the expression levels of p-p38 in the tissues after injection. In line with previous studies, we also found that expression levels of p-p38 were significantly increased by injection of CFA at 1 d, 4 d, 7 d, and 14 d after injection (all P<0.01) (Figure 4A), confirming the activation of p38 MAPK in inflammatory pain. Then we applied SB203580, the inhibitor of p38, to the rats on the basis of overexpression of miR-16. The behavioral tests at 4 d after CFA injection were analyzed. As shown in Figure 4B–4D, administration of SB203580 could significantly reduce pain response and raise pain threshold (MWTs, TWLs, and frequency responses to cold stimulation) on the basis of miR-16 compared to only administration of miR-16 (all P<0.05). These results suggested that miR-16 alleviates inflammatory pain by inhibition of p38 MAPK.
Figure 4.
MiR-16 alleviates inflammatory pain by inhibition of p38 MAPK. (A) Relative p-p38 expression level after infection of CFA; (B) Mechanical withdrawal threshold after injection; (C) Thermal withdrawal latencies after injection; (D) Frequency responses to cold stimulation after injection. MiR – microRNA; CFA – complete Freund’s adjuvant; MAPK – mitogen-activated protein kinase; p-p38 – phosphorylation of p38; * P<0.05 compared to control group; ** P<0.01 compared to 0 h after injection or control group; # P<0.05 compared to CFA group; & P<0.05 compared to CFA + miR-16 group.
Discussion
In the present study, we investigated the functional role of miR-16 in inflammatory pain and explored its underlying associated mechanism. The results indicate that miR-16 is significantly decreased in CFA-induced inflammatory pain. Additionally, we demonstrat that RAB23 is a target gene of miR-16 and is negatively regulated by miR-16. Administration of miR-16 or RAB23 via intrathecal injection can release the pain response and raise the pain threshold. Furthermore, we found that miR-16 alleviates inflammatory pain by inhibition of the p38 MAPK signaling pathway. These results show that overexpression of miR-16 may be a new target for treatment of inflammatory pain.
Chronic inflammatory pain is still a severe clinical problem, and the underlying specific molecular mechanisms remain largely unknown. Recently, the critical role of miRNAs in pain has received substantial research interest. Many miRNAs have been identified to be expressed at low levels [11,12] or high levels [13] in pain. However, little research has been conducted on the functional role of miR-16 in inflammatory pain. Therefore, in our study we aimed to clarify the effect of miR-16 on inflammatory pain as well as the underlying associated mechanisms. We first induced a model of inflammatory pain by intra-plantar injection of 100 μl CFA and evaluated the expression pattern of miR-16 in the inflammatory samples. The data showed that miR-16 was down-regulated in the inflammatory samples, suggesting that miR-16 might play a significant role in the inflammatory pain. To prove the assumption, we next analyzed the behavioral test results after injection of CFA and intrathecal injection of miR-16. The threshold of mechanical hyperalgesia, the latencies of hyperalgesia, and frequency of cold hyperalgesia were all significantly increased compared to the CFA group, indicating a release of pain by overexpression of miR-16.
To explore the underlying molecular mechanism through which miR-16 alleviates CFA-induced inflammatory pain, we predicted that RAB23 gene mRNA 3′-UTR might contain a miR-16 binding site. RAB23, first identified in 1994, is a member of the Rab family of monomeric small guanosine triphosphatases (GTPases). It has been reported that RAB23 is involved in small GTPase-mediated signal transduction and intracellular protein transportation [20]. It is expressed ubiquitously in a variety of tissues, but with the predominant expression in the brain [21,22]. In addition to the functional role in embryonic development, RAB23 has been reported to be involved in gastric cancer [23,24], hepatocellular carcinoma [25], breast cancer [26], and renal disease [27]. Zhang et al. found that miR-367 inhibits the invasion and metastasis of gastric cancer by directly suppressing the expression of RAB23 [24]. In addition, RAB23 is an important negative regulator of the SHH signaling pathway [15] that regulates nociceptive sensitization [16]. Therefore, we speculated that RAB23 might be involved in the development of inflammatory pain. We assessed the mRNA levels of RAB23 in inflammatory pain and determined the effect of RAB23 in behavior tests. Our data suggest that RAB23 is significantly increased in inflammatory pain, confirming the functional role of RAB23 in inflammatory pain. After administration of RAB23 via intrathecal injection, we observed that RAB23 significantly aggravated the pain response and reduced the pain threshold. Moreover, our bioinformatic algorithms results showed that the 3′-UTR of RAB23 mRNA comprises several predicted miR-16-binding sites, demonstrating that RAB23 is one of the target genes of miR-16. To further verify the relationship between miR-16 and RAB23, the expression mRNA and protein levels of RAB23 were investigated when the levels of miR-16 were overexpressed or silenced. The data confirmed that the levels of RAB23 were inversely correlated with miR-16. Taken together, the above results suggest that miR-16 alleviates inflammatory pain by negatively regulating the expression of RAB23.
It has been well-documented that activation of p38 MAPK is involved in inflammatory pain [18,28]. An increase in the levels of p-p38, the active form of p38, has been reported in inflammation and neuropathic pain [29–31]. Inflammatory pain could be treated by inhibition of the p38 MAPK signaling pathway [32,33]. SB203580, an inhibitor of p38 MAPK, can relieve mechanical allodynia and thermal hyperalgesia [34]. In line with the above studies, our research also showed a significant increase in the levels of p-p38 in inflammatory pain. To verify that miR-16 alleviated inflammatory pain involving with the p38 MAPK signaling pathway, we simultaneously administrated SB203580 and miR-16, and then the behavioral tests at 4 d were analyzed. Our data showed that SB203580 enhanced the analgesic effect of miR-16 and raised the pain threshold compared to only administration of miR-16, demonstrating that miR-16 alleviates inflammatory pain by inhibition of p38 MAPK.
Conclusions
Our results suggest that miR-16 alleviates inflammatory pain by targeting RAB23 and inhibiting p38 MAPK activation.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: Departmental sources
References
- 1.Hinman RS, Bennell KL, Crossley KM, McConnell J. Immediate effects of adhesive tape on pain and disability in individuals with knee osteoarthritis. Rheumatology. 2003;42:865–69. doi: 10.1093/rheumatology/keg233. [DOI] [PubMed] [Google Scholar]
- 2.Janssens HJ, Janssen M, van de Lisdonk EH, et al. Use of oral prednisolone or naproxen for the treatment of gout arthritis: A double-blind, randomised equivalence trial. Lancet. 2008;371:1854–60. doi: 10.1016/S0140-6736(08)60799-0. [DOI] [PubMed] [Google Scholar]
- 3.Cheppudira BP, Garza TH, Petz LN, et al. Anti-hyperalgesic effects of AG490, a Janus kinase inhibitor, in a rat model of inflammatory pain. Biomed Rep. 2015;3:703–6. doi: 10.3892/br.2015.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasquinelli AE. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–82. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Krichevsky A, Grad Y, et al. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci USA. 2004;101:360–65. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–20. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 10.Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol. 2013;9:328–39. doi: 10.1038/nrneurol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai G, Ambalavanar R, Wei D, Dessem D. Downregulation of selective microRNAs in trigeminal ganglion neurons following inflammatory muscle pain. Mol Pain. 2007;3:15. doi: 10.1186/1744-8069-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldrich BT, Frakes EP, Kasuya J, et al. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience. 2009;164:711–23. doi: 10.1016/j.neuroscience.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poh KW, Yeo JF, Ong WY. MicroRNA changes in the mouse prefrontal cortex after inflammatory pain. Eur J Pain. 2011;15:801.e1–12. doi: 10.1016/j.ejpain.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–72. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 15.Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–98. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 16.Babcock DT, Shi S, Jo J, et al. Hedgehog signaling regulates nociceptive sensitization. Curr Biol. 2011;21:1525–33. doi: 10.1016/j.cub.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galko MJ, Babcock DT, Gutstein HB. Methods of treating pain and morphine tolerance via modulation of hedgehog signalling pathway. Google Patents. 2011 [Google Scholar]
- 18.Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy. 2004;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- 19.Svensson CI, Marsala M, Westerlund A, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–44. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 20.Gual-Soler M, Taguchi T, Stow JL, Wicking C. Encyclopedia of Signaling Molecules. Springer; 2012. Rab23; pp. 1532–36. [Google Scholar]
- 21.Olkkonen VM, Peterson JR, Dupree P, et al. Isolation of a mouse cDNA encoding Rab23, a small novel GTPase expressed predominantly in the brain. Gene. 1994;138:207–11. doi: 10.1016/0378-1119(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 22.Guo A, Wang T, Ng EL, et al. Open brain gene product Rab23: Expression pattern in the adult mouse brain and functional characterization. J Neurosci Res. 2006;83:1118–27. doi: 10.1002/jnr.20788. [DOI] [PubMed] [Google Scholar]
- 23.Hou Q, Wu YH, Grabsch H, et al. Integrative genomics identifies RAB23 as an invasion mediator gene in diffuse-type gastric cancer. Cancer Res. 2008;68:4623–30. doi: 10.1158/0008-5472.CAN-07-5870. [DOI] [PubMed] [Google Scholar]
- 24.Bin Z, Dedong H, Xiangjie F, et al. The microRNA-367 inhibits the invasion and metastasis of gastric cancer by directly repressing Rab23. Genet Test Mol Biomarkers. 2015;19:69–74. doi: 10.1089/gtmb.2014.0210. [DOI] [PubMed] [Google Scholar]
- 25.Liu YJ, Wang Q, Li W, et al. Rab23 is a potential biological target for treating hepatocellular carcinoma. World J Gastroenterol. 2007;13:1010–17. doi: 10.3748/wjg.v13.i7.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Zeng C, Bao N, et al. Effect of Rab23 on the proliferation and apoptosis in breast cancer. Oncol Rep. 2015;34:1835–44. doi: 10.3892/or.2015.4152. [DOI] [PubMed] [Google Scholar]
- 27.Huang TH, Shui HA, Ka SM, et al. Rab 23 is expressed in the glomerulus and plays a role in the development of focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2009;24:743–54. doi: 10.1093/ndt/gfn570. [DOI] [PubMed] [Google Scholar]
- 28.Dong Y, Li P, Ni Y, et al. Decreased microRNA-125a-3p contributes to upregulation of p38 MAPK in rat trigeminal ganglions with orofacial inflammatory pain. PLoS One. 2014;9:e111594. doi: 10.1371/journal.pone.0111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji RR, Samad TA, Jin SX, et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 30.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–22. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crown ED, Gwak YS, Ye Z, et al. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol. 2008;213:257–67. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang JQ, Du JY, Liang Y, Fang JF. Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1 pathway in treating inflammatory pain induced by CFA in rats. Mol Pain. 2013;9:13. doi: 10.1186/1744-8069-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin X, Wang M, Zhang J, Xu R. p38 MAPK: A potential target of chronic pain. Curr Med Chem. 2014;21:4405–18. doi: 10.2174/0929867321666140915143040. [DOI] [PubMed] [Google Scholar]
- 34.Ma W, Quirion R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin Ther Targets. 2005;9:699–713. doi: 10.1517/14728222.9.4.699. [DOI] [PubMed] [Google Scholar]